| Revision as of 11:12, 9 August 2011 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'UNII').← Previous edit | Latest revision as of 12:56, 29 January 2023 edit undoEntranced98 (talk | contribs)Extended confirmed users, Pending changes reviewers, Rollbackers170,249 edits Importing Wikidata short description: "Chemical compound"Tag: Shortdesc helper | ||
| (28 intermediate revisions by 20 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Chemical compound}} | |||
| {{Orphan|date=April 2011}} | |||
| {{Drugbox | {{Drugbox | ||
| | verifiedrevid = |
| verifiedrevid = 443853119 | ||
| | IUPAC_name |
| IUPAC_name = 7,12-bis(1-hydroxyethyl)-3,8,13,17-tetramethyl-21''H'',23''H''-porphine-2,18-dipropanoic acid | ||
| | image |
| image = Hematoporphyrin.png | ||
| <!--Clinical data--> | |||
| | InChI = 1/C34H38N4O6/c1-15-21(7-9-31(41)42)27-14-28-22(8-10-32(43)44)16(2)24(36-28)12-29-34(20(6)40)18(4)26(38-29)13-30-33(19(5)39)17(3)25(37-30)11-23(15)35-27/h11-14,19-20,37-40H,7-10H2,1-6H3,(H,41,42)(H,43,44)/b23-11-,24-12-,25-11-,26-13-,27-14-,28-14-,29-12-,30-13- | |||
| | tradename = | |||
| | InChIKey = KFKRXESVMDBTNQ-AMPAVEGJBD | |||
| ⚫ | | pregnancy_category = | ||
| ⚫ | | |
||
| ⚫ | | legal_status = Rx-only | ||
| ⚫ | | routes_of_administration = Oral | ||
| <!--Pharmacokinetic data--> | |||
| ⚫ | | bioavailability = | ||
| ⚫ | | metabolism = | ||
| ⚫ | | elimination_half-life = | ||
| ⚫ | | excretion = | ||
| <!--Identifiers--> | |||
| ⚫ | | CAS_number = 14459-29-1 | ||
| ⚫ | | ATC_prefix = none | ||
| ⚫ | | ATC_suffix = | ||
| ⚫ | | PubChem = 11103 | ||
| ⚫ | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| ⚫ | | ChemSpiderID = 10632 | ||
| ⚫ | | UNII_Ref = {{fdacite|correct|FDA}} | ||
| ⚫ | | UNII = HBT6M5H379 | ||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | | ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| | ChEMBL = 317840 | | ChEMBL = 317840 | ||
| <!--Chemical data--> | |||
| ⚫ | | C=34 | H=38 | N=4 | O=6 | ||
| | smiles = CC1=C(C2=CC3=NC(=CC4=NC(=CC5=C(C(=C(N5)C=C1N2)C(C)O)C)C(=C4CCC(=O)O)C)C(=C3C)CCC(=O)O)C(C)O | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/C34H38N4O6/c1-15-21(7-9-31(41)42)27-14-28-22(8-10-32(43)44)16(2)24(36-28)12-29-34(20(6)40)18(4)26(38-29)13-30-33(19(5)39)17(3)25(37-30)11-23(15)35-27/h11-14,19-20,37-40H,7-10H2,1-6H3,(H,41,42)(H,43,44)/b23-11-,24-12-,25-11-,26-13-,27-14-,28-14-,29-12-,30-13- | | StdInChI = 1S/C34H38N4O6/c1-15-21(7-9-31(41)42)27-14-28-22(8-10-32(43)44)16(2)24(36-28)12-29-34(20(6)40)18(4)26(38-29)13-30-33(19(5)39)17(3)25(37-30)11-23(15)35-27/h11-14,19-20,37-40H,7-10H2,1-6H3,(H,41,42)(H,43,44)/b23-11-,24-12-,25-11-,26-13-,27-14-,28-14-,29-12-,30-13- | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = KFKRXESVMDBTNQ-AMPAVEGJSA-N | | StdInChIKey = KFKRXESVMDBTNQ-AMPAVEGJSA-N | ||
| | melting_point = 172.5 | |||
| ⚫ | | CAS_number |
||
| ⚫ | | ATC_prefix |
||
| ⚫ | | ATC_suffix |
||
| ⚫ | | UNII = HBT6M5H379 | ||
| ⚫ | | PubChem |
||
| ⚫ | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| ⚫ | | ChemSpiderID |
||
| ⚫ | | C |
||
| | molecular_weight = 598.69 g/mol | |||
| | smiles = O=C(O)CCC=5c2nc(cc4c(c(c(cc1c(c(c(n1)cc/3nc(c2)\C(=C\3C)CCC(=O)O)C(O)C)C)n4)C(O)C)C)C=5C | |||
| ⚫ | | bioavailability |
||
| ⚫ | | metabolism |
||
| ⚫ | | elimination_half-life |
||
| ⚫ | | excretion |
||
| ⚫ | | pregnancy_category |
||
| ⚫ | | legal_status |
||
| ⚫ | | routes_of_administration = Oral | ||
| }} | }} | ||
| '''Hematoporphyrin''' ('''Photodyn''', '''Sensibion''') is |
'''Hematoporphyrin''' ('''Photodyn''', '''Sensibion''') is a ] prepared from ]. It is a derivative of ], where the two vinyl groups have been hydrated (converted to alcohols). It is a deeply colored solid that is usually encountered as a solution. Its ] was determined in 1900.<ref name="urlSpringerLink - Journal Article">{{cite journal | vauthors = Luzgina VN, Filippovich EI, Evstigneeva RP | title = Hematoporphyrin IX | journal = Pharmaceutical Chemistry Journal | date = May 1977 | volume = 11 | issue = 5 | pages = 613–20 | doi = 10.1007/BF00780815 | s2cid = 44554826 | url = https://link.springer.com/article/10.1007%2FBF00780815 }}</ref> | ||
| It is used as a photosensitizer in ]. Acetylation of hematoporphyrin followed by hydrolysis of the product of that reaction affords a mixture called ''hematoporphyrin derivative'' (HPD), which is also used in photodynamic therapy.<ref>{{cite journal | vauthors = Kessel D | title = Hematoporphyrin and HPD: photophysics, photochemistry and phototherapy | journal = Photochemistry and Photobiology | volume = 39 | issue = 6 | pages = 851–9 | date = June 1984 | pmid = 6235529 | doi = 10.1111/j.1751-1097.1984.tb08871.x | s2cid = 20172683 | doi-access = free }}</ref> | |||
| ⚫ | Hematoporphyrin has been used as an ] and ] since the 1920s.<ref name="isbn0-911910-13-1">{{cite book | |
||
| ⚫ | Hematoporphyrin has also been used as an ] and ] since the 1920s.<ref name="isbn0-911910-13-1">{{cite book | last = O'Neil | first = Maryadele J. | name-list-style = vanc | title = The Merck index: an encyclopedia of chemicals, drugs, and biologicals | publisher = Merck Research Laboratories | location = Rahway, NJ | year = 2001 | isbn = 0-911910-13-1 | url = https://archive.org/details/merckindexency00onei | url-access = registration }}</ref><ref name="Strecker_1934">{{cite journal | vauthors = Strecker EA, Palmer HP, Braceland FJ | title = Hematoporphyrin as a Therapeutic Agent in the Psychoses | journal = American Journal of Psychiatry | volume = 90 | issue = 6 | pages = 1157–1173 | doi = 10.1176/ajp.90.6.1157 | date = May 1934 }}</ref> | ||
| == See also == | |||
| * ] | |||
| == References == | == References == | ||
| {{ |
{{reflist|30em}} | ||
| {{Antidepressants}} | {{Antidepressants}} | ||
| Line 50: | Line 51: | ||
| ] | ] | ||
| ] | ] | ||
| {{nervous-system-drug-stub}} | {{nervous-system-drug-stub}} | ||
| ] | |||
Latest revision as of 12:56, 29 January 2023
Chemical compound Pharmaceutical compound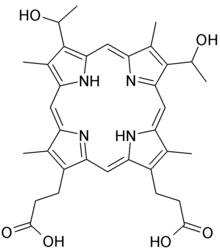 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.034.939 |
| Chemical and physical data | |
| Formula | C34H38N4O6 |
| Molar mass | 598.700 g·mol |
| 3D model (JSmol) | |
| Melting point | 172.5 °C (342.5 °F) |
SMILES
| |
InChI
| |
| (verify) | |
Hematoporphyrin (Photodyn, Sensibion) is a porphyrin prepared from hemin. It is a derivative of protoporphyrin IX, where the two vinyl groups have been hydrated (converted to alcohols). It is a deeply colored solid that is usually encountered as a solution. Its chemical structure was determined in 1900.
It is used as a photosensitizer in photodynamic therapy. Acetylation of hematoporphyrin followed by hydrolysis of the product of that reaction affords a mixture called hematoporphyrin derivative (HPD), which is also used in photodynamic therapy.
Hematoporphyrin has also been used as an antidepressant and antipsychotic since the 1920s.
References
- Luzgina VN, Filippovich EI, Evstigneeva RP (May 1977). "Hematoporphyrin IX". Pharmaceutical Chemistry Journal. 11 (5): 613–20. doi:10.1007/BF00780815. S2CID 44554826.
- Kessel D (June 1984). "Hematoporphyrin and HPD: photophysics, photochemistry and phototherapy". Photochemistry and Photobiology. 39 (6): 851–9. doi:10.1111/j.1751-1097.1984.tb08871.x. PMID 6235529. S2CID 20172683.
- O'Neil MJ (2001). The Merck index: an encyclopedia of chemicals, drugs, and biologicals. Rahway, NJ: Merck Research Laboratories. ISBN 0-911910-13-1.
- Strecker EA, Palmer HP, Braceland FJ (May 1934). "Hematoporphyrin as a Therapeutic Agent in the Psychoses". American Journal of Psychiatry. 90 (6): 1157–1173. doi:10.1176/ajp.90.6.1157.
| Antipsychotics (N05A) | |
|---|---|
| Typical |
|
| Disputed |
|
| Atypical |
|
| Others |
|
| |
This drug article relating to the nervous system is a stub. You can help Misplaced Pages by expanding it. |