| Revision as of 09:41, 10 April 2011 editGrutness (talk | contribs)Autopatrolled, Administrators316,588 editsmNo edit summary← Previous edit |
Latest revision as of 07:31, 7 January 2022 edit undoJJMC89 bot III (talk | contribs)Bots, Administrators3,708,346 editsm Moving Category:O-Methylated phenylpropanoids to Category:O-methylated phenylpropanoids per Misplaced Pages:Categories for discussion/Speedy |
| (17 intermediate revisions by 14 users not shown) |
| Line 1: |
Line 1: |
|
{{chembox |
|
{{chembox |
|
|
| Verifiedfields = changed |
| ⚫ |
| verifiedrevid = 400096692 |
|
|
|
| Watchedfields = changed |
| ⚫ |
|Reference=<ref> at ]</ref> |
|
|
⚫ |
| verifiedrevid = 423307752 |
| ⚫ |
|ImageFile=Homovanillyl alcohol.png |
|
|
⚫ |
| Reference =<ref> at ]</ref> |
| ⚫ |
|ImageSize=200px |
|
|
⚫ |
| ImageFile =Homovanillyl alcohol.png |
| ⚫ |
|IUPACName=4-(2-Hydroxyethyl)-2-methoxyphenol |
|
|
⚫ |
| ImageSize = |
| ⚫ |
|OtherNames=Homovanillic alcohol; MOPET; 3-Methoxy-4-hydroxyphenylethanol; 3-Methoxy-4-hydroxyphenethyl alcohol; 4-Hydroxy-3-methoxyphenethanol, 4-Hydroxy-3-methoxyphenethyl alcohol |
|
|
⚫ |
| PIN =4-(2-Hydroxyethyl)-2-methoxyphenol |
|
⚫ |
| OtherNames =Homovanillic alcohol; MOPET; 3-Methoxy-4-hydroxyphenylethanol; 3-Methoxy-4-hydroxyphenethyl alcohol; 4-Hydroxy-3-methoxyphenethanol, 4-Hydroxy-3-methoxyphenethyl alcohol |
|
|Section1={{Chembox Identifiers |
|
|Section1={{Chembox Identifiers |
|
|
| CASNo_Ref = {{cascite|correct|??}} |
|
| CASNo=2380-78-1 |
|
| CASNo =2380-78-1 |
|
| PubChem=16928 |
|
|
|
| ChEBI = 173769 |
|
| SMILES=COC1=C(C=CC(=C1)CCO)O |
|
|
|
| ChEMBL = 3747068 |
|
|
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|
|
| ChemSpiderID = 16039 |
|
|
| EINECS = 219-175-1 |
|
|
| PubChem =16928 |
|
|
| UNII_Ref = {{fdacite|correct|FDA}} |
|
|
| UNII = 9A7EE8MS6A |
|
|
| InChI = 1/C9H12O3/c1-12-9-6-7(4-5-10)2-3-8(9)11/h2-3,6,10-11H,4-5H2,1H3 |
|
|
| InChIKey = XHUBSJRBOQIZNI-UHFFFAOYAP |
|
|
| StdInChI_Ref = {{stdinchicite|changed|chemspider}} |
|
|
| StdInChI = 1S/C9H12O3/c1-12-9-6-7(4-5-10)2-3-8(9)11/h2-3,6,10-11H,4-5H2,1H3 |
|
|
| StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} |
|
|
| StdInChIKey = XHUBSJRBOQIZNI-UHFFFAOYSA-N |
|
|
| SMILES = Oc1ccc(cc1OC)CCO |
|
}} |
|
}} |
|
|Section2={{Chembox Properties |
|
|Section2={{Chembox Properties |
|
| Formula=C<sub>9</sub>H<sub>12</sub>O<sub>3</sub> |
|
| Formula =C<sub>9</sub>H<sub>12</sub>O<sub>3</sub> |
|
| MolarMass=168.19 g/mol |
|
| MolarMass =168.19 g/mol |
|
| Appearance= |
|
| Appearance = |
|
| Density= |
|
| Density = |
|
|
| MeltingPtC = 40 to 42 |
|
| MeltingPt=40-42 °C |
|
|
|
| MeltingPt_notes = |
|
| BoilingPt= |
|
|
|
| BoilingPt = |
|
| Solubility= |
|
|
|
| Solubility = |
|
}} |
|
}} |
|
|Section3={{Chembox Hazards |
|
|Section3={{Chembox Hazards |
|
| MainHazards= |
|
| MainHazards = |
|
| FlashPt=113 °C |
|
| FlashPtC = 113 |
|
|
| AutoignitionPtC = |
|
| Autoignition= |
|
|
|
| GHS_ref=<ref>{{cite web |title=Homovanillyl alcohol |url=https://pubchem.ncbi.nlm.nih.gov/compound/16928#section=Safety-and-Hazards |website=pubchem.ncbi.nlm.nih.gov |access-date=13 December 2021 |language=en}}</ref> |
|
| RPhrases = {{R36/37/38}} |
|
|
| SPhrases = {{S26}} {{S36}} |
|
| GHSPictograms = {{GHS07}} |
|
|
| GHSSignalWord = Warning |
|
|
| HPhrases = {{H-phrases|315|319|335}} |
|
|
| PPhrases = {{P-phrases|261|264|271|280|302+352|304+340|305+351+338|312|321|332+313|337+313|362|403+233|405|501}} |
|
}} |
|
}} |
|
}} |
|
}} |
| Line 31: |
Line 51: |
|
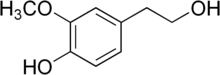
'''Homovanillyl alcohol''' is a ] of ], which in turn is a metabolite of the ] ]. |
|
'''Homovanillyl alcohol''' is a ] of ], which in turn is a metabolite of the ] ]. |
|
|
|
|
|
==See also== |
|
== See also == |
|
* ] |
|
* ] |
|
|
|
|
|
==References== |
|
== References == |
|
{{reflist}} |
|
{{reflist}} |
|
|
|
|
|
{{Neurotransmitter metabolism intermediates}} |
|
{{Neurotransmitter metabolism intermediates}} |
|
|
|
|
|
] |
|
] |
|
|
] |
|
|
|
|
|
{{Natural-phenol-stub}} |
|
{{Aromatic-stub}} |