| Revision as of 00:57, 12 August 2011 editSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,236 edits trim procedural details (WP:NOTMANUAL)← Previous edit | Latest revision as of 18:20, 9 September 2022 edit undo88.115.34.218 (talk) →Preparation | ||
| (30 intermediate revisions by 20 users not shown) | |||
| Line 1: | Line 1: | ||
| {{ |
{{Chembox | ||
| | Verifiedfields = changed | |||
| | verifiedrevid = 293599230 | |||
| | Watchedfields = changed | |||
| | Name = Isopropyl iodide | |||
| | verifiedrevid = 444362078 | |||
| | ImageFile1 = isopropyl iodide stick.png | |||
| | ImageFile = isopropyl iodide stick.png | |||
| | ImageSize1 = 150px | |||
| | ImageFile_Ref = {{chemboximage|correct|??}} | |||
| | ImageName1 = | |||
| | ImageSize = 100 | |||
| | ImageFile = IsopropylIodide.png | |||
| | ImageName = Skeletal formula of isopropyl iodide | |||
| | ImageSize =200px | |||
| | ImageFile1 = IsopropylIodide.png | |||
| | ImageName = | |||
| | ImageFile1_Ref = {{chemboximage|correct|??}} | |||
| | IUPACName = 2-iodopropane | |||
| | ImageSize1 = 160 | |||
| | OtherNames = iododimethylmethane, isopropyl iodide, 2-propyliodide, sec-propyl iodide | |||
| | ImageName1 = Spacefill model of isopropyl iodide | |||
| | Section1 = {{Chembox Identifiers | |||
| | PIN = 2-Iodopropane<ref>{{Cite web|title=isopropyl iodide - Compound Summary|url=https://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=6362&loc=ec_rcs|work=PubChem Compound|publisher=National Center for Biotechnology Information|access-date=3 March 2012|location=USA|date=27 March 2005|at=Identification and Related Records}}</ref> | |||
| | SMILES = CC(I)C | |||
| |Section1={{Chembox Identifiers | |||
| | CASNo = 75-30-9 | |||
| | CASNo = 75-30-9 | |||
| | CASNo_Ref = {{cascite}} | |||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | RTECS = TZ4200000 | |||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| }} | |||
| | UNII = 67K05OPZ0E | |||
| | Section2 = {{Chembox Properties | |||
| | PubChem = 6362 | |||
| | Formula = C<sub>3</sub>H<sub>7</sub>I | |||
| | |
| ChemSpiderID = 6122 | ||
| | ChemSpiderID_Ref = {{chemspidercite|changed|chemspider}} | |||
| | Appearance = Colourless liquid | |||
| | |
| EINECS = 200-859-3 | ||
| | UNNumber = 2392 | |||
| | Solubility = 0.14 g/100 ml at 12.5 °C | |||
| | MeSHName = isopropyl+iodide | |||
| | Solubility1 = fully ] | |||
| | |
| RTECS = TZ4200000 | ||
| | Beilstein = 1098244 | |||
| | Solubility2 = fully ] | |||
| | SMILES = CC(C)I | |||
| | Solvent2 = diethyl ether | |||
| | StdInChI_Ref = {{stdinchicite|changed|chemspider}} | |||
| | Solubility3 = fully ] | |||
| | StdInChI = 1S/C3H7I/c1-3(2)4/h3H,1-2H3 | |||
| | Solvent3 = chloroform | |||
| | StdInChIKey = FMKOJHQHASLBPH-UHFFFAOYSA-N | |||
| | Solubility4 = fully ] | |||
| | StdInChIKey_Ref = {{chemspidercite|correct|chemspider}} | |||
| | Solvent4 = benzene | |||
| | RefractIndex = 1.4997 | |||
| | MeltingPtC = -90.0 | |||
| | BoilingPtC = 89.5 | |||
| | pKa = | |||
| | Viscosity = 8.841 c] at 0 °C<br/>6.971 c] at 20 °C | |||
| | Dipole = | |||
| }} | |||
| | Section7 = {{Chembox Hazards | |||
| | ExternalMSDS = | |||
| | FlashPt = 42 °C | |||
| | MainHazards = Possible carcinogen. Harmful if swallowed, inhaled and in contact with skin. Eye, respiratory and mucous membrane irritant. | |||
| | NFPA-H = | |||
| | NFPA-F = | |||
| | NFPA-R = | |||
| | NFPA-O = | |||
| | RPhrases = R20 R21 R22 R36 R37 R38 R40 | |||
| | SPhrases = S16 S23 S26 S36 | |||
| }} | |||
| | Section8 = {{Chembox Related | |||
| | Function = ]s | |||
| | OtherFunctn = ]<br>]<br>] }} | |||
| }} | }} | ||
| |Section2={{Chembox Properties | |||
| | C=3 | H=7 | I=1 | |||
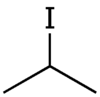
| '''Isopropyl iodide''' is the ] with the ] (CH<sub>3</sub>)<sub>2</sub>CHI. It is ], ], and volatile. Organic iodides are light-sensitive and take on a yellow colour upon storage, owing to the formation of ]. | |||
| | Appearance = Colourless liquid | |||
| | Density = 1.703 g mL<sup>−1</sup> | |||
| | BoilingPtK = 361.9 to 362.9 | |||
| | MeltingPtK = 183.15 | |||
| | Solubility = 1.4 g L<sup>−1</sup> (at 12.5 °C) | |||
| | Solvent1 = chloroform | |||
| | Solubility1 = Miscible | |||
| | Solvent2 = ethanol | |||
| | Solubility2 = Miscible | |||
| | Solvent3 = diethyl ether | |||
| | Solubility3 = Miscible | |||
| | Solvent4 = benzene | |||
| | Solubility4 = Miscible | |||
| | HenryConstant = 890 nmol Pa<sup>−1</sup> kg<sup>−1</sup> | |||
| | RefractIndex = 1.4997 | |||
| | Viscosity = 6.971 mPa (at 20 °C) | |||
| }} | |||
| |Section3={{Chembox Thermochemistry | |||
| | DeltaHf = −77.2–−72.6 kJ mol<sup>−1</sup> | |||
| | HeatCapacity = 137.3 J K<sup>−1</sup> mol<sup>−1</sup> | |||
| }} | |||
| |Section4={{Chembox Hazards | |||
| | GHSPictograms = {{GHS flame}} {{GHS exclamation mark}} | |||
| | GHSSignalWord = '''WARNING''' | |||
| | HPhrases = {{H-phrases|226|302}} | |||
| | FlashPtC = 42 | |||
| }} | |||
| |Section5={{Chembox Related | |||
| | OtherFunction_label = alkanes | |||
| | OtherFunction = {{Unbulleted list|]|]|]|]|]|]}} | |||
| | OtherCompounds = ] | |||
| }} | |||
| }} | |||
| '''Isopropyl iodide''' is the ] with the ] (CH<sub>3</sub>)<sub>2</sub>CHI. It is ], ], and volatile. Organic iodides are light-sensitive and take on a yellow colour upon storage, owing to the formation of ]. | |||
| ==Preparation== | ==Preparation== | ||
| Isopropyl iodide is prepared by iodination of ] using ] or, equivalently, with a mixture of ], ], and ].<ref name="merck">Merck Index of Chemicals and Drugs, 9th ed., monograph 5074</ref> An alternative preparation involves the reaction of 2-propyl bromide with an acetone solution of |
Isopropyl iodide is prepared by iodination of ] using ] or, equivalently, with a mixture of ], ], and ].<ref name="merck">Merck Index of Chemicals and Drugs, 9th ed., monograph 5074</ref> An alternative preparation involves the reaction of ] with an acetone solution of ] (]):<ref>Textbook of Practical Organic Chemistry, 5th Edition, Prentice Hall, 1989</ref> | ||
| :(CH<sub>3</sub>)<sub>2</sub>CHBr + |
:(CH<sub>3</sub>)<sub>2</sub>CHBr + NaI → (CH<sub>3</sub>)<sub>2</sub>CHI + NaBr | ||
| ==References== | ==References== | ||
| {{ |
{{Reflist}} | ||
| {{Organohalide-stub}} | |||
| ] | |||
| {{organohalide-stub}} | |||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 18:20, 9 September 2022
| |

| |
| Names | |
|---|---|
| Preferred IUPAC name 2-Iodopropane | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 1098244 |
| ChemSpider | |
| ECHA InfoCard | 100.000.782 |
| EC Number |
|
| MeSH | isopropyl+iodide |
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 2392 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C3H7I |
| Molar mass | 169.993 g·mol |
| Appearance | Colourless liquid |
| Density | 1.703 g mL |
| Melting point | −90.00 °C; −130.00 °F; 183.15 K |
| Boiling point | 88.8 to 89.8 °C; 191.7 to 193.5 °F; 361.9 to 362.9 K |
| Solubility in water | 1.4 g L (at 12.5 °C) |
| Solubility in chloroform | Miscible |
| Solubility in ethanol | Miscible |
| Solubility in diethyl ether | Miscible |
| Solubility in benzene | Miscible |
| Henry's law constant (kH) |
890 nmol Pa kg |
| Refractive index (nD) | 1.4997 |
| Viscosity | 6.971 mPa (at 20 °C) |
| Thermochemistry | |
| Heat capacity (C) | 137.3 J K mol |
| Std enthalpy of formation (ΔfH298) |
−77.2–−72.6 kJ mol |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Warning |
| Hazard statements | H226, H302 |
| Flash point | 42 °C (108 °F; 315 K) |
| Related compounds | |
| Related alkanes | |
| Related compounds | Diiodohydroxypropane |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Isopropyl iodide is the organoiodine compound with the formula (CH3)2CHI. It is colorless, flammable, and volatile. Organic iodides are light-sensitive and take on a yellow colour upon storage, owing to the formation of iodine.
Preparation
Isopropyl iodide is prepared by iodination of isopropyl alcohol using hydrogen iodide or, equivalently, with a mixture of glycerol, iodine, and phosphorus. An alternative preparation involves the reaction of 2-propyl bromide with an acetone solution of sodium iodide (Finkelstein reaction):
- (CH3)2CHBr + NaI → (CH3)2CHI + NaBr
References
- "isopropyl iodide - Compound Summary". PubChem Compound. USA: National Center for Biotechnology Information. 27 March 2005. Identification and Related Records. Retrieved 3 March 2012.
- Merck Index of Chemicals and Drugs, 9th ed., monograph 5074
- Textbook of Practical Organic Chemistry, 5th Edition, Prentice Hall, 1989
This article about an organic halide is a stub. You can help Misplaced Pages by expanding it. |