| Revision as of 19:16, 29 June 2011 edit85.173.197.193 (talk) →Uses← Previous edit | Latest revision as of 16:36, 14 November 2024 edit undo65.242.29.19 (talk) grammarTag: Visual edit | ||
| (162 intermediate revisions by 98 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Iodo-derivative of fluorone used as a pink dye}} | |||
| {{use dmy dates |date=November 2023}} | |||
| {{redirect|Red 3|the techno release called Red Three|Dave Clarke (DJ)}} | |||
| {{chembox | {{chembox | ||
| | Verifiedfields = changed | |||
| | Watchedfields = changed | | Watchedfields = changed | ||
| | verifiedrevid = |
| verifiedrevid = 442926733 | ||
| | |
| Name = Erythrosine | ||
| | |
| ImageFile = Erythrosine.svg | ||
| | |
| ImageSize = 230px | ||
| | |
| ImageName = Erythrosine | ||
| | |
| IUPACName = 2-(6-Hydroxy-2,4,5,7-tetraiodo-3-oxo-xanthen-9-yl)benzoic acid | ||
| | |
|Section1={{Chembox Identifiers | ||
| | |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 3144 | | ChemSpiderID = 3144 | ||
| | |
| ChEMBL_Ref = {{ebicite|changed|EBI}} | ||
| | |
| ChEMBL = 1332616 | ||
| | UNII_Ref = {{fdacite|changed|FDA}} | |||
| | UNII = 8TL7LH93FM | |||
| | InChI = 1/C20H8I4O5/c21-11-5-9-17(13(23)15(11)25)28-18-10(6-12(22)16(26)14(18)24)20(9)8-4-2-1-3-7(8)19(27)29-20/h1-6,25-26H | | InChI = 1/C20H8I4O5/c21-11-5-9-17(13(23)15(11)25)28-18-10(6-12(22)16(26)14(18)24)20(9)8-4-2-1-3-7(8)19(27)29-20/h1-6,25-26H | ||
| | InChIKey = OALHHIHQOFIMEF-UHFFFAOYAB | | InChIKey = OALHHIHQOFIMEF-UHFFFAOYAB | ||
| Line 19: | Line 25: | ||
| | StdInChIKey = OALHHIHQOFIMEF-UHFFFAOYSA-N | | StdInChIKey = OALHHIHQOFIMEF-UHFFFAOYSA-N | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | CASNo = 16423-68-0 | | CASNo = 16423-68-0 | ||
| | |
| SMILES = C(=O)C1=CC=CC=C1C1=C2C=C(I)C(=O)C(I)=C2OC2=C1C=C(I)C()=C2I.. | ||
| | |
| PubChem = 3259 | ||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| | |
| Formula = C<sub>20</sub>H<sub>6</sub>I<sub>4</sub>Na<sub>2</sub>O<sub>5</sub> | ||
| | |
| MolarMass = 879.86 g/mol | ||
| | |
| Density = | ||
| | MeltingPtC = 142 to 144 | |||
| | MeltingPt = | |||
| | MeltingPt_ref = <ref>{{cite web | title = Erythrosine B product description | url = http://www.chemicalbook.com/ChemicalProductProperty_US_CB0393696.aspx | work = The Chemical Book }}</ref> | |||
| | |
| BoilingPt = | ||
| }} | }} | ||
| |Section3={{Chembox Hazards | |||
| | NFPA-H = 2 | |||
| | NFPA-F = 1 | |||
| | NFPA-R = 0 | |||
| }} | |||
| }} | }} | ||
| '''Erythrosine''', also known as Red No. 3, is an ], specifically a derivative of ]. |
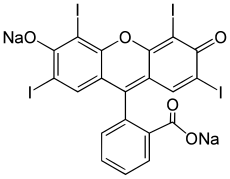
'''Erythrosine''', also known as '''Red No. 3''', is an ], specifically a derivative of ]. It is a pink dye which is primarily used for ].<ref name=Ullmann>{{cite book | first = Phyllis A. | last = Lyday | name-list-style = vanc | chapter = Iodine and Iodine Compounds | title = Ullmann's Encyclopedia of Industrial Chemistry | date = 2005 | publisher = Wiley-VCH | location = Weinheim }}</ref> It is the disodium ] of 2,4,5,7-tetraiodo]. Its maximum absorbance is at 530 ]<ref>{{cite web | first = Robert John | last = Lancashire | name-list-style = vanc | publisher = Department of Chemistry, University of the West Indies |url= http://wwwchem.uwimona.edu.jm:1104/lectures/ecode.html |title=Food Color Additives<!-- Bot generated title --> |access-date=2007-01-29 | archive-url= https://web.archive.org/web/20070128165006/http://wwwchem.uwimona.edu.jm:1104/lectures/ecode.html |archive-date=2007-01-28 |url-status=dead }}</ref> in an aqueous solution, and it is subject to ]. | ||
| ==Uses== | ==Uses== | ||
| It is used as a ],<ref>{{cite journal | vauthors = Chequer FM, Venâncio VP, Bianchi ML, Antunes LM | title = Genotoxic and mutagenic effects of erythrosine B, a xanthene food dye, on HepG2 cells | journal = Food and Chemical Toxicology | volume = 50 | issue = 10 | pages = 3447–51 | date = October 2012 | pmid = 22847138 | doi = 10.1016/j.fct.2012.07.042 | doi-access = free }}</ref> ] ink,<ref>{{cite book |title=Synthetic Dyes in Biology, Medicine And Chemistry |last=Gurr |first=Edward | name-list-style = vanc |publisher=elsevier|year=2012|pages=197, 198}}</ref> biological stain,<ref name=":0" /> ] disclosing agent,<ref>{{cite journal | vauthors = Wood S, Metcalf D, Devine D, Robinson C | title = Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms | journal = The Journal of Antimicrobial Chemotherapy | volume = 57 | issue = 4 | pages = 680–4 | date = April 2006 | pmid = 16464894 | doi = 10.1093/jac/dkl021 | doi-access = free }}</ref> ] medium,<ref name=":0">{{cite book |title=Dictionary of Analytical Reagents |publisher=CRC Press |year=1993 |isbn= 978-0-412-35150-1 |pages=474 }}</ref> sensitizer for ] photographic films, and visible light photoredox catalyst.<ref>{{cite journal | vauthors = Rogers DA, Brown RG, Brandeburg ZC, Ko EY, Hopkins MD, LeBlanc G, Lamar AA | title = N-Bromosuccinimide | journal = ACS Omega | volume = 3 | issue = 10 | pages = 12868–12877 | date = October 2018 | pmid = 31458011 | pmc = 6644467 | doi = 10.1021/acsomega.8b02320 }}</ref> | |||
| It is used as a ], in ] inks, as a biological stain, a ] disclosing agent and a ] medium. Erythrosine is commonly used in sweets such as some candies and popsicles, and even more widely used in cake-decorating gels. It is also used to color ] shells.<ref></ref><ref>Blue Diamond Ultra Premium Blend Mixed Nuts, distributed by Diamond Foods, Inc. Stockton, CA</ref> As a food additive, it has the ] E127. | |||
| Erythrosine is commonly used in sweets such as some candies and ], and even more widely used in cake-decorating gels. It was also used to color ] shells.<ref>{{Cite web |last=Day |first=Chris |date=2023-01-16 |title=The Almost-Forgotten Era Of Red Pistachios |url=https://www.thedailymeal.com/1168708/the-almost-forgotten-era-of-red-pistachios/ |access-date=2023-11-17 |website=The Daily Meal |language=en-US}}</ref> As a food additive, it has the ] E127. | |||
| While commonly used in many countries of the world, erythrosine is less commonly used in the United States because ] (Red #40) is generally used instead. However, Allura Red AC is banned in many European countries because it is an ], despite the fact that it has fewer known health risks than erythrosine.{{Citation needed|date=April 2010}} | |||
| ==Regulation and prevalence== | |||
| As a result of efforts begun in the 1970s, in 1990 the U.S. FDA had instituted a partial ban on erythrosine, citing research that high doses have been found to cause cancer in rats.<ref>, ''The Washington Post'', February 7, 1990</ref> In June of 2008, the ] (CSPI) petitioned the FDA for a complete ban on erythrosine in the United States.<ref>, CBS News, June 3, 2008</ref> | |||
| Erythrosine is restricted as a food additive in the European Union, China, and the United Kingdom.<ref>{{cite news |url=https://www.npr.org/2021/10/15/1046348573/sprinklegate-sinks-a-u-k-bakerys-top-sellers-after-topping-is-found-to-be-illega |title='Sprinklegate' sinks a U.K. bakery's top sellers after topping is found to be illegal |date=October 15, 2021 |first=Bill |last=Chappell |access-date=29 November 2023 }}</ref> Its usage is limited in Australia, and New Zealand.<ref>{{Citation |last=FSANZ |title=Australia New Zealand Food Standards Code – Schedule 8 – Food additive names and code numbers (for statement of ingredients) |date=2021-03-26 |url=https://www.legislation.gov.au/F2015L00478/latest/text |access-date=2024-09-19 |publisher=Office Parliamentary Counsel; Locked Bag 30 Kingston ACT 2604}}</ref> Erythrosine can be used in colored food and ingested drugs in the U.S. without any restriction; however, its use is banned in cosmetics and ] drugs. It is less commonly used in the United States because ] (Red #40) is generally used instead.<ref>{{cite web | url=https://www.fda.gov/ForIndustry/ColorAdditives/ColorAdditiveInventories/ucm106626.htm | title=Color Additive Status List | work=Food & Drug Administration | date=December 2015 | access-date=May 16, 2016}}</ref> The ] variant is also banned from use in the United States. | |||
| The ] only allows erythrosine in processed cherries<ref>{{cite journal |last1=EFSA |title=Scientific Opinion on the re-evaluation of Erythrosine (E 127) as a food additive |journal=EFSA Journal |date=2011 |volume=9 |issue=1 |page=1854 |doi=10.2903/j.efsa.2011.1854|url=https://phaidra.univie.ac.at/o:245195 |author1-link=EFSA }} | |||
| A series of toxicology tests combined with a review of other reported studies concluded that erythrosine is non-].<ref>{{Cite journal | doi = 10.1093/mutage/1.4.253 | title = Mutagenicity studies on FD&C Red No.3 | year = 1986 | last1 = Lin | first1 = George H. Y. | last2 = Brusick | first2 = David J. | journal = Mutagenesis | volume = 1 | issue = 4 | pages = 253–259 | pmid = 2457780}}</ref> Up to now, erythrosine can be used in colored food in USA without any restriction (). | |||
| Erythrosine is exclusively authorised for use in cocktail and candied cherries, and Bigarreaux cherries</ref> and pet foods.<ref>{{cite journal |last1=EFSA |title=Update of the Scientific Opinion on the safety and efficacy of erythrosine in feed for cats, dogs, reptiles and ornamental fish |journal=EFSA Journal |date=2015 |volume=13 |issue=9 |page=4233 |doi=10.2903/j.efsa.2015.4233|author1-link=EFSA |doi-access=free |hdl=2434/559021 |hdl-access=free }}</ref><ref>{{cite journal |last1=EFSA|title=Safety of erythrosine for ornamental fish |journal=EFSA Journal |date=2019 |volume=17 |issue=5 |page=5699 |doi=10.2903/j.efsa.2019.5699|pmid=32626322 | pmc=7009114 |author1-link=EFSA }}</ref> | |||
| ⚫ | ==Synonyms== |
||
| ⚫ | Erythrosine B; Erythrosin B; Acid Red 51; C.I. 45430; FD |
||
| ===United States=== | |||
| ⚫ | 2',4',5',7'-Tetraiodo-3',6'-dihydroxy-spiro-3-one disodium salt; Tetraiodofluorescein |
||
| As a result of efforts begun in the 1970s, in 1990, the U.S. ] (FDA) instituted a partial ban on erythrosine, citing research that high doses cause cancer in rats.<ref>, ''The Washington Post'', February 7, 1990</ref> A 1990 study concluded that "chronic erythrosine ingestion may promote thyroid tumor formation in rats via chronic stimulation of the thyroid by TSH." with 4% of total daily dietary intake consisting of erythrosine B.<ref>{{cite journal | vauthors = Jennings AS, Schwartz SL, Balter NJ, Gardner D, Witorsch RJ | title = Effects of oral erythrosine (2',4',5',7'-tetraiodofluorescein) on the pituitary-thyroid axis in rats | journal = Toxicology and Applied Pharmacology | volume = 103 | issue = 3 | pages = 549–56 | date = May 1990 | pmid = 2160137 | doi = 10.1016/0041-008x(90)90327-q | doi-access = free }}</ref> A series of toxicology tests combined with a review of other reported studies concluded that erythrosine is non-] and any increase in tumors is caused by a non-genotoxic mechanism.<ref>{{cite journal | vauthors = Lin GH, Brusick DJ | title = Mutagenicity studies on FD&C red No.3 | journal = Mutagenesis | volume = 1 | issue = 4 | pages = 253–9 | date = July 1986 | pmid = 2457780 | doi = 10.1093/mutage/1.4.253 }}</ref> | |||
| In June 2008, the ] (CSPI) petitioned the FDA for a complete ban on erythrosine in the United States,<ref>{{cite web|url=https://www.cbsnews.com/news/fda-urged-to-ban-some-food-dyes/|title=FDA Urged To Ban Some Food Dyes|publisher=CBS News|date=June 3, 2008|archive-url=https://web.archive.org/web/20120827181228/http://www.cbsnews.com/stories/2008/06/03/health/main4151092.shtml|archive-date=2012-08-27|access-date=2022-11-14|url-status=live}}</ref> but the FDA has not taken any further action. | |||
| As of May 2023, the U.S. state of ] is considering banning the use of Red Dye No. 3 in foods (it was already banned from cosmetics as of 1990) because it has been shown to cause cancer in animals and because of claims that it, and other synthetic food dyes, may contribute to child behavioral problems such as hyperactivity.<ref name=NJTimesMay2023> by Dana G. Smith, April 13, 2023 on the New York Times website. Last access 5/23/2023.</ref> ] plans to ban the manufacture, sale, and distribution of foods containing Red Dye No. 3 starting in 2027, following a bill signed into law in October 2023 that also bans three other food additives: ], ], and ].<ref>{{cite web|title = California becomes first US state to ban 4 potentially harmful chemicals in food | work = CNN | url = https://www.cnn.com/2023/10/09/health/california-governor-bans-red-dye-no-3-wellness/index.html | date = October 10, 2023}}</ref><ref>{{cite web|url=https://leginfo.legislature.ca.gov/faces/billCompareClient.xhtml?bill_id=202320240AB418&showamends=false|title=AB-418 The California Food Safety Act.|publisher=]|lang=en}}</ref><ref> by Marlene Cimons, Washington Post, Oct. 11, 2023. The article notes that Red dye No. 3, bromated vegetable oil, potassium bromate and propyl paraben all have been linked to risk of cancer and hyperactivity in children.</ref> | |||
| ⚫ | ==Synonyms== | ||
| ⚫ | Erythrosine B; Erythrosin B; Acid Red 51; C.I. 45430; FD&C Red No. 3; E127; | ||
| ⚫ | 2',4',5',7'-Tetraiodo-3',6'-dihydroxy-spiro-3-one disodium salt; Tetraiodofluorescein sodium salt; Calcoid Erythrosine N; 2,4,5,7-Tetraiodo-3,6-dihydroxyxanthene-9-spiro-1'-3H-isobenzofuran-3'-one disodium salt; 2',4',5',7'-Tetraiodofluorescein, disodium salt; C.I. Food Red 14; Aizen Erythrosine; Tetraiodifluorescein, disodium salt; Spiroxanthen]-3-one, 3',6'-dihydroxy-2',4',5',7'-tetraiodo-, disodium salt.<ref> {{Webarchive|url=https://web.archive.org/web/20100317184306/http://www.usca.edu/chemistry/spectra/erythrosin.htm |date=2010-03-17 }}, University of South Carolina</ref><ref>, chemicalland21.com</ref> | ||
| ==Classification== | ==Classification== | ||
| Line 52: | Line 74: | ||
| * ] E127 (Food Red 14) | * ] E127 (Food Red 14) | ||
| * ] no. 45430 (Acid Red 51) | * ] no. 45430 (Acid Red 51) | ||
| * ] No. 1697 | * ] No. 1697 | ||
| ==References== | == References == | ||
| {{reflist}} | {{reflist}} | ||
| ==External links== | == External links == | ||
| * | * | ||
| * | * | ||
| Line 64: | Line 86: | ||
| ] | ] | ||
| ] | ] | ||
| ⚫ | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | ] | ||
| ⚫ | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 16:36, 14 November 2024
Iodo-derivative of fluorone used as a pink dye"Red 3" redirects here. For the techno release called Red Three, see Dave Clarke (DJ).
| |
| Names | |
|---|---|
| IUPAC name 2-(6-Hydroxy-2,4,5,7-tetraiodo-3-oxo-xanthen-9-yl)benzoic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.036.390 |
| E number | E127 (colours) |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C20H6I4Na2O5 |
| Molar mass | 879.86 g/mol |
| Melting point | 142 to 144 °C (288 to 291 °F; 415 to 417 K) |
| Hazards | |
| NFPA 704 (fire diamond) |
 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Erythrosine, also known as Red No. 3, is an organoiodine compound, specifically a derivative of fluorone. It is a pink dye which is primarily used for food coloring. It is the disodium salt of 2,4,5,7-tetraiodofluorescein. Its maximum absorbance is at 530 nm in an aqueous solution, and it is subject to photodegradation.
Uses
It is used as a food coloring, printing ink, biological stain, dental plaque disclosing agent, radiopaque medium, sensitizer for orthochromatic photographic films, and visible light photoredox catalyst.
Erythrosine is commonly used in sweets such as some candies and ice pops, and even more widely used in cake-decorating gels. It was also used to color pistachio shells. As a food additive, it has the E number E127.
Regulation and prevalence
Erythrosine is restricted as a food additive in the European Union, China, and the United Kingdom. Its usage is limited in Australia, and New Zealand. Erythrosine can be used in colored food and ingested drugs in the U.S. without any restriction; however, its use is banned in cosmetics and topical drugs. It is less commonly used in the United States because Allura Red AC (Red #40) is generally used instead. The lake variant is also banned from use in the United States.
The European Food Safety Authority only allows erythrosine in processed cherries and pet foods.
United States
As a result of efforts begun in the 1970s, in 1990, the U.S. Food and Drug Administration (FDA) instituted a partial ban on erythrosine, citing research that high doses cause cancer in rats. A 1990 study concluded that "chronic erythrosine ingestion may promote thyroid tumor formation in rats via chronic stimulation of the thyroid by TSH." with 4% of total daily dietary intake consisting of erythrosine B. A series of toxicology tests combined with a review of other reported studies concluded that erythrosine is non-genotoxic and any increase in tumors is caused by a non-genotoxic mechanism.
In June 2008, the Center for Science in the Public Interest (CSPI) petitioned the FDA for a complete ban on erythrosine in the United States, but the FDA has not taken any further action.
As of May 2023, the U.S. state of New York is considering banning the use of Red Dye No. 3 in foods (it was already banned from cosmetics as of 1990) because it has been shown to cause cancer in animals and because of claims that it, and other synthetic food dyes, may contribute to child behavioral problems such as hyperactivity. California plans to ban the manufacture, sale, and distribution of foods containing Red Dye No. 3 starting in 2027, following a bill signed into law in October 2023 that also bans three other food additives: propylparaben, potassium bromate, and brominated vegetable oil.
Synonyms
Erythrosine B; Erythrosin B; Acid Red 51; C.I. 45430; FD&C Red No. 3; E127; 2',4',5',7'-Tetraiodo-3',6'-dihydroxy-spiro-3-one disodium salt; Tetraiodofluorescein sodium salt; Calcoid Erythrosine N; 2,4,5,7-Tetraiodo-3,6-dihydroxyxanthene-9-spiro-1'-3H-isobenzofuran-3'-one disodium salt; 2',4',5',7'-Tetraiodofluorescein, disodium salt; C.I. Food Red 14; Aizen Erythrosine; Tetraiodifluorescein, disodium salt; Spiroxanthen]-3-one, 3',6'-dihydroxy-2',4',5',7'-tetraiodo-, disodium salt.
Classification
It is listed under the following number systems:
- FD&C Red No. 3
- E number E127 (Food Red 14)
- Color Index no. 45430 (Acid Red 51)
- Bureau of Indian Standards No. 1697
References
- "Erythrosine B product description". The Chemical Book.
- Lyday PA (2005). "Iodine and Iodine Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH.
- Lancashire RJ. "Food Color Additives". Department of Chemistry, University of the West Indies. Archived from the original on 28 January 2007. Retrieved 29 January 2007.
- Chequer FM, Venâncio VP, Bianchi ML, Antunes LM (October 2012). "Genotoxic and mutagenic effects of erythrosine B, a xanthene food dye, on HepG2 cells". Food and Chemical Toxicology. 50 (10): 3447–51. doi:10.1016/j.fct.2012.07.042. PMID 22847138.
- Gurr E (2012). Synthetic Dyes in Biology, Medicine And Chemistry. elsevier. pp. 197, 198.
- ^ Dictionary of Analytical Reagents. CRC Press. 1993. p. 474. ISBN 978-0-412-35150-1.
- Wood S, Metcalf D, Devine D, Robinson C (April 2006). "Erythrosine is a potential photosensitizer for the photodynamic therapy of oral plaque biofilms". The Journal of Antimicrobial Chemotherapy. 57 (4): 680–4. doi:10.1093/jac/dkl021. PMID 16464894.
- Rogers DA, Brown RG, Brandeburg ZC, Ko EY, Hopkins MD, LeBlanc G, Lamar AA (October 2018). "N-Bromosuccinimide". ACS Omega. 3 (10): 12868–12877. doi:10.1021/acsomega.8b02320. PMC 6644467. PMID 31458011.
- Day, Chris (16 January 2023). "The Almost-Forgotten Era Of Red Pistachios". The Daily Meal. Retrieved 17 November 2023.
- Chappell, Bill (15 October 2021). "'Sprinklegate' sinks a U.K. bakery's top sellers after topping is found to be illegal". Retrieved 29 November 2023.
- FSANZ (26 March 2021), Australia New Zealand Food Standards Code – Schedule 8 – Food additive names and code numbers (for statement of ingredients), Office Parliamentary Counsel; Locked Bag 30 Kingston ACT 2604, retrieved 19 September 2024
- "Color Additive Status List". Food & Drug Administration. December 2015. Retrieved 16 May 2016.
- EFSA (2011). "Scientific Opinion on the re-evaluation of Erythrosine (E 127) as a food additive". EFSA Journal. 9 (1): 1854. doi:10.2903/j.efsa.2011.1854. Erythrosine is exclusively authorised for use in cocktail and candied cherries, and Bigarreaux cherries
- EFSA (2015). "Update of the Scientific Opinion on the safety and efficacy of erythrosine in feed for cats, dogs, reptiles and ornamental fish". EFSA Journal. 13 (9): 4233. doi:10.2903/j.efsa.2015.4233. hdl:2434/559021.
- EFSA (2019). "Safety of erythrosine for ornamental fish". EFSA Journal. 17 (5): 5699. doi:10.2903/j.efsa.2019.5699. PMC 7009114. PMID 32626322.
- FDA: Red Dye's Reluctant Regulator; Partial Ban Points to Limitations of 30-Year-Old Delaney Clause, The Washington Post, February 7, 1990
- Jennings AS, Schwartz SL, Balter NJ, Gardner D, Witorsch RJ (May 1990). "Effects of oral erythrosine (2',4',5',7'-tetraiodofluorescein) on the pituitary-thyroid axis in rats". Toxicology and Applied Pharmacology. 103 (3): 549–56. doi:10.1016/0041-008x(90)90327-q. PMID 2160137.
- Lin GH, Brusick DJ (July 1986). "Mutagenicity studies on FD&C red No.3". Mutagenesis. 1 (4): 253–9. doi:10.1093/mutage/1.4.253. PMID 2457780.
- "FDA Urged To Ban Some Food Dyes". CBS News. 3 June 2008. Archived from the original on 27 August 2012. Retrieved 14 November 2022.
- Two States Have Proposed Bans on Common Food Additives Linked to Health Concerns by Dana G. Smith, April 13, 2023 on the New York Times website. Last access 5/23/2023.
- "California becomes first US state to ban 4 potentially harmful chemicals in food". CNN. 10 October 2023.
- "AB-418 The California Food Safety Act". ca.gov.
- California isn’t banning Skittles, but four additives will be removed by Marlene Cimons, Washington Post, Oct. 11, 2023. The article notes that Red dye No. 3, bromated vegetable oil, potassium bromate and propyl paraben all have been linked to risk of cancer and hyperactivity in children.
- Erythrosin B Archived 2010-03-17 at the Wayback Machine, University of South Carolina
- Erythrosine, chemicalland21.com
External links
- Bureau of Indian Standards: List of Indian Standards under mandatory certification
- Some more details, other common names