| Revision as of 16:37, 23 July 2011 editMartinBotIII (talk | contribs)136,346 editsm fix MSDS link (ilo.org) using AWB← Previous edit | Latest revision as of 11:17, 5 March 2024 edit undo192.167.164.69 (talk) The antiferromagnetic temperature is now expressed in Kelvin degrees that is the measurement unit used from the article cited. In general, Kelvin degrees are used more often for magnetic order temperatures. | ||
| (65 intermediate revisions by 51 users not shown) | |||
| Line 1: | Line 1: | ||
| {{redirect|CoO||COO (disambiguation)}} | |||
| {{chembox | {{chembox | ||
| | Watchedfields = changed | |||
| | verifiedrevid = |
| verifiedrevid = 441022830 | ||
| | |
| Name = Cobalt(II) oxide | ||
| | ImageFile =NaCl polyhedra.png | |||
| | |
| ImageFile = Cobalt(II)-oxide-3D-vdW.png | ||
| | |
| ImageName = Cobalt(II) oxide | ||
| | |
| IUPACName = Cobalt(II) oxide | ||
| | OtherNames = Cobaltous oxide<br/>Cobalt monoxide | |||
| ⚫ | | Section1 = {{Chembox Identifiers | ||
| | SystematicName = | |||
| ⚫ | | |
||
| ⚫ | | Section1 = {{Chembox Identifiers | ||
| ⚫ | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 8117730 | | ChemSpiderID = 8117730 | ||
| | InChI = 1/Co.O/rCoO/c1-2 | | InChI = 1/Co.O/rCoO/c1-2 | ||
| Line 20: | Line 23: | ||
| | StdInChIKey = IUYLTEAJCNAMJK-UHFFFAOYSA-N | | StdInChIKey = IUYLTEAJCNAMJK-UHFFFAOYSA-N | ||
| | CASNo = 1307-96-6 | | CASNo = 1307-96-6 | ||
| | |
| CASNo_Ref = {{cascite|correct|CAS}} | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | PubChem = 9942118 | |||
| | |
| UNII = V9X9644V7Q | ||
| | |
| PubChem = 9942118 | ||
| | |
| RTECS = GG2800000 | ||
| | UNNumber = 3288 | |||
| | EINECS = 215-154-6 | |||
| }} | }} | ||
| | Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| | |
| Formula = CoO | ||
| | |
| MolarMass = 74.9326 g/mol | ||
| | |
| Appearance = olive or gray powder | ||
| | |
| Odor = odorless | ||
| | Density = 6.45 g/cm<sup>3</sup> <ref>{{RubberBible87th}}</ref> | |||
| | Density = 6.44 g/cm<sup>3</sup> <ref>{{cite book | last =Patnaik | first =Pradyot | year = 2003 | title =Handbook of Inorganic Chemical Compounds | publisher = McGraw-Hill | page =| isbn =0070494398 | url= http://books.google.com/?id=Xqj-TTzkvTEC&pg=PA119 | accessdate = 2009-06-06}}</ref> | |||
| | |
| Solubility = insoluble in water<ref> {{Webarchive|url=https://web.archive.org/web/20110719172732/http://www.alfa.com/content/msds/german/44354.pdf |date=2011-07-19 }}. Alfa.com. Retrieved on 2011-11-19.</ref> | ||
| | |
| MeltingPtC = 1933 | ||
| | |
| BoilingPt = | ||
| | MagSus = +4900.0·10<sup>−6</sup> cm<sup>3</sup>/mol | |||
| }} | }} | ||
| | Section3 = {{Chembox Structure | | Section3 = {{Chembox Structure | ||
| | |
| CrystalStruct = ], ] | ||
| | |
| SpaceGroup = Fm<u style="text-decoration:overline">3</u>m, No. 225 | ||
| }} | }} | ||
| | Section4 = | |||
| ⚫ | | Section7 = {{Chembox Hazards | ||
| | Section5 = | |||
| ⚫ | | |
||
| | Section6 = | |||
| | EUIndex = 027-002-00-4 | |||
| ⚫ | | Section7 = {{Chembox Hazards | ||
| | EUClass = Harmful ('''Xn''')<br/>Dangerous for the environment ('''N''') | |||
| ⚫ | | ExternalSDS = | ||
| | RPhrases = {{R22}}, {{R43}}, {{R50/53}} | |||
| | MainHazards = | |||
| | SPhrases = {{S2}}, {{S24}}, {{S37}}, {{S60}}, {{S61}} | |||
| | GHSPictograms = {{GHS07}}{{GHS09}} | |||
| ⚫ | | |
||
| | GHSSignalWord = Warning | |||
| ⚫ | | |
||
| | HPhrases = {{H-phrases|302|317|410}} | |||
| | NFPA-R = | |||
| | PPhrases = {{P-phrases|260|280|284|301+310+330|304+340+310|342+311|403+233}} | |||
| ⚫ | | |
||
| | |
| NFPA-H = 3 | ||
| ⚫ | | NFPA-F = 0 | ||
| ⚫ | | NFPA-R = 0 | ||
| ⚫ | | FlashPt = Non-flammable | ||
| | LD50 = 202 mg/kg | |||
| }} | }} | ||
| | Section8 = {{Chembox Related | | Section8 = {{Chembox Related | ||
| | |
| OtherAnions = ]<br/>] | ||
| | |
| OtherCations = ]<br/>] | ||
| | |
| OtherCompounds = ]<br/>] | ||
| }} | }} | ||
| }} | }} | ||
| '''Cobalt(II) oxide |
'''Cobalt(II) oxide''' is an ] that has been described as an ]<ref name=G&E/> or ]<ref name=Ullmann/> ]. It is used extensively in the ]s industry as an additive to create blue-colored ] and ], as well as in the chemical industry for producing cobalt(II) salts. A related material is ], a black solid with the ] Co<sub>3</sub>O<sub>4</sub>. | ||
| == Structure and properties == | == Structure and properties == | ||
| CoO crystals adopt the ] (]) structure with a lattice constant of 4. |
CoO crystals adopt the ] (]) structure with a lattice constant of 4.2615 Å.<ref>{{cite journal|doi=10.1103/PhysRevB.35.6847|title=Percolation effects and magnetic properties of the randomly diluted fcc system CopMg1-pO|year=1987|author1=Kannan, R. |author2=Seehra, Mohindar S. |journal=Physical Review B|volume=35|pages=6847–6853|issue=13|pmid=9940938 |bibcode=1987PhRvB..35.6847K }}</ref> | ||
| It is ] below |
It is ] below 289 K.<ref>{{cite journal|doi=10.1103/PhysRevB.24.419|title=Principal magnetic susceptibilities and uniaxial stress experiments in CoO|year=1981|author1=Silinsky, P. S. |author2=Seehra, Mohindar S. |journal=Physical Review B|volume=24|issue=1 |pages=419–423|bibcode=1981PhRvB..24..419S }}</ref> | ||
| ==Preparation== | ==Preparation== | ||
| Cobalt(II) oxide is prepared by oxidation of cobalt powder with air or by thermal decomposition of ] or the carbonate.<ref name=G&E>{{Greenwood&Earnshaw2nd}}</ref><ref name=Ullmann>{{Ullmann |doi=10.1002/14356007.a07_281.pub2|title=Cobalt and Cobalt Compounds|year=2005|last1=Donaldson|first1=John Dallas|last2=Beyersmann|first2=Detmar}}</ref> | |||
| ⚫ | ] decomposes to cobalt(II) oxide at 950 |
||
| Fisher, William B. (Chester, VA) 1983. http://www.freepatentsonline.com/4389339.html</ref> | |||
| ⚫ | ] decomposes to cobalt(II) oxide at 950 °C:<ref>{{cite patent | title = Process for making a cobalt oxide catalyst | invent1 = James, Leonard E. | invent2 = Crescentini, Lamberto | invent3 = Fisher, William B. | pubdate = 1983-06-21 | number = 4389339 | country = US }}</ref> | ||
| :2 Co<sub>3</sub>O<sub>4</sub> → 6 CoO + O<sub>2</sub> | :2 Co<sub>3</sub>O<sub>4</sub> → 6 CoO + O<sub>2</sub> | ||
| ⚫ | It may also be prepared by precipitating the hydroxide, followed by thermal dehydration:{{cn|date=March 2021}} | ||
| Though commercially available, cobalt(II) oxide may be prepared in the laboratory by electrolyzing a solution of cobalt(II) chloride<ref>Kern, S.; J. Chem. Soc.; 1876. Part 1. p880.</ref>: | |||
| ⚫ | : CoX<sub>2</sub> + 2 KOH → Co(OH)<sub>2</sub> + 2 KX | ||
| : Co(OH)<sub>2</sub> → CoO + H<sub>2</sub>O | |||
| ⚫ | It may also be prepared by precipitating the hydroxide, followed by thermal |
||
| ⚫ | : CoX + 2 |
||
| : Co(OH)<sub>2</sub> → CoO + H<sub>2</sub>O | |||
| ==Reactions== | ==Reactions== | ||
| As can be expected, cobalt(II) oxide reacts with mineral acids to form the corresponding cobalt salts: | As can be expected, cobalt(II) oxide reacts with mineral acids to form the corresponding cobalt salts:{{cn|date=March 2021}} | ||
| : CoO + 2 HX → CoX<sub>2</sub> + H<sub>2</sub>O | : CoO + 2 HX → CoX<sub>2</sub> + H<sub>2</sub>O | ||
| ==Applications== | ==Applications== | ||
| Cobalt(II) oxide has for centuries used as a coloring agent on ] fired pottery |
Cobalt(II) oxide has for centuries been used as a coloring agent on ] fired pottery. The additive provides a deep shade of blue named ]. The band gap (CoO) is around 2.4 eV.{{Citation Needed|date=October 2017}} | ||
| It also is used in ]. | It also is used in ]. | ||
| ==See also== | |||
| *] | |||
| ⚫ | *] | ||
| ⚫ | *] | ||
| == References == | == References == | ||
| Line 93: | Line 103: | ||
| {{Cobalt compounds}} | {{Cobalt compounds}} | ||
| {{Oxygen compounds}} | |||
| {{Oxides}} | |||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ⚫ | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ⚫ | ] | ||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 11:17, 5 March 2024
"CoO" redirects here. For other uses, see COO (disambiguation).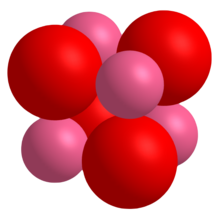
| |
| Names | |
|---|---|
| IUPAC name Cobalt(II) oxide | |
| Other names
Cobaltous oxide Cobalt monoxide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| ECHA InfoCard | 100.013.777 |
| EC Number |
|
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 3288 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | CoO |
| Molar mass | 74.9326 g/mol |
| Appearance | olive or gray powder |
| Odor | odorless |
| Density | 6.45 g/cm |
| Melting point | 1,933 °C (3,511 °F; 2,206 K) |
| Solubility in water | insoluble in water |
| Magnetic susceptibility (χ) | +4900.0·10 cm/mol |
| Structure | |
| Crystal structure | cubic, cF8 |
| Space group | Fm3m, No. 225 |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Warning |
| Hazard statements | H302, H317, H410 |
| Precautionary statements | P260, P280, P284, P301+P310+P330, P304+P340+P310, P342+P311, P403+P233 |
| NFPA 704 (fire diamond) |
 |
| Flash point | Non-flammable |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 202 mg/kg |
| Safety data sheet (SDS) | ICSC 1551 |
| Related compounds | |
| Other anions | Cobalt(II) sulfide Cobalt(II) hydroxide |
| Other cations | Iron(II) oxide Nickel(II) oxide |
| Related compounds | Cobalt(II,III) oxide Cobalt(III) oxide |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Cobalt(II) oxide is an inorganic compound that has been described as an olive-green or gray solid. It is used extensively in the ceramics industry as an additive to create blue-colored glazes and enamels, as well as in the chemical industry for producing cobalt(II) salts. A related material is cobalt(II,III) oxide, a black solid with the formula Co3O4.
Structure and properties
CoO crystals adopt the periclase (rock salt) structure with a lattice constant of 4.2615 Å.
It is antiferromagnetic below 289 K.
Preparation
Cobalt(II) oxide is prepared by oxidation of cobalt powder with air or by thermal decomposition of cobalt(II) nitrate or the carbonate.
Cobalt(II,III) oxide decomposes to cobalt(II) oxide at 950 °C:
- 2 Co3O4 → 6 CoO + O2
It may also be prepared by precipitating the hydroxide, followed by thermal dehydration:
- CoX2 + 2 KOH → Co(OH)2 + 2 KX
- Co(OH)2 → CoO + H2O
Reactions
As can be expected, cobalt(II) oxide reacts with mineral acids to form the corresponding cobalt salts:
- CoO + 2 HX → CoX2 + H2O
Applications
Cobalt(II) oxide has for centuries been used as a coloring agent on kiln fired pottery. The additive provides a deep shade of blue named cobalt blue. The band gap (CoO) is around 2.4 eV. It also is used in cobalt blue glass.
See also
References
- Lide, David R., ed. (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, Florida: CRC Press. ISBN 0-8493-0487-3.
- Advanced Search – Alfa Aesar – A Johnson Matthey Company Archived 2011-07-19 at the Wayback Machine. Alfa.com. Retrieved on 2011-11-19.
- ^ Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. ISBN 978-0-08-037941-8.
- ^ Donaldson, John Dallas; Beyersmann, Detmar (2005). "Cobalt and Cobalt Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a07_281.pub2. ISBN 978-3527306732.
- Kannan, R.; Seehra, Mohindar S. (1987). "Percolation effects and magnetic properties of the randomly diluted fcc system CopMg1-pO". Physical Review B. 35 (13): 6847–6853. Bibcode:1987PhRvB..35.6847K. doi:10.1103/PhysRevB.35.6847. PMID 9940938.
- Silinsky, P. S.; Seehra, Mohindar S. (1981). "Principal magnetic susceptibilities and uniaxial stress experiments in CoO". Physical Review B. 24 (1): 419–423. Bibcode:1981PhRvB..24..419S. doi:10.1103/PhysRevB.24.419.
- US 4389339, James, Leonard E.; Crescentini, Lamberto & Fisher, William B., "Process for making a cobalt oxide catalyst", published 1983-06-21
| Cobalt compounds | |
|---|---|
| Cobalt(I) | |
| Cobalt(II) | |
| Cobalt(0,III) | |
| Cobalt(II,III) | |
| Cobalt(III) | |
| Cobalt(III,IV) | |
| Cobalt(IV) | |
| Cobalt(V) | |