| Revision as of 10:45, 9 August 2011 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'DrugBank', 'ChEBI').← Previous edit | Latest revision as of 12:31, 14 August 2024 edit undoPreimage (talk | contribs)Extended confirmed users960 edits →Chembox_Properties: Add pKb | ||
| (173 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Distinguish|Guanine|Guanosine|Guanfacine}} | |||
| {{chembox | |||
| {{Chembox | |||
| | verifiedrevid = 415512619 | |||
| |Watchedfields = changed | |||
| | Name = Guanidine | |||
| |verifiedrevid = 443849964 | |||
| | ImageFile = Guanidine-2D-skeletal.png | |||
| |ImageFileL1 = Guanidin.svg | |||
| <!-- | ImageSize = 150px --> | |||
| |ImageFileL1_Ref = {{chemboximage|correct|??}} | |||
| | ImageName = Skeletal formula of guanidine | |||
| |ImageNameL1 = Skeletal formula of guanidine | |||
| | ImageFile1 = Guanidine-3D-balls.png | |||
| |ImageFileR1 = Guanidine-2D.png | |||
| <!-- | ImageSize1 = 150px --> | |||
| |ImageFileR1_Ref = {{chemboximage|correct|??}} | |||
| | ImageName1 = Ball-and-stick model of guanidine | |||
| |ImageNameR1 = Skeletal formula of guanidine with the implicit carbon shown, and all explicit hydrogens added. | |||
| | IUPACName = Guanidine | |||
| |ImageFileL2 = Guanidine-3D-balls.png | |||
| | Section1 = {{Chembox Identifiers | |||
| | |
|ImageFileL2_Ref = {{chemboximage|correct|??}} | ||
| |ImageNameL2 = Ball and stick model of guanidine | |||
| | ChemSpiderID = 3400 | |||
| |ImageFileR2 = Guanidine-3D-vdW.png | |||
| | PubChem = 3520 | |||
| | |
|ImageFileR2_Ref = {{chemboximage|correct|??}} | ||
| |ImageNameR2 = Spacefill model of guanidine | |||
| | UNII = JU58VJ6Y3B | |||
| |PIN = Guanidine<ref>{{cite book |author=] |date=2014 |title=Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013 |publisher=] |pages=883 |doi=10.1039/9781849733069 |isbn=978-0-85404-182-4}}</ref> | |||
| | InChI = 1/CH5N3/c2-1(3)4/h(H5,2,3,4) | |||
| |OtherNames = Iminomethanediamine | |||
| | InChIKey = ZRALSGWEFCBTJO-UHFFFAOYAY | |||
| |Section1={{Chembox Identifiers | |||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| |IUPHAR_ligand = 4783 | |||
| | ChEMBL = 821 | |||
| |CASNo = 113-00-8 | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| |CASNo_Ref = {{cascite|correct|CAS}} | |||
| | StdInChI = 1S/CH5N3/c2-1(3)4/h(H5,2,3,4) | |||
| |PubChem = 3520 | |||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| |ChemSpiderID = 3400 | |||
| | StdInChIKey = ZRALSGWEFCBTJO-UHFFFAOYSA-N | |||
| |ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | CASNo = 113-00-8 | |||
| |UNII = JU58VJ6Y3B | |||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| |UNII_Ref = {{fdacite|correct|FDA}} | |||
| | DrugBank = DB00536 | |||
| | |
|EINECS = 204-021-8 | ||
| |DrugBank = DB00536 | |||
| | SMILES = C(=N)(N)N | |||
| |DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| }} | |||
| |MeSHName = Guanidine | |||
| | Section2 = {{Chembox Properties | |||
| |ChEBI = 42820 | |||
| | Formula = CH<sub>5</sub>N<sub>3</sub> | |||
| |ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | MolarMass = 59.07 g/mol | |||
| | |
|ChEMBL = 821 | ||
| |ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| | MeltingPt = 50 °C | |||
| |Beilstein = 506044 | |||
| | BoilingPt = | |||
| |Gmelin = 100679 | |||
| | pKa = 13.6 (guanidinium cation)<ref name=Perrin/> | |||
| |SMILES = NC(N)=N | |||
| }} | |||
| |StdInChI = 1S/CH5N3/c2-1(3)4/h(H5,2,3,4) | |||
| | Section7 = {{Chembox Hazards | |||
| |StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | ExternalMSDS = | |||
| |StdInChIKey = ZRALSGWEFCBTJO-UHFFFAOYSA-N | |||
| | EUIndex = Not listed | |||
| |StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | MainHazards = | |||
| | NFPA-H = | |||
| | NFPA-F = | |||
| | NFPA-R = | |||
| | FlashPt = | |||
| | RPhrases = | |||
| | SPhrases = | |||
| }} | |||
| | Section8 = {{Chembox Related | |||
| | OtherFunctn = | |||
| | Function = | |||
| | OtherCpds = ]<br/>] | |||
| }} | |||
| }} | }} | ||
| |Section2={{Chembox Properties | |||
| |C=1 | H=5 | N=3 | |||
| |MeltingPtC = 50 | |||
| |pKb = 0.4<ref name=Perrin/> | |||
| |ConjugateAcid = Guanidinium | |||
| |LogP = −1.251 | |||
| }} | |||
| |Section3={{Chembox Thermochemistry | |||
| |DeltaHf = −57 – −55 kJ mol<sup>−1</sup> | |||
| |DeltaHc = −1.0511 – −1.0531 MJ mol<sup>−1</sup> | |||
| }} | |||
| |Section4={{Chembox Pharmacology | |||
| |HalfLife = 7–8 hours | |||
| }} | |||
| |Section5={{Chembox Hazards | |||
| |LD50 = 475 mg/kg (oral, rat)<ref>{{cite web|url=https://chem.nlm.nih.gov/chemidplus/rn/50-01-1|website=ChemIDplus|publisher=]|title=Guanidine hydrochloride|access-date=2014-08-10|archive-date=2014-08-12|archive-url=https://web.archive.org/web/20140812205443/https://chem.nlm.nih.gov/chemidplus/rn/50-01-1|url-status=live}}</ref> | |||
| }} | |||
| |Section6={{Chembox Related | |||
| |OtherCompounds = {{Unbulleted list|]|]}} | |||
| }} | |||
| }} | |||
| '''Guanidine''' is the compound with the formula HNC(NH<sub>2</sub>)<sub>2</sub>. It is a colourless solid that dissolves in ] solvents. It is a ] that is used in the production of ] and ]. It is found in ] predominantly in patients experiencing renal failure.<ref>{{cite journal | vauthors = Sawynok J, Dawborn JK | title = Plasma concentration and urinary excretion of guanidine derivatives in normal subjects and patients with renal failure | journal = Clinical and Experimental Pharmacology & Physiology | volume = 2 | issue = 1 | pages = 1–15 | year = 1975 | pmid = 1126056 | doi = 10.1111/j.1440-1681.1975.tb02368.x | s2cid = 41794868 }}</ref> A guanidine ] also appears in larger organic molecules, including on the side chain of ]. | |||
| == Structure == | |||
| '''Guanidine''' is a ] compound of strong ] formed by the ] of ]. It is used in the manufacture of ] and ]. It is found in ] as a normal product of protein ]. The molecule was first synthesized in 1861 by the oxidative degradation of an aromatic natural product, ], isolated from Peruvian ].<ref>A. Strecker, ''Liebigs Ann. Chem.'' '''1861''', ''118'', 151.</ref> Despite the simplicity of the molecule, the crystal structure was first described 148 years later.<ref>T. Yamada, X. Liu, U. Englert, H. Yamane, R. Dronskowski, ''Chem. Eur. J.'' '''2009''', ''15'', 5651.</ref> | |||
| Guanidine can be thought of as a nitrogenous analogue of ]. That is, the C=O group in carbonic acid is replaced by a C=NH group, and each OH is replaced by a {{chem|NH|2}} group.<ref>{{cite journal | vauthors = Göbel M, Klapötke TM | title = First structural characterization of guanidine, HN=C(NH(2))(2) | journal = Chemical Communications | volume = 43 | issue = 30 | pages = 3180–3182 | date = August 2007 | pmid = 17653381 | doi = 10.1039/B705100J }}</ref> ] can be seen as the carbon analogue in much the same way. A detailed crystallographic analysis of guanidine was elucidated 148 years after its first synthesis, despite the simplicity of the molecule.<ref>{{cite journal | vauthors = Yamada T, Liu X, Englert U, Yamane H, Dronskowski R | title = Solid-state structure of free base guanidine achieved at last | journal = Chemistry: A European Journal | volume = 15 | issue = 23 | pages = 5651–5655 | date = June 2009 | pmid = 19388036 | doi = 10.1002/chem.200900508 }}</ref> In 2013, the positions of the hydrogen atoms and their displacement parameters were accurately determined using single-crystal neutron diffraction.<ref>{{cite journal | vauthors = Sawinski PK, Meven M, Englert U, Dronskowski R | journal = Crystal Growth & Design | year = 2013 | volume = 13 |issue=4 | pages = 1730–5 | doi = 10.1021/cg400054k | title = Single-Crystal Neutron Diffraction Study on Guanidine, CN<sub>3</sub>H<sub>5</sub> |doi-access=free }}</ref> | |||
| ] | |||
| ==Production== | |||
| ==Guanidinium cation== | |||
| Guanidine can be obtained from natural sources, being first isolated in 1861 by ] via the oxidative degradation of an aromatic natural product, ], isolated from Peruvian ].<ref name=Ullmann/><ref>{{cite journal | vauthors = Strecker A | author-link = Adolph Strecker | journal = Liebigs Ann. Chem. | year = 1861 | volume = 118 | pages = 151–177 | issue = 2 | title = Untersuchungen über die chemischen Beziehungen zwischen Guanin, Xanthin, Theobromin, Caffeïn und Kreatinin | trans-title = Studies on the chemical relationships between guanine, xanthine, theobromine, caffeine and creatinine | doi = 10.1002/jlac.18611180203 | url = https://zenodo.org/record/1427163 | access-date = 2019-07-02 | archive-date = 2021-07-16 | archive-url = https://web.archive.org/web/20210716154230/https://zenodo.org/record/1427163 | url-status = live }}</ref> | |||
| Guanidine is protonated in physiological conditions. This ] is called the '''guanidinium''' ], <sup>+</sup>. The guanidinium cation has a charge of ]. It is a highly stable cation in aqueous solution due to the efficient resonance stabilization of the charge and efficient solvation by water molecules. As a result, its ] is 13.6<ref name=Perrin>Perrin, D.D., ''Dissociation Constants of Organic Bases in Aqueous Solution'', Butterworths, London, 1965; Supplement, 1972.</ref> meaning that guanidine is a very strong base in water. | |||
| A laboratory method of producing guanidine is gentle (180-190 °C) thermal decomposition of dry ] in anhydrous conditions: | |||
| :{{chem2|3 NH4SCN -> 2 CH5N3 + H2S + CS2}} | |||
| The commercial route involves a two step process starting with the reaction of ] with ] salts. Via the intermediacy of ], this ] step affords salts of the guanidinium cation (see below). In the second step, the salt is treated with base, such as ].<ref name=Ullmann>{{Ullmann| vauthors = Güthner T, Mertschenk B, Schulz B |title=Guanidine and Derivatives |doi=10.1002/14356007.a12_545.pub2}}</ref> | |||
| ]s (S-alkylated ]s) react with ]s to give ] salts:<ref>{{cite journal|last1=Palmer|first1=David C.|title=''S''-Methylisothiourea|journal=E-EROS Encyclopedia of Reagents for Organic Synthesis|year=2001|doi=10.1002/047084289X.rm199s|isbn=0471936235}}</ref> | |||
| :RNH<sub>2</sub> + <sup>+</sup>X<sup>−</sup> → <sup>+</sup>X<sup>−</sup> + CH<sub>3</sub>SH | |||
| The resulting guanidinium ions can often be deprotonated to give the guanidine. This approach is sometimes called the Rathke synthesis, in honor of its discoverer. after ]<ref>{{cite journal|title=Heinrich Bernhard Rathke. (1840-1923)|journal=Berichte der Deutschen Chemischen Gesellschaft (A and B Series)|date=8 October 1924|volume=57|issue=9|pages=A83–A92|doi=10.1002/cber.19240570929|doi-access=free}}</ref><ref>{{cite journal|last1=Rathke|first1=B.|title=Ueber Derivate und Constitution des Schwefelharnstoffs|journal=Berichte der Deutschen Chemischen Gesellschaft|date=July 1881|volume=14|issue=2|pages=1774–1780|doi=10.1002/cber.18810140247|url=https://zenodo.org/record/1425246}}</ref> | |||
| ==Chemistry== | |||
| ===Guanidinium cation=== | |||
| The ] is called the '''guanidinium''' ], ({{chem|C(NH|2|)|3|+}}). This planar, symmetric ion consists of three ] groups each bonded to the central carbon atom with a covalent bond of ] 4/3. It is a highly stable ] cation in aqueous solution due to the efficient ] of the charge and efficient ] by water molecules. As a result, its ] is 13.6<ref name=Perrin>{{cite book| vauthors = Perrin DD |title=Dissociation Constants of Organic Bases in Aqueous Solution |publisher=Butterworths |location=London |date=1972 |edition=Supplement }}</ref> (p''K''<sub>b</sub> of 0.4) meaning that guanidine is a very strong base in water; in neutral water, it exists almost exclusively as guanidinium. Due to this, most guanidine derivatives are salts containing the conjugate acid. | |||
| <gallery> | <gallery> | ||
| Image:Guanidinium-ion-3D-balls.png| |
Image:Guanidinium-ion-3D-balls.png|{{center|]}} | ||
| Image:Guanidinium-ion-2D-skeletal.png| |
Image:Guanidinium-ion-2D-skeletal.png|{{center|]}} | ||
| Image:Guanidinium-ion-canonical-forms-2D-skeletal.png| |
Image:Guanidinium-ion-canonical-forms-2D-skeletal.png|{{center|canonical forms}} | ||
| </gallery> | </gallery> | ||
| ===Testing for guanidine=== | |||
| Notable guanidinium salts include ] (GndCl), which has ] properties and is used to denature proteins. Empirically, guanidine hydrochloride is known to denature proteins with a linear relationship between concentration and ] of unfolding. Another such salt is ]. | |||
| Guanidine can be selectively detected using sodium 1,2-naphthoquinone-4-sulfonic acid (]) and acidified urea.<ref>{{Cite journal | vauthors = Sullivan MX |date=1935-10-01 |title=A Colorimetric Test for Guanidine |journal=Proceedings of the Society for Experimental Biology and Medicine |language=en |volume=33 |issue=1 |pages=106–108 |doi=10.3181/00379727-33-8270C |s2cid=88290359 |issn=0037-9727}}</ref> | |||
| ==Uses== | |||
| ===Industry=== | |||
| The main salt of commercial interest is ] {{chem|NO|3}}. It is used as a propellant, for example in ]s. | |||
| ===Medicine=== | |||
| Since the Middle Ages in Europe, guanidine has been used to treat diabetes as the active ] ingredient in ]. Due to its long-term ], further research for blood sugar control was suspended at first after the discovery of insulin. Later development of nontoxic, safe ] led to the long-used first-line diabetes control medicine ], introduced to Europe in the 1950s & United States in 1995 and now prescribed to over 17 million patients per year in the US.<ref name="Treatment approach to type 2 diabet">{{cite journal | vauthors = Blaslov K, Naranđa FS, Kruljac I, Renar IP | title = Treatment approach to type 2 diabetes: Past, present and future | journal = World Journal of Diabetes | volume = 9 | issue = 12 | pages = 209–219 | date = December 2018 | pmid = 30588282 | pmc = 6304295 | doi = 10.4239/wjd.v9.i12.209 | doi-access = free }}</ref><ref>{{Cite web|title=The Top 300 of 2019|url=https://clincalc.com/DrugStats/Top300Drugs.aspx|access-date=2022-02-17|website=clincalc.com|archive-date=2021-02-12|archive-url=https://web.archive.org/web/20210212142534/https://clincalc.com/DrugStats/Top300Drugs.aspx|url-status=live}}</ref> | |||
| Guanidinium chloride<ref name="Treatment approach to type 2 diabet"/> is a now-controversial ] in treatment of ]. Recent studies have shown some significant subsets of patients who see no improvement after the administration of this drug.<ref>{{cite book| vauthors = Brook I |title=Pediatric Anaerobic Infections: Diagnosis and Management|publisher=Taylor & Francis|year=2001|isbn=0824741862|edition=3rd|page=529}}<!-- Please confirm page correct for edition--></ref> | |||
| ===Biochemistry=== | |||
| Guanidine exists protonated, as guanidinium, in solution at physiological pH. | |||
| ] (also known as guanidine hydrochloride) has ] properties and is used to denature proteins. Guanidinium chloride is known to denature proteins with a linear relationship between concentration and ] of unfolding. In aqueous solutions containing 6 ] guanidinium chloride, almost all ] lose their entire ] and become ] peptide chains. ] is also used for its denaturing effect on various biological samples. | |||
| Recent studies suggest that guanidinium is produced by bacteria as a toxic byproduct. To alleviate the toxicity of guanidinium, bacteria have developed a class of transporters known as guanidinium exporters or Gdx proteins to expel the extra amounts of this ion to the outside of the cell.<ref>{{cite journal | vauthors = Kermani AA, Macdonald CB, Gundepudi R, Stockbridge RB | title = Guanidinium export is the primal function of SMR family transporters | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 115 | issue = 12 | pages = 3060–3065 | date = March 2018 | pmid = 29507227 | pmc = 5866581 | doi = 10.1073/pnas.1719187115 | bibcode = 2018PNAS..115.3060K | doi-access = free }}</ref> Gdx proteins, are highly selective for guanidinium and mono-substituted guanidinyl compounds and share an overlapping set of non-canonical substrates with drug exporter EmrE.<ref>{{cite journal | vauthors = Kermani AA, Macdonald CB, Burata OE, Ben Koff B, Koide A, Denbaum E, Koide S, Stockbridge RB | display-authors = 6 | title = The structural basis of promiscuity in small multidrug resistance transporters | journal = Nature Communications | volume = 11 | issue = 1 | pages = 6064 | date = November 2020 | pmid = 33247110 | doi = 10.1038/s41467-020-19820-8 | pmc = 7695847 | bibcode = 2020NatCo..11.6064K }}</ref> | |||
| ===Other=== | |||
| Guanidinium hydroxide is the active ingredient in some non-lye ]s. | |||
| ==Guanidine derivatives== | ==Guanidine derivatives== | ||
| ] | ] | ||
| '''Guanidines''' are a group of ]s sharing a common ] with the general structure (R |
'''Guanidines''' are a group of ]s sharing a common ] with the general structure {{chem|(R|1|R|2|N)(R|3|R|4|N)C{{=}}N−R|5}}. The central bond within this group is that of an ], and the group is related structurally to amidines and ureas. Examples of guanidines are ], ], ], and ]. | ||
| '''Guanidinium salts''' are well known for their denaturing action on proteins; ] is one of the most effective ]. In 6 M aqueous GndHCl almost all ] lose their ordered "secondary structure" (that results from intramolecular noncovalent interactions) and become "randomly coiled"; that is, their secondary structure interactions are disrupted by the dissolved guanidinium, leaving only the primary covalent structure of their polyamide backbones. | |||
| ] is an ] guanidine.<ref name=Witters2001>{{cite journal | vauthors = Witters LA | title = The blooming of the French lilac | journal = The Journal of Clinical Investigation | volume = 108 | issue = 8 | pages = 1105–1107 | date = October 2001 | pmid = 11602616 | pmc = 209536 | doi = 10.1172/JCI14178 }}</ref> | |||
| {{Clear|left}} | |||
| == See also == | |||
| *] | |||
| *] | |||
| *] | |||
| == |
== References== | ||
| *] | |||
| *] | |||
| {{Reflist}} | |||
| ==References== | |||
| <references/> | |||
| {{Antidiarrheals, intestinal anti-inflammatory/anti-infective agents}} | |||
| {{Antihypertensives and diuretics}} | {{Antihypertensives and diuretics}} | ||
| {{Authority control}} | |||
| ] | |||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 12:31, 14 August 2024
Not to be confused with Guanine, Guanosine, or Guanfacine.
| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name Guanidine | |||
| Other names Iminomethanediamine | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| Beilstein Reference | 506044 | ||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| DrugBank | |||
| ECHA InfoCard | 100.003.656 | ||
| EC Number |
| ||
| Gmelin Reference | 100679 | ||
| IUPHAR/BPS | |||
| MeSH | Guanidine | ||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | CH5N3 | ||
| Molar mass | 59.072 g·mol | ||
| Melting point | 50 °C (122 °F; 323 K) | ||
| log P | −1.251 | ||
| Basicity (pKb) | 0.4 | ||
| Conjugate acid | Guanidinium | ||
| Thermochemistry | |||
| Std enthalpy of formation (ΔfH298) |
−57 – −55 kJ mol | ||
| Std enthalpy of combustion (ΔcH298) |
−1.0511 – −1.0531 MJ mol | ||
| Pharmacology | |||
| Pharmacokinetics: | |||
| Biological half-life | 7–8 hours | ||
| Hazards | |||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) | 475 mg/kg (oral, rat) | ||
| Related compounds | |||
| Related compounds | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Guanidine is the compound with the formula HNC(NH2)2. It is a colourless solid that dissolves in polar solvents. It is a strong base that is used in the production of plastics and explosives. It is found in urine predominantly in patients experiencing renal failure. A guanidine moiety also appears in larger organic molecules, including on the side chain of arginine.
Structure
Guanidine can be thought of as a nitrogenous analogue of carbonic acid. That is, the C=O group in carbonic acid is replaced by a C=NH group, and each OH is replaced by a NH
2 group. Isobutene can be seen as the carbon analogue in much the same way. A detailed crystallographic analysis of guanidine was elucidated 148 years after its first synthesis, despite the simplicity of the molecule. In 2013, the positions of the hydrogen atoms and their displacement parameters were accurately determined using single-crystal neutron diffraction.
Production
Guanidine can be obtained from natural sources, being first isolated in 1861 by Adolph Strecker via the oxidative degradation of an aromatic natural product, guanine, isolated from Peruvian guano.
A laboratory method of producing guanidine is gentle (180-190 °C) thermal decomposition of dry ammonium thiocyanate in anhydrous conditions:
- 3 NH4SCN → 2 CH5N3 + H2S + CS2
The commercial route involves a two step process starting with the reaction of dicyandiamide with ammonium salts. Via the intermediacy of biguanidine, this ammonolysis step affords salts of the guanidinium cation (see below). In the second step, the salt is treated with base, such as sodium methoxide.
Isothiouronium salts (S-alkylated thioureas) react with amines to give guanidinium salts:
- RNH2 + X → X + CH3SH
The resulting guanidinium ions can often be deprotonated to give the guanidine. This approach is sometimes called the Rathke synthesis, in honor of its discoverer. after Bernhard Rathke
Chemistry
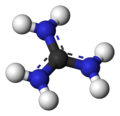
Guanidinium cation
The conjugate acid is called the guanidinium cation, (C(NH
2)
3). This planar, symmetric ion consists of three amino groups each bonded to the central carbon atom with a covalent bond of order 4/3. It is a highly stable +1 cation in aqueous solution due to the efficient resonance stabilization of the charge and efficient solvation by water molecules. As a result, its pKaH is 13.6 (pKb of 0.4) meaning that guanidine is a very strong base in water; in neutral water, it exists almost exclusively as guanidinium. Due to this, most guanidine derivatives are salts containing the conjugate acid.
-
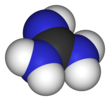
 ball-and-stick model
ball-and-stick model
-
 resonance hybrid
resonance hybrid
-
 canonical forms
canonical forms
Testing for guanidine
Guanidine can be selectively detected using sodium 1,2-naphthoquinone-4-sulfonic acid (Folin's reagent) and acidified urea.
Uses
Industry
The main salt of commercial interest is the nitrate NO
3. It is used as a propellant, for example in air bags.
Medicine
Since the Middle Ages in Europe, guanidine has been used to treat diabetes as the active antihyperglycemic ingredient in French lilac. Due to its long-term hepatotoxicity, further research for blood sugar control was suspended at first after the discovery of insulin. Later development of nontoxic, safe biguanides led to the long-used first-line diabetes control medicine metformin, introduced to Europe in the 1950s & United States in 1995 and now prescribed to over 17 million patients per year in the US.
Guanidinium chloride is a now-controversial adjuvant in treatment of botulism. Recent studies have shown some significant subsets of patients who see no improvement after the administration of this drug.
Biochemistry
Guanidine exists protonated, as guanidinium, in solution at physiological pH.
Guanidinium chloride (also known as guanidine hydrochloride) has chaotropic properties and is used to denature proteins. Guanidinium chloride is known to denature proteins with a linear relationship between concentration and free energy of unfolding. In aqueous solutions containing 6 M guanidinium chloride, almost all proteins lose their entire secondary structure and become randomly coiled peptide chains. Guanidinium thiocyanate is also used for its denaturing effect on various biological samples.
Recent studies suggest that guanidinium is produced by bacteria as a toxic byproduct. To alleviate the toxicity of guanidinium, bacteria have developed a class of transporters known as guanidinium exporters or Gdx proteins to expel the extra amounts of this ion to the outside of the cell. Gdx proteins, are highly selective for guanidinium and mono-substituted guanidinyl compounds and share an overlapping set of non-canonical substrates with drug exporter EmrE.
Other
Guanidinium hydroxide is the active ingredient in some non-lye hair relaxers.
Guanidine derivatives
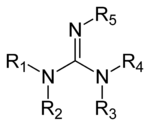
Guanidines are a group of organic compounds sharing a common functional group with the general structure (R
1R
2N)(R
3R
4N)C=N−R
5. The central bond within this group is that of an imine, and the group is related structurally to amidines and ureas. Examples of guanidines are arginine, triazabicyclodecene, saxitoxin, and creatine.
Galegine is an isoamylene guanidine.
See also
References
- International Union of Pure and Applied Chemistry (2014). Nomenclature of Organic Chemistry: IUPAC Recommendations and Preferred Names 2013. The Royal Society of Chemistry. p. 883. doi:10.1039/9781849733069. ISBN 978-0-85404-182-4.
- ^ Perrin DD (1972). Dissociation Constants of Organic Bases in Aqueous Solution (Supplement ed.). London: Butterworths.
- "Guanidine hydrochloride". ChemIDplus. National Library of Medicine. Archived from the original on 2014-08-12. Retrieved 2014-08-10.
- Sawynok J, Dawborn JK (1975). "Plasma concentration and urinary excretion of guanidine derivatives in normal subjects and patients with renal failure". Clinical and Experimental Pharmacology & Physiology. 2 (1): 1–15. doi:10.1111/j.1440-1681.1975.tb02368.x. PMID 1126056. S2CID 41794868.
- Göbel M, Klapötke TM (August 2007). "First structural characterization of guanidine, HN=C(NH(2))(2)". Chemical Communications. 43 (30): 3180–3182. doi:10.1039/B705100J. PMID 17653381.
- Yamada T, Liu X, Englert U, Yamane H, Dronskowski R (June 2009). "Solid-state structure of free base guanidine achieved at last". Chemistry: A European Journal. 15 (23): 5651–5655. doi:10.1002/chem.200900508. PMID 19388036.
- Sawinski PK, Meven M, Englert U, Dronskowski R (2013). "Single-Crystal Neutron Diffraction Study on Guanidine, CN3H5". Crystal Growth & Design. 13 (4): 1730–5. doi:10.1021/cg400054k.
- ^ Güthner T, Mertschenk B, Schulz B. "Guanidine and Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a12_545.pub2. ISBN 978-3527306732.
- Strecker A (1861). "Untersuchungen über die chemischen Beziehungen zwischen Guanin, Xanthin, Theobromin, Caffeïn und Kreatinin" [Studies on the chemical relationships between guanine, xanthine, theobromine, caffeine and creatinine]. Liebigs Ann. Chem. 118 (2): 151–177. doi:10.1002/jlac.18611180203. Archived from the original on 2021-07-16. Retrieved 2019-07-02.
- Palmer, David C. (2001). "S-Methylisothiourea". E-EROS Encyclopedia of Reagents for Organic Synthesis. doi:10.1002/047084289X.rm199s. ISBN 0471936235.
- "Heinrich Bernhard Rathke. (1840-1923)". Berichte der Deutschen Chemischen Gesellschaft (A and B Series). 57 (9): A83 – A92. 8 October 1924. doi:10.1002/cber.19240570929.
- Rathke, B. (July 1881). "Ueber Derivate und Constitution des Schwefelharnstoffs". Berichte der Deutschen Chemischen Gesellschaft. 14 (2): 1774–1780. doi:10.1002/cber.18810140247.
- Sullivan MX (1935-10-01). "A Colorimetric Test for Guanidine". Proceedings of the Society for Experimental Biology and Medicine. 33 (1): 106–108. doi:10.3181/00379727-33-8270C. ISSN 0037-9727. S2CID 88290359.
- ^ Blaslov K, Naranđa FS, Kruljac I, Renar IP (December 2018). "Treatment approach to type 2 diabetes: Past, present and future". World Journal of Diabetes. 9 (12): 209–219. doi:10.4239/wjd.v9.i12.209. PMC 6304295. PMID 30588282.
- "The Top 300 of 2019". clincalc.com. Archived from the original on 2021-02-12. Retrieved 2022-02-17.
- Brook I (2001). Pediatric Anaerobic Infections: Diagnosis and Management (3rd ed.). Taylor & Francis. p. 529. ISBN 0824741862.
- Kermani AA, Macdonald CB, Gundepudi R, Stockbridge RB (March 2018). "Guanidinium export is the primal function of SMR family transporters". Proceedings of the National Academy of Sciences of the United States of America. 115 (12): 3060–3065. Bibcode:2018PNAS..115.3060K. doi:10.1073/pnas.1719187115. PMC 5866581. PMID 29507227.
- Kermani AA, Macdonald CB, Burata OE, Ben Koff B, Koide A, Denbaum E, et al. (November 2020). "The structural basis of promiscuity in small multidrug resistance transporters". Nature Communications. 11 (1): 6064. Bibcode:2020NatCo..11.6064K. doi:10.1038/s41467-020-19820-8. PMC 7695847. PMID 33247110.
- Witters LA (October 2001). "The blooming of the French lilac". The Journal of Clinical Investigation. 108 (8): 1105–1107. doi:10.1172/JCI14178. PMC 209536. PMID 11602616.
| Antidiarrheals, intestinal anti-inflammatory and anti-infective agents (A07) | |
|---|---|
| Rehydration | |
| Intestinal anti-infectives | |
| Intestinal adsorbents |
|
| Antipropulsives (opioids) |
|
| Intestinal anti-inflammatory agents |
|
| Antidiarrheal micro-organisms | |
| Other antidiarrheals | |
| |
| Sympatholytic (and closely related) antihypertensives (C02) | |||||
|---|---|---|---|---|---|
| Sympatholytics (antagonize α-adrenergic vasoconstriction) | |||||
| Other antagonists |
| ||||
| |||||