| Revision as of 22:18, 9 August 2011 editZéroBot (talk | contribs)704,777 editsm r2.7.1) (robot Adding: fi:Amanulliinihappo← Previous edit |
Latest revision as of 07:35, 16 March 2023 edit undoInternetArchiveBot (talk | contribs)Bots, Pending changes reviewers5,388,266 edits Rescuing 1 sources and tagging 0 as dead.) #IABot (v2.0.9.3) (Whoop whoop pull up - 12776 |
| (11 intermediate revisions by 11 users not shown) |
| Line 1: |
Line 1: |
|
{{chembox |
|
{{chembox |
|
| verifiedrevid = 399503245 |
|
| verifiedrevid = 443948153 |
|
|ImageFile=Amanullinic acid structure.png |
|
| ImageFile=Amanullinic acid structure.png |
|
|ImageSize=200px |
|
| ImageSize=200px |
|
|ImageFile1=Amanullinic acid with tube model.png |
|
| ImageFile1=Amanullinic acid with tube model.png |
|
|ImageSize1=200px |
|
| ImageSize1=200px |
|
|IUPACName= |
|
| IUPACName= |
|
|OtherNames=1-L-Aspartic acid-3-isoleucine-alpha-amanitin |
|
| OtherNames=1-L-Aspartic acid-3-isoleucine-alpha-amanitin |
|
|Section1= {{Chembox Identifiers |
|
|Section1={{Chembox Identifiers |
|
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|
| ChemSpiderID = 149798 |
|
| ChemSpiderID = 149798 |
|
| InChI = 1/C39H53N9O13S/c1-5-17(3)31-36(58)41-13-28(51)42-26-16-62(61)38-22(21-8-7-19(49)9-23(21)45-38)11-24(33(55)40-14-29(52)46-31)43-37(59)32(18(4)6-2)47-35(57)27-10-20(50)15-48(27)39(60)25(12-30(53)54)44-34(26)56/h7-9,17-18,20,24-27,31-32,45,49-50H,5-6,10-16H2,1-4H3,(H,40,55)(H,41,58)(H,42,51)(H,43,59)(H,44,56)(H,46,52)(H,47,57)(H,53,54) |
|
| InChI = 1/C39H53N9O13S/c1-5-17(3)31-36(58)41-13-28(51)42-26-16-62(61)38-22(21-8-7-19(49)9-23(21)45-38)11-24(33(55)40-14-29(52)46-31)43-37(59)32(18(4)6-2)47-35(57)27-10-20(50)15-48(27)39(60)25(12-30(53)54)44-34(26)56/h7-9,17-18,20,24-27,31-32,45,49-50H,5-6,10-16H2,1-4H3,(H,40,55)(H,41,58)(H,42,51)(H,43,59)(H,44,56)(H,46,52)(H,47,57)(H,53,54) |
|
| InChIKey = HFENEIQMWRYNGK-UHFFFAOYAM |
|
| InChIKey = HFENEIQMWRYNGK-UHFFFAOYAM |
|
| SMILES1 = O=C2N1CC(O)CC1C(=O)NC(C(=O)NC5C(=O)NCC(=O)NC(C(=O)NCC(=O)NC(C(=O)NC2CC(=O)O)CS(=O)c4c(c3ccc(O)cc3n4)C5)C(C)CC)C(C)CC |
|
|
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChI = 1S/C39H53N9O13S/c1-5-17(3)31-36(58)41-13-28(51)42-26-16-62(61)38-22(21-8-7-19(49)9-23(21)45-38)11-24(33(55)40-14-29(52)46-31)43-37(59)32(18(4)6-2)47-35(57)27-10-20(50)15-48(27)39(60)25(12-30(53)54)44-34(26)56/h7-9,17-18,20,24-27,31-32,45,49-50H,5-6,10-16H2,1-4H3,(H,40,55)(H,41,58)(H,42,51)(H,43,59)(H,44,56)(H,46,52)(H,47,57)(H,53,54) |
|
| StdInChI = 1S/C39H53N9O13S/c1-5-17(3)31-36(58)41-13-28(51)42-26-16-62(61)38-22(21-8-7-19(49)9-23(21)45-38)11-24(33(55)40-14-29(52)46-31)43-37(59)32(18(4)6-2)47-35(57)27-10-20(50)15-48(27)39(60)25(12-30(53)54)44-34(26)56/h7-9,17-18,20,24-27,31-32,45,49-50H,5-6,10-16H2,1-4H3,(H,40,55)(H,41,58)(H,42,51)(H,43,59)(H,44,56)(H,46,52)(H,47,57)(H,53,54) |
|
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChIKey = HFENEIQMWRYNGK-UHFFFAOYSA-N |
|
| StdInChIKey = HFENEIQMWRYNGK-UHFFFAOYSA-N |
|
|
| CASNo_Ref = {{cascite|correct|??}} |
|
| CASNo=54532-45-5 |
|
| CASNo=54532-45-5 |
|
| PubChem=171349 |
|
| PubChem=171349 |
|
| SMILES=O=C(NCC(N(C(NCC(N(C3)C(N(CC(O)=O)C(N54C(O)C5)=O)=O)=O)=O)()(C)CC)=O)(CC1=C(S3=O)NC2=C1C=CC(O)=C2)NC(((C)CC)()N4=O)=O |
|
| SMILES = O=C(NCC(N(C(NCC(N(C3)C(N(CC(O)=O)C(N54C(O)C5)=O)=O)=O)=O)()(C)CC)=O)(CC1=C(S3=O)NC2=C1C=CC(O)=C2)NC(((C)CC)()N4=O)=O |
|
}} |
|
}} |
|
|Section2= {{Chembox Properties |
|
|Section2={{Chembox Properties |
|
| Formula=C<sub>39</sub>H<sub>53</sub>N<sub>9</sub>O<sub>13</sub>S |
|
| Formula=C<sub>39</sub>H<sub>53</sub>N<sub>9</sub>O<sub>13</sub>S |
|
| MolarMass=887.96 g/mol |
|
| MolarMass=887.96 g/mol |
|
| Appearance= |
|
| Appearance= |
|
| Density= |
|
| Density= |
|
| MeltingPt= |
|
| MeltingPt= |
|
| BoilingPt= |
|
| BoilingPt= |
|
| Solubility= |
|
| Solubility= |
|
}} |
|
}} |
|
|Section3= {{Chembox Hazards |
|
|Section3={{Chembox Hazards |
|
| MainHazards= |
|
| MainHazards= |
|
| FlashPt= |
|
| FlashPt= |
|
|
| AutoignitionPt = |
|
| Autoignition= |
|
|
}} |
|
}} |
|
}} |
|
}} |
|
|
|
|
|
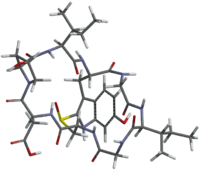
'''Amanullinic acid''' is a ] ]. It is an ], all of which are found in several members of the '']'' genus of ]s. The oral {{LD50}} of amanullinic acid is approximately 0.1 mg/kg in mice.<ref>{{cite journal | author = T. Wieland and Faulstich H. | journal = CRC Critical Reviews in Biochemistry | volume = 5 | pages = 185 | year = 1978 | doi = 10.3109/10409237809149870 | title = Amatoxins, Phallotoxins, Phallolysin, and Antamanide: the Biologically Active Components of Poisonous Amanita Mushrooms | pmid = 363352 | issue = 3}}</ref> |
|
'''Amanullinic acid''' is a ] ]. It is an ], all of which are found in several members of the ] genus '']''. Amanullinic acid is relatively non-toxic( oral {{LD50}} >20 mg/kg in mice).<ref>{{cite journal |author1 = T. Wieland |author2 =Faulstich H. | journal = CRC Critical Reviews in Biochemistry | volume = 5 | pages = 185–260 | year = 1978 | doi = 10.3109/10409237809149870 | title = Amatoxins, Phallotoxins, Phallolysin, and Antamanide: the Biologically Active Components of Poisonous Amanita Mushrooms | pmid = 363352 | issue = 3}}</ref> |
|
|
|
|
|
==Toxicology== |
|
==Toxicology== |
| Line 50: |
Line 50: |
|
|
|
|
|
==External links== |
|
==External links== |
|
* |
|
* {{Webarchive|url=https://web.archive.org/web/20090113180522/http://dohs.ors.od.nih.gov/pdf/Amatoxins%20REVISED.pdf |date=2009-01-13 }} |
|
|
|
|
|
{{Poisonous Amanitas}} |
|
{{Poisonous Amanitas}} |
| Line 57: |
Line 57: |
|
] |
|
] |
|
] |
|
] |
|
] |
|
] |
|
|
|
|
] |
|