| Revision as of 07:02, 30 May 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (changes to verified fields - updated '') per Chem/Drugbox validation (report errors or bugs)← Previous edit | Latest revision as of 20:22, 24 November 2024 edit undoAescley Ezca (talk | contribs)50 editsm added link to parathion methylTag: Visual edit | ||
| (133 intermediate revisions by 89 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | Verifiedfields = changed | | Verifiedfields = changed | ||
| | Watchedfields = changed | |||
| | verifiedrevid = 417767900 | |||
| | verifiedrevid = 431610740 | |||
| | Name = Parathion | |||
| | Name = Parathion | |||
| | ImageFile = Methyl&Ethylparathion.png | |||
| | ImageFile=Methyl&Ethylparathion.png | |||
| | ImageSize = 220px | |||
| | ImageFile1=Ethyl-parathion-from-AHRLS-2011-3D-balls.png | |||
| | ImageName = | |||
| | |
| PIN = ''O'',''O''-Diethyl ''O''-(4-nitrophenyl) phosphorothioate | ||
| | |
| OtherNames = E605 | ||
| | |
|Section1={{Chembox Identifiers | ||
| | ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| | SMILES = S=P(Oc1ccc(cc1)()=O)(OCC)OCC | |||
| | ChEBI = 27928 | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | SMILES = S=P(Oc1ccc(cc1)()=O)(OCC)OCC | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | ChemSpiderID = 13844817 | | ChemSpiderID = 13844817 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | | UNII_Ref = {{fdacite|correct|FDA}} | ||
| Line 24: | Line 26: | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | CASNo = 56-38-2 | | CASNo = 56-38-2 | ||
| | KEGG_Ref = {{keggcite| |
| KEGG_Ref = {{keggcite|correct|kegg}} | ||
| | KEGG = C06604 | | KEGG = C06604 | ||
| | |
| PubChem = 991 | ||
| | RTECS = TF4550000 | |||
| | EC_number = 200-271-7 | |||
| | UNNumber = 3018 2783 | |||
| | Beilstein = 2059093 | |||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| | C=10 | H=14 | N=1 | O=5 | P=1 | S=1 | |||
| | Formula = C<sub>10</sub>H<sub>14</sub>NO<sub>5</sub>PS | |||
| | Appearance = White crystals (pure form) | |||
| | MolarMass = 291.3 g/mol | |||
| | Solubility = 24 mg/L | |||
| | Appearance = White crystals (pure form) | |||
| | |
| SolubleOther = high solubility | ||
| in ] |
in ] and ] | ||
| | Solvent = other solvents | |||
| | MeltingPt = 6 °C | |||
| | MeltingPtC = 6 | |||
| }} | |||
| | Section7 = {{Chembox Hazards | |||
| | ExternalMSDS = | |||
| | FlashPt = 120 °C | |||
| | RPhrases = {{R24}}, {{R26/28}}, {{R48/25}}, {{R50/53}} | |||
| | SPhrases = {{S28}}, {{S36/37}}, {{S45}}, {{S60}}, {{S61}} | |||
| }} | }} | ||
| |Section7={{Chembox Hazards | |||
| | ExternalSDS = | |||
| | NFPA-H = 4 | |||
| | NFPA-F = 1 | |||
| | NFPA-R = 2 | |||
| | NFPA-S = | |||
| | FlashPtC = 120 | |||
| | GHSPictograms = {{GHS06}}{{GHS08}}{{GHS09}} | |||
| | GHSSignalWord = Danger | |||
| | HPhrases = {{H-phrases|300|311|330|372|410}} | |||
| | PPhrases = {{P-phrases|260|264|270|271|273|280|284|301+310|302+352|304+340|310|312|314|320|321|322|330|361|363|391|403+233|405|501}} | |||
| | PEL = none (methyl parathion),<ref name=PGCH|0427>{{PGCH|0427}}</ref> TWA 0.1 mg/m<sup>3</sup> (ethyl parathion)<ref name=PGCH|0479/> | |||
| | REL = TWA 0.2 mg/m<sup>3</sup> (methyl parathion)<ref name=PGCH|0427/> TWA 0.05 mg/m3 (ethyl parathion)<ref name=PGCH|0479/> | |||
| | IDLH = N.D. (methyl parathion)<ref name=PGCH|0427/> 10 mg/m<sup>3</sup> (ethyl parathion)<ref name=PGCH|0479>{{PGCH|0479}}</ref> | |||
| | LCLo = 50 mg/m<sup>3</sup> (rabbit, 2 hr)<br/>14 mg/m<sup>3</sup> (guinea pig, 2 hr)<br/>15 mg/m<sup>3</sup> (mouse)<ref name=IDLH>{{IDLH|56382|Parathion}}</ref> | |||
| | LC50 = 84 mg/m<sup>3</sup> (rat, 4 hr)<ref name=IDLH/> | |||
| | LD50 = 5 mg/kg (mouse, oral)<br/>10 mg/kg (rabbit, oral)<br/>3 mg/kg (dog, oral)<br/>0.93 mg/kg (cat, oral)<br/>5 mg/kg (horse, oral)<br/>8 mg/kg (guinea pig, oral)<br/>2 mg/kg (rat, oral)<ref name=IDLH/> | |||
| }}<ref>{{cite web |url=http://www.ehs.neu.edu/laboratory_safety/general_information/nfpa_hazard_rating/documents/NFPAratingJR.htm |title=Hazard Rating Information for NFPA Fire Diamonds |access-date=2015-03-13 |url-status=dead |archive-url=https://web.archive.org/web/20150217114741/http://www.ehs.neu.edu/laboratory_safety/general_information/nfpa_hazard_rating/documents/NFPAratingJR.htm |archive-date=2015-02-17 }}</ref> | |||
| }} | }} | ||
| '''Parathion''', also called '''parathion-ethyl''' or '''diethyl parathion''', is an ]. It is a potent ] and ]. It was originally developed by ] in the 1940s. It is highly toxic to non-target organisms, including humans. Its use is banned or restricted in many countries, and there are proposals to ban it from all use. Closely related is "methyl parathion" (see below). | |||
| ==Parathion-methyl== | |||
| "Parathion-methyl" (CAS#298-00-0), also known as methyl parathion or dimethyl parathion, was also developed and is marketed for similar uses. It is a distinct compound with diminished toxicity. Some trade names of parathion-methyl include '''Bladan M''', '''Metaphos''', '''ME605''', and '''E601'''. | |||
| '''Parathion''', also called '''parathion-ethyl''' or '''diethyl parathion''', is an ] ] and ]. It was originally developed by ] in the 1940s. It is highly toxic to non-target organisms, including humans, so its use has been banned or restricted in most countries. In response to safety concerns, the less toxic but still dangerous analogue ] was later developed.<ref>{{Cite web|url=http://www.fao.org/3/w5715e/w5715e05.htm|title=Parathion|website=www.fao.org|access-date=2020-04-17}}</ref> | |||
| <center>]</center> | |||
| ==History== | ==History== | ||
| ] | ] | ||
| Parathion was developed by |
Parathion was developed by ] for the German trust ] in the 1940s. After ] and the collapse of IG Farben due to the war crime trials, the Western allies seized the ], and parathion was marketed worldwide by different companies and under different brand names. The most common German brand was '''E605''' (banned in Germany after 2002); this was not a food-additive "]" as used in the EU today. "E" stands for ''Entwicklungsnummer'' (German for "development number"). It is an irreversible ]. | ||
| Safety concerns later led to the development of ], which is somewhat less toxic. | |||
| In the ], Parathion was banned after 2001. <ref> </ref> In ], the substance is no longer approved as a pesticide. | |||
| ==Handling properties== | ==Handling properties== | ||
| Pure parathion is a white crystalline solid. It is commonly distributed as a brown ] that smells of rotting ]s or ]. The insecticide is somewhat stable, although it darkens when exposed to sunlight. | |||
| ==Industrial synthesis== | ==Industrial synthesis== | ||
| Parathion is synthesized from ] ( |
Parathion is synthesized from ] {{chem2|(C2H5O)2PS2H}} by ] to generate diethylthiophosphoryl chloride ({{chem2|(C2H5O)2P(S)Cl}}), and then the chloride is treated with ] (the ] ] of ]).<ref name = fee>{{cite book |last1=Fee |first1=D. C. |last2=Gard |first2=D. R. |last3=Yang |first3=C. |chapter=Phosphorus Compounds |title=Kirk-Othmer Encyclopedia of Chemical Technology |publisher=John Wiley & Sons |location=New York |year=2005 |doi=10.1002/0471238961.16081519060505.a01.pub2 |isbn=978-0471238966 }}</ref> | ||
| : |
:{{chem2|2 (C2H5O)2P(S)SH + 3 Cl2 → 2 (C2H5O)2P(S)Cl + S2Cl2 + 2 HCl}} | ||
| :{{chem2|(C2H5O)2P(S)Cl + NaOC6H4NO2 → (C2H5O)2P(S)OC6H4NO2 + NaCl}} | |||
| : (C2H5O)<sub>2</sub>P(S)Cl + NaOC<sub>6</sub>H<sub>4</sub>NO<sub>2</sub> → (C<sub>2</sub>H<sub>5</sub>O)<sub>2</sub>P(S)OC<sub>6</sub>H<sub>4</sub>NO<sub>2</sub> + NaCl | |||
| ==Applications== | ==Applications== | ||
| Line 67: | Line 86: | ||
| ==Insecticidal activity== | ==Insecticidal activity== | ||
| Parathion acts on the enzyme ] |
Parathion acts on the enzyme ] indirectly. After an insect (or a human) ingests parathion, an ] replaces the double bonded sulfur with oxygen to give ].<ref name = metcalf>{{cite book |last=Metcalf |first=R. L. |chapter=Insect Control |title=Ullmann's Encyclopedia of Industrial Chemistry |publisher=Wiley-VCH Verlag GmbH & Co. KGaA |location=New York |year=2002 |doi=10.1002/14356007.a14_263 |isbn=978-3527306732 }}</ref> | ||
| :{{chem2|(C2H5O)2P(S)OC6H4NO2 + 1/2 O2 → (C2H5O)2P(O)OC6H4NO2 + S}} | |||
| :(C<sub>2</sub>H<sub>5</sub>O)<sub>2</sub>P(S)OC<sub>6</sub>H<sub>4</sub>NO<sub>2</sub> + 1/2 O<sub>2</sub> → (C<sub>2</sub>H<sub>5</sub>O)<sub>2</sub>P(O)OC<sub>6</sub>H<sub>4</sub>NO<sub>2</sub> + S | |||
| The phosphate ester is more reactive in organisms than the phosphorothiolate ester, as the phosphorus atoms become much more |
The phosphate ester is more reactive in organisms than the phosphorothiolate ester, as the phosphorus atoms become much more electropositive.<ref name = metcalf/> | ||
| Parathion {{visible anchor|Resistance|text=resistance}} is a special case of ]. | |||
| ==Degradation== | ==Degradation== | ||
| Degradation of parathion leads to more water |
Degradation of parathion leads to more water-soluble products. ], which deactivates the molecule, occurs at the ] bond resulting in ] and ].<ref name = metcalf/> | ||
| :{{chem2|(C2H5O)2P(S)OC6H4NO2 + H2O → HOC6H4NO2 + (C2H5O)2P(S)OH}} | |||
| :(C<sub>2</sub>H<sub>5</sub>O)<sub>2</sub>P(S)OC<sub>6</sub>H<sub>4</sub>NO<sub>2</sub> + H<sub>2</sub>O → HOC<sub>6</sub>H<sub>4</sub>NO<sub>2</sub> + (C<sub>2</sub>H<sub>5</sub>O)<sub>2</sub>P(S)OH | |||
| Degradation proceeds differently under ] conditions: the nitro group on parathion is reduced to the ]. | Degradation proceeds differently under ] conditions: the nitro group on parathion is reduced to the ]. | ||
| :{{chem2|(C2H5O)2P(S)OC6H4NO2 + 6 H → (C2H5O)2P(S)OC6H4NH2 + 2 H2O}} | |||
| :(C<sub>2</sub>H<sub>5</sub>O)<sub>2</sub>P(S)OC<sub>6</sub>H<sub>4</sub>NO<sub>2</sub> + 6 H → (C<sub>2</sub>H<sub>5</sub>O)<sub>2</sub>P(S)OC<sub>6</sub>H<sub>4</sub>NH<sub>2</sub> + 2 H<sub>2</sub>O | |||
| ==Safety== | ==Safety== | ||
| Parathion is a ]. It generally disrupts the ] by inhibiting |
Parathion is a ]. It generally disrupts the ] by inhibiting ]. It is absorbed via skin, mucous membranes, and orally. Absorbed parathion is rapidly metabolized to paraoxon, as described in ]. Paraoxon exposure can result in ]s, ], poor vision, ], abdominal pain, severe ], ], ], ], and finally ] as well as respiratory arrest. Symptoms of poisoning are known to last for extended periods, sometimes months. The most common and very specific antidote is ], in doses of up to 100 mg daily. Because atropine may also be toxic, it is recommended that small frequently repeated doses be used in treatment. If human poisoning is detected early and the treatment is prompt (atropine and artificial respiration), fatalities are infrequent. Insufficient oxygen will lead to ] and permanent brain damage. ] including ] is noticed as late ] after recovery from acute intoxication. Parathion and related organophosphorus pesticides are used in hundreds of thousands of poisonings annually, especially suicides.<ref>Litchfield, M.H. "Estimates of acute pesticide poisoning in agricultural workers in less developed countries" Toxicology Reviews 2005, volume 24, pp. 271-8. {{PMID|16499408}}</ref> It is known as ''Schwiegermuttergift'' (mother-in-law poison) in Germany. For this reason, most formulations contain a blue dye providing warning. | ||
| Parathion was used as a ] agent, most notably by an element of the ] attached to the ] during the ]. They used it to poison clothing that was then supplied to anti-government guerrillas. When the enemy soldiers put on the clothes, they were poisoned by absorption through the skin.<ref>{{Cite web|url=https://openaccess.leidenuniv.nl/bitstream/handle/1887/68698/Poison%20in%20Rhodesia%2C%20Colm%20Wittenberg.pdf?sequence=1|title = Poison in Rhodesia|date = 31 January 2019}}</ref><ref>{{Cite web|url=http://cco.ndu.edu/News/Article/1506904/dirty-war-rhodesia-and-chemical-biological-warfare-1975-1980-book-review/|title=Dirty War: Rhodesia and Chemical Biological Warfare 1975-1980 (Book Review)|website=PRISM | National Defense University}}</ref><ref>{{cite book|title=Dirty War: Rhodesia and Chemical Biological Warfare, 1975–1980|last=Cross|first=Glenn|year=2017|location=Solihull, UK|publisher=Helion & Company|isbn=978-1-911512-12-7}}</ref> | |||
| Parathion has been used as a ], most notably by the ] during the ].<ref>Moorcraft, Paul and McLaughlin, Peter. ''The Rhodesian War: A Military History''. Yorkshire: Pen & Sword, 2008, p. 106</ref> | |||
| Based on animal studies, parathion is considered by the ] to be a possible human ].<ref name=epa>{{cite web | title = Parathion | work = Integrated Risk Information System | publisher = ] | date = 26 January 2007 | url = http://www.epa.gov/iris/subst/0327.htm}}</ref> Studies show that parathion is toxic to fetuses, but does not cause birth defects.<ref name = extoxnet>{{cite web | work = Extension Toxicology Network | title = Pesticide Information Profiles - Parathion | publisher = ] | |
Based on animal studies, parathion is considered by the ] to be a possible human ].<ref name=epa>{{cite web | title = Parathion | work = Integrated Risk Information System | publisher = ] | date = 26 January 2007 | url = http://www.epa.gov/iris/subst/0327.htm}}</ref> Studies show that parathion is toxic to fetuses, but does not cause birth defects.<ref name = extoxnet>{{cite web | work = Extension Toxicology Network | title = Pesticide Information Profiles - Parathion | publisher = ] |date=September 1993 | url = http://extoxnet.orst.edu/pips/parathio.htm}}</ref> | ||
| It is classified |
It is classified by the ] as a ]{{cn|date=February 2018}} and by the ] as ] (extremely hazardous).{{cn|date=February 2018}} | ||
| Parathion is |
Parathion is toxic to ], ], ]s, and other forms of wildlife.<ref name = extoxnet/> | ||
| ===Protection against poisoning=== | ===Protection against poisoning=== | ||
| To |
To provide the end user with a minimum standard of protection, suitable protective gloves, clothing, and a respirator with organic-vapour cartridges is normally worn. ] during the production process requires special ventilation and continuous measurement of air contamination in order not to exceed PEL levels, as well as careful attention to personal hygiene. Frequent analysis of workers' serum acetylcholinesterase activity is also helpful with regards to occupational safety, because the action of parathion is cumulative. Also, atropine has been used as a specific antidote.{{fact|date=January 2024}} | ||
| === Use in suicides === | |||
| ==Proposals to ban== | |||
| A chemist {{who?|date=March 2024}} swallowed {{convert|.00424|oz|g}} of parathion {{when?|date=March 2024}} to find the most lethal means of exposure to humans, intending to take an antidote afterwards, but was paralyzed and so died before he could reach it.<ref name=":0">{{Cite book |last=Carson |first=Rachel |url=http://worldcat.org/oclc/1346358856 |title=Silent Spring |publisher= HarperCollins|year=1962 |isbn=978-0-547-52762-8 |oclc=1346358856}}</ref> | |||
| According to the non-governmental organisation ] or PAN, parathion is one of the most dangerous pesticides. This organization lists parathion also as a 'bad actor chemical'.<ref name = panid>{{cite web | title = Parathion - Identification, toxicity, use, water pollution potential, ecological toxicity and regulatory information | publisher = ] | author = S. Kegley, B. Hill, S. Orme | url = http://pesticideinfo.org/Detail_Chemical.jsp?Rec_Id=PC35122}}</ref> In the US alone more than 650 agricultural workers have been poisoned since 1966, of which 100 died. In underdeveloped countries many more people have suffered fatal and nonfatal intoxications. The ], PAN and numerous environmental organisations propose a general and global ban. Its use is banned or restricted in 23 countries and its import is illegal in a total of 50 countries.<ref name=panid/> | |||
| Parathion was commonly used for suicides in the 1950s and 1960s.<ref name=":0" /> | |||
| ==See also== | ==See also== | ||
| * ] | * ] | ||
| * ] | |||
| ==References== | ==References== | ||
| Line 103: | Line 127: | ||
| ==External links== | ==External links== | ||
| * {{PPDB|506}} | |||
| * U.S. ] (public domain) | * U.S. ] (public domain) | ||
| * U.S. ] (public domain) | |||
| * Ethyl parathion: {{ICSC|0006}} | * Ethyl parathion: {{ICSC|0006}} | ||
| * Methyl parathion: {{ICSC|0626}} | * Methyl parathion: {{ICSC|0626}} | ||
| {{Insecticides}} | {{Insecticides}} | ||
| {{Acetylcholine metabolism and transport modulators}} | |||
| {{Cholinergics}} | |||
| {{Authority control}} | |||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 20:22, 24 November 2024
| |

| |
| Names | |
|---|---|
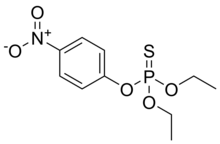
| Preferred IUPAC name O,O-Diethyl O-(4-nitrophenyl) phosphorothioate | |
| Other names E605 | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 2059093 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.000.247 |
| EC Number |
|
| KEGG | |
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 3018 2783 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C10H14NO5PS |
| Molar mass | 291.26 g·mol |
| Appearance | White crystals (pure form) |
| Melting point | 6 °C (43 °F; 279 K) |
| Solubility in water | 24 mg/L |
| Solubility in other solvents | high solubility |
| Hazards | |
| GHS labelling: | |
| Pictograms |   
|
| Signal word | Danger |
| Hazard statements | H300, H311, H330, H372, H410 |
| Precautionary statements | P260, P264, P270, P271, P273, P280, P284, P301+P310, P302+P352, P304+P340, P310, P312, P314, P320, P321, P322, P330, P361, P363, P391, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) |
 |
| Flash point | 120 °C (248 °F; 393 K) |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 5 mg/kg (mouse, oral) 10 mg/kg (rabbit, oral) 3 mg/kg (dog, oral) 0.93 mg/kg (cat, oral) 5 mg/kg (horse, oral) 8 mg/kg (guinea pig, oral) 2 mg/kg (rat, oral) |
| LC50 (median concentration) | 84 mg/m (rat, 4 hr) |
| LCLo (lowest published) | 50 mg/m (rabbit, 2 hr) 14 mg/m (guinea pig, 2 hr) 15 mg/m (mouse) |
| NIOSH (US health exposure limits): | |
| PEL (Permissible) | none (methyl parathion), TWA 0.1 mg/m (ethyl parathion) |
| REL (Recommended) | TWA 0.2 mg/m (methyl parathion) TWA 0.05 mg/m3 (ethyl parathion) |
| IDLH (Immediate danger) | N.D. (methyl parathion) 10 mg/m (ethyl parathion) |
| Safety data sheet (SDS) | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Parathion, also called parathion-ethyl or diethyl parathion, is an organophosphate insecticide and acaricide. It was originally developed by IG Farben in the 1940s. It is highly toxic to non-target organisms, including humans, so its use has been banned or restricted in most countries. In response to safety concerns, the less toxic but still dangerous analogue parathion methyl was later developed.
History

Parathion was developed by Gerhard Schrader for the German trust IG Farben in the 1940s. After World War II and the collapse of IG Farben due to the war crime trials, the Western allies seized the patent, and parathion was marketed worldwide by different companies and under different brand names. The most common German brand was E605 (banned in Germany after 2002); this was not a food-additive "E number" as used in the EU today. "E" stands for Entwicklungsnummer (German for "development number"). It is an irreversible acetylcholinesterase inhibitor.
Safety concerns later led to the development of parathion methyl, which is somewhat less toxic.
In the EU, Parathion was banned after 2001. In Switzerland, the substance is no longer approved as a pesticide.
Handling properties
Pure parathion is a white crystalline solid. It is commonly distributed as a brown liquid that smells of rotting eggs or garlic. The insecticide is somewhat stable, although it darkens when exposed to sunlight.
Industrial synthesis
Parathion is synthesized from diethyl dithiophosphoric acid (C2H5O)2PS2H by chlorination to generate diethylthiophosphoryl chloride ((C2H5O)2P(S)Cl), and then the chloride is treated with sodium 4-nitrophenolate (the sodium salt of 4-nitrophenol).
- 2 (C2H5O)2P(S)SH + 3 Cl2 → 2 (C2H5O)2P(S)Cl + S2Cl2 + 2 HCl
- (C2H5O)2P(S)Cl + NaOC6H4NO2 → (C2H5O)2P(S)OC6H4NO2 + NaCl
Applications
As a pesticide, parathion is generally applied by spraying. It is often applied to cotton, rice and fruit trees. The usual concentrations of ready-to-use solutions are 0.05 to 0.1%. The chemical is banned for use on many food crops.
Insecticidal activity
Parathion acts on the enzyme acetylcholinesterase indirectly. After an insect (or a human) ingests parathion, an oxidase replaces the double bonded sulfur with oxygen to give paraoxon.
- (C2H5O)2P(S)OC6H4NO2 + 1/2 O2 → (C2H5O)2P(O)OC6H4NO2 + S
The phosphate ester is more reactive in organisms than the phosphorothiolate ester, as the phosphorus atoms become much more electropositive.
Parathion resistance is a special case of acetylcholinesterase inhibitor resistance.
Degradation
Degradation of parathion leads to more water-soluble products. Hydrolysis, which deactivates the molecule, occurs at the aryl ester bond resulting in diethyl thiophosphate and 4-nitrophenol.
- (C2H5O)2P(S)OC6H4NO2 + H2O → HOC6H4NO2 + (C2H5O)2P(S)OH
Degradation proceeds differently under anaerobic conditions: the nitro group on parathion is reduced to the amine.
- (C2H5O)2P(S)OC6H4NO2 + 6 H → (C2H5O)2P(S)OC6H4NH2 + 2 H2O
Safety
Parathion is a cholinesterase inhibitor. It generally disrupts the nervous system by inhibiting acetylcholinesterase. It is absorbed via skin, mucous membranes, and orally. Absorbed parathion is rapidly metabolized to paraoxon, as described in Insecticidal activity. Paraoxon exposure can result in headaches, convulsions, poor vision, vomiting, abdominal pain, severe diarrhea, unconsciousness, tremor, dyspnea, and finally pulmonary edema as well as respiratory arrest. Symptoms of poisoning are known to last for extended periods, sometimes months. The most common and very specific antidote is atropine, in doses of up to 100 mg daily. Because atropine may also be toxic, it is recommended that small frequently repeated doses be used in treatment. If human poisoning is detected early and the treatment is prompt (atropine and artificial respiration), fatalities are infrequent. Insufficient oxygen will lead to cerebral hypoxia and permanent brain damage. Peripheral neuropathy including paralysis is noticed as late sequelae after recovery from acute intoxication. Parathion and related organophosphorus pesticides are used in hundreds of thousands of poisonings annually, especially suicides. It is known as Schwiegermuttergift (mother-in-law poison) in Germany. For this reason, most formulations contain a blue dye providing warning.
Parathion was used as a chemical warfare agent, most notably by an element of the British South Africa Police attached to the Selous Scouts during the Rhodesian Bush War. They used it to poison clothing that was then supplied to anti-government guerrillas. When the enemy soldiers put on the clothes, they were poisoned by absorption through the skin.
Based on animal studies, parathion is considered by the U.S. Environmental Protection Agency to be a possible human carcinogen. Studies show that parathion is toxic to fetuses, but does not cause birth defects.
It is classified by the United Nations Environment Programme as a persistent organic pollutant and by the World Health Organization as Toxicity Class Ia (extremely hazardous).
Parathion is toxic to bees, fish, birds, and other forms of wildlife.
Protection against poisoning
To provide the end user with a minimum standard of protection, suitable protective gloves, clothing, and a respirator with organic-vapour cartridges is normally worn. Industrial safety during the production process requires special ventilation and continuous measurement of air contamination in order not to exceed PEL levels, as well as careful attention to personal hygiene. Frequent analysis of workers' serum acetylcholinesterase activity is also helpful with regards to occupational safety, because the action of parathion is cumulative. Also, atropine has been used as a specific antidote.
Use in suicides
A chemist swallowed .00424 ounces (0.120 g) of parathion to find the most lethal means of exposure to humans, intending to take an antidote afterwards, but was paralyzed and so died before he could reach it.
Parathion was commonly used for suicides in the 1950s and 1960s.
See also
References
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0427". National Institute for Occupational Safety and Health (NIOSH).
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0479". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Parathion". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- "Hazard Rating Information for NFPA Fire Diamonds". Archived from the original on 2015-02-17. Retrieved 2015-03-13.
- "Parathion". www.fao.org. Retrieved 2020-04-17.
- Non-inclusion of parathion in Annex I to Council Directive 91/414/EEC
- Fee, D. C.; Gard, D. R.; Yang, C. (2005). "Phosphorus Compounds". Kirk-Othmer Encyclopedia of Chemical Technology. New York: John Wiley & Sons. doi:10.1002/0471238961.16081519060505.a01.pub2. ISBN 978-0471238966.
- ^ Metcalf, R. L. (2002). "Insect Control". Ullmann's Encyclopedia of Industrial Chemistry. New York: Wiley-VCH Verlag GmbH & Co. KGaA. doi:10.1002/14356007.a14_263. ISBN 978-3527306732.
- Litchfield, M.H. "Estimates of acute pesticide poisoning in agricultural workers in less developed countries" Toxicology Reviews 2005, volume 24, pp. 271-8. PMID 16499408
- "Poison in Rhodesia" (PDF). 31 January 2019.
- "Dirty War: Rhodesia and Chemical Biological Warfare 1975-1980 (Book Review)". PRISM | National Defense University.
- Cross, Glenn (2017). Dirty War: Rhodesia and Chemical Biological Warfare, 1975–1980. Solihull, UK: Helion & Company. ISBN 978-1-911512-12-7.
- "Parathion". Integrated Risk Information System. U. S. Environmental Protection Agency. 26 January 2007.
- ^ "Pesticide Information Profiles - Parathion". Extension Toxicology Network. Oregon State University. September 1993.
- ^ Carson, Rachel (1962). Silent Spring. HarperCollins. ISBN 978-0-547-52762-8. OCLC 1346358856.
External links
- Parathion in the Pesticide Properties DataBase (PPDB)
- ATSDR - Methyl Parathion Expert Panel Report U.S. Department of Health and Human Services (public domain)
- CDC - NIOSH Pocket Guide to Chemical Hazards U.S. Department of Health and Human Services (public domain)
- Ethyl parathion: International Chemical Safety Card 0006
- Methyl parathion: International Chemical Safety Card 0626