| Revision as of 02:47, 16 April 2011 editRifleman 82 (talk | contribs)Extended confirmed users32,435 edits bold, unwikilink← Previous edit | Latest revision as of 21:17, 27 November 2024 edit undoIra Leviton (talk | contribs)Extended confirmed users331,876 edits Added a chem2 template. Appearance unchanged. | ||
| (26 intermediate revisions by 22 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Chembox | {{Chembox | ||
| | Verifiedfields = changed | |||
| ⚫ | | verifiedrevid = |
||
| | Watchedfields = changed | |||
| ⚫ | | verifiedrevid = 424305424 | ||
| | ImageFile = Nitrosonium-tetrafluoroborate-2D.png | | ImageFile = Nitrosonium-tetrafluoroborate-2D.png | ||
| | ImageSize = | |||
| | ImageAlt = | |||
| | IUPACName = nitrosonium tetrafluoroborate | | IUPACName = nitrosonium tetrafluoroborate | ||
| | PIN = | |||
| | OtherNames = nitrosyl tetrafluoroborate | | OtherNames = nitrosyl tetrafluoroborate | ||
| | |
|Section1={{Chembox Identifiers | ||
| | CASNo_Ref = {{cascite|correct|??}} | |||
| | CASNo = 14635-75-7 | |||
| | |
| CASNo = 14635-75-7 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | SMILES = }} | |||
| | UNII = B99S282SUJ | |||
| ⚫ | | |
||
| | PubChem = 151929 | |||
| ⚫ | | N |
||
| | ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} | |||
| ⚫ | | |
||
| | ChemSpiderID = 9312255 | |||
| ⚫ | | |
||
| | SMILES = (F)(F)(F)F.N# | |||
| | MeltingPt = 250 °C (sublimes) | |||
| | StdInChI_Ref = {{stdinchicite|changed|chemspider}} | |||
| | BoilingPt = | |||
| | StdInChI = 1S/BF4.NO/c2-1(3,4)5;1-2/q-1;+1 | |||
| ⚫ | | |
||
| | StdInChIKey_Ref = {{stdinchicite|changed|chemspider}} | |||
| | Section3 = {{Chembox Hazards | |||
| | StdInChIKey = KGCNVGDHOSFKFT-UHFFFAOYSA-N }} | |||
| | MainHazards = | |||
| ⚫ | |Section2={{Chembox Properties | ||
| | FlashPt = | |||
| ⚫ | | N=1 | O=1 | B=1 | F=4 | ||
| | Autoignition = }} | |||
| ⚫ | | Appearance = colourless crystalline solid | ||
| ⚫ | | Density = 2.185 g cm<sup>−3</sup> | ||
| | MeltingPtC = 250 | |||
| | MeltingPt_notes = (sublimes) | |||
| ⚫ | | Solubility = decomposes}} | ||
| }} | }} | ||
| '''Nitrosonium tetrafluoroborate''', also called '''nitrosyl tetrafluoroborate''', is a ] with the ] NOBF<sub>4</sub>. This colourless solid |
'''Nitrosonium tetrafluoroborate''', also called '''nitrosyl tetrafluoroborate''', is a ] with the ] NOBF<sub>4</sub>. This colourless solid is used in ] as a ], ] and a mild oxidant.<ref>{{Citation |last=Olah |first=George A. |title=Nitrosonium Tetrafluoroborate |date=2004-10-15 |work=Encyclopedia of Reagents for Organic Synthesis |editor-last=John Wiley & Sons, Ltd |url=https://onlinelibrary.wiley.com/doi/10.1002/047084289X.rn058.pub2 |access-date=2024-11-27 |place=Chichester, UK |publisher=John Wiley & Sons, Ltd |language=en |doi=10.1002/047084289x.rn058.pub2 |isbn=978-0-471-93623-7 |last2=Surya Prakash |first2=G. K. |last3=Wang |first3=Qi |last4=Li |first4=Xing-ya |last5=Surya Prakash |first5=G. K. |last6=Hu |first6=Jinbo}}</ref> | ||
| NOBF<sub>4</sub> is the ] of ], and is composed of a ] ], <sup>+</sup>, and a ] ], <sup>−</sup>. | NOBF<sub>4</sub> is the ] of ], and is composed of a ] ], <sup>+</sup>, and a ] ], <sup>−</sup>.<ref>{{Cite journal |last=Lozinšek |first=Matic |date=2021-11-28 |title=Nitrosonium tetrafluoridoborate, NOBF4 |url=https://scripts.iucr.org/cgi-bin/paper?S2414314621012153 |journal=IUCrData |volume=6 |issue=11 |doi=10.1107/S2414314621012153 |issn=2414-3146 |pmc=9462292 |pmid=36337464}}</ref> | ||
| ==Reactions== | ==Reactions== | ||
| The dominant property of NOBF<sub>4</sub> is the oxidizing power and electrophilic character of the nitrosonium cation. It forms colored ]es with hexamethylbenzene and with ]. The latter, a deep yellow species, provides a means to dissolve NOBF<sub>4</sub> in dichloromethane.<ref>{{cite journal |doi=10.1021/ic00346a008|title=Redox equilibria of the nitrosonium cation and of its nonbonded complexes|year=1990|last1=Lee|first1=K. Y.|last2=Kuchynka|first2=D. J.|last3=Kochi|first3=Jay K.|journal=Inorganic Chemistry|volume=29|issue=21|pages=4196–4204}}</ref> | |||
| ⚫ | Nitrosonium tetrafluoroborate may be used to prepare metal salts of the type <sub>2</sub> (M = Cr, Mn, Fe, Co, Ni, Cu). The nitrosonium cation acts as the oxidizer, itself being reduced to nitric oxide gas:<ref>{{cite journal | doi = 10.1002/0471224502.ch2 | journal = ] | volume = 33 | pages = 75–83 | title = 11. Homoleptic Transition Metal Acetonitrile Cations with Tetrafluoroborate or Trifluoromethanesulfonate Anions | |
||
| ⚫ | Nitrosonium tetrafluoroborate may be used to prepare metal salts of the type <sub>2</sub> (M = Cr, Mn, Fe, Co, Ni, Cu). The nitrosonium cation acts as the oxidizer, itself being reduced to nitric oxide gas:<ref>{{cite journal | doi = 10.1002/0471224502.ch2 | journal = ] | volume = 33 | pages = 75–83 | title = 11. Homoleptic Transition Metal Acetonitrile Cations with Tetrafluoroborate or Trifluoromethanesulfonate Anions | first1=Robert A. | last1=Heintz | first2=Jennifer A. | last2=Smith | first3=Paul S. | last3=Szalay | first4=Amy | last4=Weisgerber | first5=Kim R. | last5=Dunbar | date=August 2004 | isbn=978-0-471-46075-6}}</ref> | ||
| : M + |
: {{chem2|M + 2NOBF4 + ''x''CH3CN → (BF4)2 + 2NO}} | ||
| With ] the ] is formed.<ref>{{cite journal | title = Solvent and electrolyte effects on the kinetics of ferrocenium-ferrocene self-exchange. A reevaluation |author1=Roger M. Nielson |author2=George E. McManis |author3=Lance K. Safford |author4=Michael J. Weaver | journal = ] | doi = 10.1021/j100342a086 | year = 1989 | volume = 93 | issue = 5 | pages = 2152}}</ref> | |||
| In its ] of this salt, ν<sub>NO</sub> is a strong peak at 2387 cm<sup>−1</sup>.<ref>{{cite journal |doi=10.1039/JR9630003557 |title=670. The Infrared Spectrum of the Nitrosonium Ion |date=1963 |last1=Sharp |first1=D. W. A. |last2=Thorley |first2=J. |journal=Journal of the Chemical Society (Resumed) |page=3557 }}</ref> | |||
| ==References== | ==References== | ||
| {{reflist}} | {{reflist}} | ||
| {{Tetrafluoroborates}} | |||
| ⚫ | {{inorganic-compound-stub}} | ||
| ] | ] | ||
| ] | ] | ||
| ⚫ | {{inorganic-compound-stub}} | ||
Latest revision as of 21:17, 27 November 2024
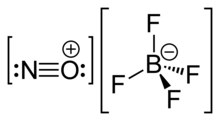
| |
| Names | |
|---|---|
| IUPAC name nitrosonium tetrafluoroborate | |
| Other names nitrosyl tetrafluoroborate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.035.148 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | BF4NO |
| Molar mass | 116.81 g·mol |
| Appearance | colourless crystalline solid |
| Density | 2.185 g cm |
| Melting point | 250 °C (482 °F; 523 K) (sublimes) |
| Solubility in water | decomposes |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Nitrosonium tetrafluoroborate, also called nitrosyl tetrafluoroborate, is a chemical compound with the chemical formula NOBF4. This colourless solid is used in organic synthesis as a nitrosating agent, diazotizing agent and a mild oxidant.
NOBF4 is the nitrosonium salt of fluoroboric acid, and is composed of a nitrosonium cation, , and a tetrafluoroborate anion, .
Reactions
The dominant property of NOBF4 is the oxidizing power and electrophilic character of the nitrosonium cation. It forms colored charge transfer complexes with hexamethylbenzene and with 18-crown-6. The latter, a deep yellow species, provides a means to dissolve NOBF4 in dichloromethane.
Nitrosonium tetrafluoroborate may be used to prepare metal salts of the type 2 (M = Cr, Mn, Fe, Co, Ni, Cu). The nitrosonium cation acts as the oxidizer, itself being reduced to nitric oxide gas:
- M + 2NOBF4 + xCH3CN → [M(CH3CN)x](BF4)2 + 2NO
With ferrocene the ferrocenium tetrafluoroborate is formed.
In its infrared spectrum of this salt, νNO is a strong peak at 2387 cm.
References
- Olah, George A.; Surya Prakash, G. K.; Wang, Qi; Li, Xing-ya; Surya Prakash, G. K.; Hu, Jinbo (2004-10-15), John Wiley & Sons, Ltd (ed.), "Nitrosonium Tetrafluoroborate", Encyclopedia of Reagents for Organic Synthesis, Chichester, UK: John Wiley & Sons, Ltd, doi:10.1002/047084289x.rn058.pub2, ISBN 978-0-471-93623-7, retrieved 2024-11-27
- Lozinšek, Matic (2021-11-28). "Nitrosonium tetrafluoridoborate, NOBF4". IUCrData. 6 (11). doi:10.1107/S2414314621012153. ISSN 2414-3146. PMC 9462292. PMID 36337464.
- Lee, K. Y.; Kuchynka, D. J.; Kochi, Jay K. (1990). "Redox equilibria of the nitrosonium cation and of its nonbonded complexes". Inorganic Chemistry. 29 (21): 4196–4204. doi:10.1021/ic00346a008.
- Heintz, Robert A.; Smith, Jennifer A.; Szalay, Paul S.; Weisgerber, Amy; Dunbar, Kim R. (August 2004). "11. Homoleptic Transition Metal Acetonitrile Cations with Tetrafluoroborate or Trifluoromethanesulfonate Anions". Inorg. Synth. 33: 75–83. doi:10.1002/0471224502.ch2. ISBN 978-0-471-46075-6.
- Roger M. Nielson; George E. McManis; Lance K. Safford; Michael J. Weaver (1989). "Solvent and electrolyte effects on the kinetics of ferrocenium-ferrocene self-exchange. A reevaluation". J. Phys. Chem. 93 (5): 2152. doi:10.1021/j100342a086.
- Sharp, D. W. A.; Thorley, J. (1963). "670. The Infrared Spectrum of the Nitrosonium Ion". Journal of the Chemical Society (Resumed): 3557. doi:10.1039/JR9630003557.
| Salts and covalent derivatives of the tetrafluoroborate ion | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |