| Revision as of 18:46, 16 July 2011 editEmausBot (talk | contribs)Bots, Template editors2,858,169 editsm r2.6.4) (robot Adding: kk:Валидол← Previous edit | Latest revision as of 15:50, 25 June 2022 edit undoPlastikspork (talk | contribs)Edit filter managers, Administrators258,994 edits Undid revision 1094641127 by 2A02:3038:0:C767:1:2:9D4:38D1 (talk)Tag: Undo | ||
| (20 intermediate revisions by 14 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | Watchedfields = changed | |||
| | verifiedrevid = |
| verifiedrevid = 439818432 | ||
| | Name = Methyl pentanoate | | Name = Methyl pentanoate | ||
| | Reference =<ref></ref> | | Reference =<ref></ref> | ||
| | ImageFile = methyl pentanoate. |
| ImageFile = methyl pentanoate.svg | ||
| | ImageSize = 200px | |||
| | PIN = Methyl pentanoate | |||
| | ImageName = | |||
| | |
| OtherNames = Methyl valerate | ||
| | OtherNames = Methyl valerate<br/>Mentholum valerianicum | |||
| | Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| Line 18: | Line 18: | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = HNBDRPTVWVGKBR-UHFFFAOYSA-N | | StdInChIKey = HNBDRPTVWVGKBR-UHFFFAOYSA-N | ||
| | CASNo_Ref = {{cascite|correct|??}} | |||
| | CASNo = 624-24-8 | | CASNo = 624-24-8 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | UNII = ZW21JJJ9VN | |||
| | SMILES = O=C(OC)CCCC | | SMILES = O=C(OC)CCCC | ||
| }} | }} | ||
| | Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| | |
| C=6|H=12|O=2 | ||
| | MolarMass = 116.16 g/mol | |||
| | Density = 0.89 g/cm<sup>3</sup> | | Density = 0.89 g/cm<sup>3</sup> | ||
| | MeltingPt =<25 °C | | MeltingPt =<25 °C | ||
| | |
| BoilingPtC = 126 | ||
| }} | }} | ||
| }} | }} | ||
| Line 32: | Line 34: | ||
| '''Methyl pentanoate''', commonly known as '''methyl valerate''', is the ] ] of ] (valeric acid) with a fruity ]. | '''Methyl pentanoate''', commonly known as '''methyl valerate''', is the ] ] of ] (valeric acid) with a fruity ]. | ||
| Methyl pentanoate is commonly used in fragrances, beauty care, ], laundry ]s at levels of 0. |
Methyl pentanoate is commonly used in fragrances, beauty care, ], laundry ]s at levels of 0.1–1%. | ||
| In a very pure form (greater than 99.5%) it is used as a ] in the manufacture of ]s. | In a very pure form (greater than 99.5%) it is used as a ] in the manufacture of ]s. | ||
| It is also used as an ]. | It is also used as an ].{{cn|date=November 2014}} | ||
| == See also == | == See also == | ||
| * ] | |||
| * ] | * ] | ||
| == References == | == References == | ||
| {{Reflist}} | {{Reflist}} | ||
| {{Esters}} | |||
| ] | ] | ||
| ] | ] | ||
| {{ester-stub}} | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 15:50, 25 June 2022
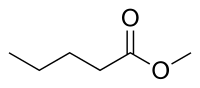
| |
| Names | |
|---|---|
| Preferred IUPAC name Methyl pentanoate | |
| Other names Methyl valerate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.009.853 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C6H12O2 |
| Molar mass | 116.160 g·mol |
| Density | 0.89 g/cm |
| Melting point | <25 °C |
| Boiling point | 126 °C (259 °F; 399 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Methyl pentanoate, commonly known as methyl valerate, is the methyl ester of pentanoic acid (valeric acid) with a fruity odor.
Methyl pentanoate is commonly used in fragrances, beauty care, soap, laundry detergents at levels of 0.1–1%.
In a very pure form (greater than 99.5%) it is used as a plasticizer in the manufacture of plastics.
It is also used as an insecticide.
See also
References
| Esters | |
|---|---|
| Methyl esters | |
| Ethyl esters | |
| Propyl esters | |
| Butyl esters | |
| Amyl esters | |
| Hexyl esters | |
| Phenyl esters | |
| Heptyl esters | |
| Benzyl esters | |
This article about an ester is a stub. You can help Misplaced Pages by expanding it. |