| Revision as of 07:35, 9 August 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:Wi← Previous edit | Latest revision as of 22:12, 3 September 2024 edit undoBD2412 (talk | contribs)Autopatrolled, IP block exemptions, Administrators2,458,882 editsm →top: Clean up spacing around commas and other punctuation fixes, replaced: ,S → , STag: AWB | ||
| (88 intermediate revisions by 35 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | Watchedfields = changed | |||
| | verifiedrevid = |
| verifiedrevid = 443829334 | ||
| ⚫ | |ImageFileL1=Fulvene. |
||
| | Name = | |||
| ⚫ | |ImageSizeL1=120px | ||
| | ImageFile = | |||
| ⚫ | |ImageFileR1=Fulvene_3D.png | ||
| ⚫ | | ImageFileL1 = Fulvene with hydrogens.svg | ||
| ⚫ | |ImageSizeR1=120px | ||
| ⚫ | | ImageSizeL1 = 120px | ||
| ⚫ | |IUPACName= |
||
| ⚫ | | ImageFileR1 = Fulvene_3D.png | ||
| ⚫ | |OtherNames=5-Methylene-1,3-cyclopentadiene |
||
| ⚫ | | ImageSizeR1 = 120px | ||
| ⚫ | |Section1={{Chembox Identifiers | ||
| | PIN = 5-Methylidenecyclopenta-1,3-diene<ref name=iupac2013>{{cite book | title = Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book) | publisher = ] | date = 2014 | location = Cambridge | page = 379 | doi = 10.1039/9781849733069-FP001 | isbn = 978-0-85404-182-4}}</ref> | |||
| ⚫ | | |
||
| | SystematicName = | |||
| ⚫ | | OtherNames = Fulvene<ref name=iupac2013 /><br />5-Methylene-1,3-cyclopentadiene | ||
| ⚫ | | IUPACName = | ||
| ⚫ | | Section1 = {{Chembox Identifiers | ||
| ⚫ | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 120097 | | ChemSpiderID = 120097 | ||
| | InChI = 1/C6H6/c1-6-4-2-3-5-6/h2-5H,1H2 | | InChI = 1/C6H6/c1-6-4-2-3-5-6/h2-5H,1H2 | ||
| Line 16: | Line 21: | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = PGTKVMVZBBZCKQ-UHFFFAOYSA-N | | StdInChIKey = PGTKVMVZBBZCKQ-UHFFFAOYSA-N | ||
| ⚫ | | CASNo_Ref = {{cascite|correct|??}} | ||
| | CASNo=497-20-1 | | CASNo=497-20-1 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| ⚫ | | |
||
| | UNII = 19W699IKIE | |||
| ⚫ | | |
||
| ⚫ | | PubChem=136323 | ||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | ChEBI = 51999 | | ChEBI = 51999 | ||
| | SMILES = C=C1\C=C/C=C1 | | SMILES = C=C1\C=C/C=C1 | ||
| }} | }} | ||
| |Section2={{Chembox Properties | | Section2 = {{Chembox Properties | ||
| | C=6|H=6 | |||
| | Formula=C<sub>6</sub>H<sub>6</sub> | |||
| | Appearance= | |||
| | MolarMass=78.11 g/mol | |||
| | |
| Density= | ||
| | |
| MeltingPt= | ||
| | |
| BoilingPt= | ||
| | |
| Solubility= | ||
| | MagSus = -42.9·10<sup>−6</sup> cm<sup>3</sup>/mol | |||
| | Solubility= | |||
| }} | }} | ||
| |Section3={{Chembox Hazards | | Section3 = {{Chembox Hazards | ||
| | |
| MainHazards= | ||
| | |
| FlashPt= | ||
| | AutoignitionPt = | |||
| | Autoignition= | |||
| }} | }} | ||
| | Section4 = | |||
| | Section5 = | |||
| | Section6 = | |||
| }} | }} | ||
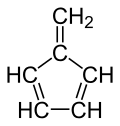
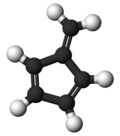
| '''Fulvene''' (pentafulvene) is a ] with the formula (CH=CH)<sub>2</sub>C=CH<sub>2</sub>. It is a prototype of a ] hydrocarbon.<ref>{{cite journal|author1=Preethanuj Preethalayam |author2=Syam krishnan, K.|author3=Sreeja Thulasi |author4= Sarath Chand, S. |author5=Jomy Joseph | |||
| |author6= Vijay Nair | |||
| '''Fulvene''' is one of several ]s with the same formula as ], C<sub>6</sub>H<sub>6</sub>. Fulvenes include the derivatives of this simple hydrocarbon, which itself is rarely encountered.<ref>{{cite journal | |||
| |author7=Florian Jaroschik|author8=K.V.Radhakrishnan | |||
| |title= Recent Advances in the Chemistry of Pentafulvenes. | |||
| ⚫ | |journal = Chemical Reviews | ||
| |year=2017|volume= 117|issue=5|pages= 3930–3989 | |||
| |doi=10.1021/acs.chemrev.6b00210|pmid=28151643}}</ref> Fulvene is rarely encountered,<ref>{{cite journal | |||
| | author = Bergmann, E. D. | | author = Bergmann, E. D. | ||
| | title = Fulvenes and Substituted Fulvenes | | title = Fulvenes and Substituted Fulvenes | ||
| Line 45: | Line 61: | ||
| | pages = 41–84 | | pages = 41–84 | ||
| | year = 1968 | | year = 1968 | ||
| | doi = 10.1021/cr60251a002}}</ref> |
| doi = 10.1021/cr60251a002}}</ref> but substituted derivatives (]) are numerous. They are mainly of interest as ]s and precursors to ligands in ]. | ||
| | author = Thiele, J. | |||
| Fulvene is an isomer of ], which when irradiated at 237 to 254 nm forms small amounts of fulvene along with ].<ref>{{Cite journal |last=Kaplan |first=Louis |last2=Wilzbach |first2=K. E. |date=1968 |title=Photolysis of benzene vapor. Benzvalene formation at wavelengths 2537-2370 A |url=https://pubs.acs.org/doi/abs/10.1021/ja01014a086 |journal=Journal of the American Chemical Society |language=en |volume=90 |issue=12 |pages=3291–3292 |doi=10.1021/ja01014a086 |issn=0002-7863}}</ref> | |||
| | title = <!--no umlaut-->Ueber Ketonreactionen bei dem Cyclopentadiën | |||
| | journal = ] | |||
| | volume = 33 | |||
| | pages = 666–673 | |||
| | year = 1900 | |||
| | doi = 10.1002/cber.190003301113}}</ref> Most fulvenes are prepared by the reactions starting from cyclopentadiene or sodium cyclopentadienyl.<ref>{{cite journal | |||
| | author = Hafner, K.; Vöpel, K. H.; Ploss, G.; König, C. | |||
| | title = 6-(Dimethylamino)fulvene | |||
| | journal = Organic Syntheses Coll. Vol. | |||
| | volume = 5 | |||
| | pages = 431 | |||
| | year = 1973 | |||
| | url = http://www.orgsyn.org/orgsyn/pdfs/CV5P0431.pdf}}</ref> | |||
| == See also == | |||
| 2,3,4,5-Tetramethylfulvene, abbreviated Me<sub>4</sub>Fv, is a relatively common ligand in ]. It typically results from the deprotonation of cationic ] complexes.<ref>{{cite journal | |||
| *] | |||
| | author = Kreindlin, A. Z.; Rybinskaya, M. A. | |||
| *] | |||
| | title = Cationic and Neutral Transition Metal Complexes with a Tetramethylfulvene or Trimethylallyldiene Ligand | |||
| ⚫ | | |
||
| | volume = 73 | |||
| | pages = 417–432 | |||
| | year = 2004 | |||
| | doi = 10.1070/RC2004v073n05ABEH000842}}</ref> | |||
| ==References== | ==References== | ||
| {{reflist}} | {{reflist}} | ||
| {{Authority control}} | |||
| ⚫ | ] | ||
| ⚫ | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 22:12, 3 September 2024
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 5-Methylidenecyclopenta-1,3-diene | |||
| Other names
Fulvene 5-Methylene-1,3-cyclopentadiene | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C6H6 | ||
| Molar mass | 78.114 g·mol | ||
| Magnetic susceptibility (χ) | -42.9·10 cm/mol | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Fulvene (pentafulvene) is a hydrocarbon with the formula (CH=CH)2C=CH2. It is a prototype of a cross-conjugated hydrocarbon. Fulvene is rarely encountered, but substituted derivatives (fulvenes) are numerous. They are mainly of interest as ligands and precursors to ligands in organometallic chemistry.
Fulvene is an isomer of benzene, which when irradiated at 237 to 254 nm forms small amounts of fulvene along with benzvalene.
See also
References
- ^ Nomenclature of Organic Chemistry : IUPAC Recommendations and Preferred Names 2013 (Blue Book). Cambridge: The Royal Society of Chemistry. 2014. p. 379. doi:10.1039/9781849733069-FP001. ISBN 978-0-85404-182-4.
- Preethanuj Preethalayam; Syam krishnan, K.; Sreeja Thulasi; Sarath Chand, S.; Jomy Joseph; Vijay Nair; Florian Jaroschik; K.V.Radhakrishnan (2017). "Recent Advances in the Chemistry of Pentafulvenes". Chemical Reviews. 117 (5): 3930–3989. doi:10.1021/acs.chemrev.6b00210. PMID 28151643.
- Bergmann, E. D. (1968). "Fulvenes and Substituted Fulvenes". Chemical Reviews. 68: 41–84. doi:10.1021/cr60251a002.
- Kaplan, Louis; Wilzbach, K. E. (1968). "Photolysis of benzene vapor. Benzvalene formation at wavelengths 2537-2370 A". Journal of the American Chemical Society. 90 (12): 3291–3292. doi:10.1021/ja01014a086. ISSN 0002-7863.