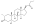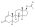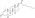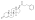| Revision as of 02:09, 1 September 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{drugbox}} (no changed fields - added verified revid - updated 'UNII_Ref', 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report errors or bugs)← Previous edit | Latest revision as of 07:01, 9 December 2024 edit undoCitation bot (talk | contribs)Bots5,414,630 edits Added bibcode. | Use this bot. Report bugs. | Suggested by Abductive | Category:Prodrugs | #UCB_Category 267/338 | ||
| (64 intermediate revisions by 38 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Chemical compound}} | |||
| {{Drugbox | {{Drugbox | ||
| | verifiedrevid = |
| verifiedrevid = 447772837 | ||
| | IUPAC_name = (2''R'',5''S'',8''R'',9''S'',10''S'',13''S'',14''S'',17''S'')-17-hydroxy-2,10,13-trimethyl-1,2,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydrocyclopentaphenanthren-3-one | | IUPAC_name = (2''R'',5''S'',8''R'',9''S'',10''S'',13''S'',14''S'',17''S'')-17-hydroxy-2,10,13-trimethyl-1,2,4,5,6,7,8,9,11,12,14,15,16,17-tetradecahydrocyclopentaphenanthren-3-one | ||
| | image = Drostanolone propionate.svg | | image = Drostanolone propionate.svg | ||
| | width = 250px | |||
| | drug_name = Dromostanolone | |||
| <!--Clinical data--> | <!--Clinical data--> | ||
| | tradename = | | tradename = Drolban, Masteril, Masteron, others | ||
| | pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | | pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | ||
| | pregnancy_US = <!-- A / B |
| pregnancy_US = <!-- A / B / C / D / X --> | ||
| | pregnancy_category = X | | pregnancy_category = X | ||
| | legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> | | legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> | ||
| | legal_CA = Schedule IV | | legal_CA = Schedule IV | ||
| | legal_UK = <!-- GSL |
| legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> | ||
| | legal_US = Schedule III | | legal_US = Schedule III | ||
| | legal_status = |
| legal_status = | ||
| | routes_of_administration = Intramuscular injection |
| routes_of_administration = ]<ref name="Llewellyn2011" /> | ||
| | class = ]; ]; ] | |||
| <!--Pharmacokinetic data--> | <!--Pharmacokinetic data--> | ||
| | bioavailability = Oral |
| bioavailability = ]: 0–2%<br />]: 100% | ||
| | protein_bound = |
| protein_bound = High | ||
| | metabolism = |
| metabolism = ] | ||
| | elimination_half-life = 2 |
| elimination_half-life = ]: 2 days<ref name="Llewellyn2011" /> | ||
| | excretion = |
| excretion = ] | ||
| <!--Identifiers--> | <!--Identifiers--> | ||
| | IUPHAR_ligand = 6947 | |||
| | CAS_number_Ref = {{cascite|correct|??}} | |||
| | CAS_number = 58-19-5 | | CAS_number = 58-19-5 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | UNII = 7DR7H00HDT | |||
| | ATC_prefix = A14 | | ATC_prefix = A14 | ||
| | ATC_suffix = |
| ATC_suffix = | ||
| | PubChem = 224004 | | PubChem = 224004 | ||
| | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| Line 35: | Line 41: | ||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | | ChEBI_Ref = {{ebicite|correct|EBI}} | ||
| | ChEBI = 31523 | | ChEBI = 31523 | ||
| | synonyms = Dromostanolone propionate; NSC-12198; Drostanolone 17β-propionate; 2α-Methyl-4,5α-dihydrotestosterone 17β-propionate; 2α-Methyl-DHT propionate; 2α-Methyl-5α-androstan-17β-ol-3-one 17β-propionate | |||
| <!--Chemical data--> | <!--Chemical data--> | ||
| | C=23 | H=36 | O=3 | | C=23 | H=36 | O=3 | ||
| ⚫ | | SMILES = CCC(=O)O1CC21(CC32CC43(C(C(=O)C4)C)C)C | ||
| | molecular_weight = 360.53 g/mol | |||
| ⚫ | | |
||
| | InChI = 1/C23H36O3/c1-5-21(25)26-20-9-8-17-16-7-6-15-12-19(24)14(2)13-23(15,4)18(16)10-11-22(17,20)3/h14-18,20H,5-13H2,1-4H3/t14-,15+,16+,17+,18+,20+,22+,23+/m1/s1 | |||
| | InChIKey = NOTIQUSPUUHHEH-UXOVVSIBBM | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/C23H36O3/c1-5-21(25)26-20-9-8-17-16-7-6-15-12-19(24)14(2)13-23(15,4)18(16)10-11-22(17,20)3/h14-18,20H,5-13H2,1-4H3/t14-,15+,16+,17+,18+,20+,22+,23+/m1/s1 | | StdInChI = 1S/C23H36O3/c1-5-21(25)26-20-9-8-17-16-7-6-15-12-19(24)14(2)13-23(15,4)18(16)10-11-22(17,20)3/h14-18,20H,5-13H2,1-4H3/t14-,15+,16+,17+,18+,20+,22+,23+/m1/s1 | ||
| Line 47: | Line 51: | ||
| | StdInChIKey = NOTIQUSPUUHHEH-UXOVVSIBSA-N | | StdInChIKey = NOTIQUSPUUHHEH-UXOVVSIBSA-N | ||
| }} | }} | ||
| <!-- Definition and medical uses --> | |||
| '''Drostanolone propionate''', or '''dromostanolone propionate''', sold under the brand names '''Drolban''', '''Masteril''', and '''Masteron''' among others, is an ] and ] (AAS) medication which was used to treat ] in women but is now no longer marketed.<ref name="Llewellyn2011">{{cite book| vauthors = Llewellyn W |title=Anabolics|url=https://books.google.com/books?id=afKLA-6wW0oC&pg=PT517|year=2011|publisher=Molecular Nutrition Llc|isbn=978-0-9828280-1-4|pages=517–}}</ref><ref name="Drugs.com">{{cite web | title = Anabolic Agents | url = https://www.drugs.com/international/dromostanolone.html | work = Drugs.com }}</ref> It is given by ].<ref name="Llewellyn2011" /> | |||
| <!-- Side effects and mechanism of action --> | |||
| '''Drostanolone propionate''' (trade name ''Masteron'') is an ] which is the ] ] of ]. It is known to be highly ] and mildly ].{{Citation needed|date=January 2010}} It is incapable of aromatization and has similar properties to ].{{Citation needed|date=January 2010}} It has been successfully used as a breast cancer drug, but because of the high risk of virilization, safer options are prescribed.<ref>{{cite journal | journal = Clin Oncol | year = 1976 | volume = 2 | issue = 3 | pages = 203–6 | title = A comparison of drostanolone propionate (Masteril) and nandrolone decanoate (Deca-durabolin) in the treatment of breast carcinoma | author = Chowdhury MS, Banks AJ, Bond WH, Jones WG, Ward HW | pmid = 103698}}</ref> | |||
| ]s of drostanolone propionate include ]s of ] like ], ], ], and increased ].<ref name="Llewellyn2011" /> It has no risk of ].<ref name="Llewellyn2011" /> The drug is a ] androgen and anabolic steroid and hence is an ] of the ] (AR), the ] of androgens like ] and ] (DHT).<ref name="Llewellyn2011" /><ref name="pmid18500378">{{cite journal | vauthors = Kicman AT | title = Pharmacology of anabolic steroids | journal = British Journal of Pharmacology | volume = 154 | issue = 3 | pages = 502–521 | date = June 2008 | pmid = 18500378 | pmc = 2439524 | doi = 10.1038/bjp.2008.165 }}</ref> It has moderate ] effects and weak ]ic effects, which give it a mild side effect profile and make it especially suitable for use in women.<ref name="Llewellyn2011" /> The drug has no ]ic effects.<ref name="Llewellyn2011" /> Drostanolone propionate is an ] and a long-lasting ] of ] in the body.<ref name="Llewellyn2011" /> | |||
| <!-- History, society, and culture --> | |||
| == Use in sports == | |||
| Drostanolone propionate was first described in 1959 and was introduced for medical use in 1961.<ref name="Llewellyn2011" /><ref name="RingoldBatres1959" /><ref name="Publishing2013" /> In addition to its medical use, drostanolone propionate is used to ].<ref name="Llewellyn2011" /> The drug is a ] in many countries and so non-medical use is generally illicit.<ref name="Llewellyn2011" /><ref name="FFFLM2006" /> | |||
| {{Section OR|date=January 2010}} | |||
| {{Unreferenced section|date=January 2010}} | |||
| Drostanolone propionate is used primarily by athletes who need to retain strength while losing mass. It is beneficial to runners and athletes who must remain in a certain weight class.{{Citation needed|date=January 2010}} | |||
| ==Medical uses== | |||
| It has gained popularity in the bodybuilding community as a diuretic and muscle defining drug. Individuals interested in using the drostanolone propionate are those that are looking to add muscle hardness and density to their physiques, nearly always for the purpose of bodybuilding competitions.{{Citation needed|date=January 2010}} | |||
| The principal clinical indication of drostanolone propionate in the ] as well as international markets was the treatment of advanced inoperable ] in women.<ref name="Llewellyn2011" /> | |||
| ] is part of the complex therapy for some kind of ], particularly the ones associated with hormone-active tissues like breast or ]. Some types of ] cells, expressing ]s (called ER+ cancers), use ] for their growth and dissemination. That is why drugs that block estrogen receptors or decrease their expression on the cell membrane, ]s, could limit the tumor spread and size. Drostanolone propionate has been FDA approved<ref>{{Cite web|url=http://www.accessdata.fda.gov/scripts/cder/ob/docs/obdetail.cfm?Appl_No=012936&TABLE1=OB_DISC|title=Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations|website=www.accessdata.fda.gov|access-date=2016-03-15}}</ref> as an ]ic drug for the treatment of breast cancer. By the time of its release, there were not many alternatives for patients with breast cancer and drostanolone propionate was a revolution for these patients. As it has lower ]ic rate compared to ], the risk of ] is much lighter. Due to this fact, women, who usually do not respond well to any AAS, were having much greater chance to survive cancer. Drostanolone propionate can also be used for breast tumors that do not respond well to other treatments or also as ] for advanced incurable tumors. The effects of the product depend of course on the dose and period of administration. The risk of virilization becomes greater with high doses and continuous administration period. | |||
| Drostanolone propionate can increase muscle hardness and density, giving an individual a more complete appearance when competing on stage.{{Citation needed|date=January 2010}} However, an already rather low body-fat level is needed for it to take full effect.{{Citation needed|date=January 2010}} Drostanolone propionate can promote increased strength while keeping body fat the same or even lowering it, something can help to prevent muscle loss while dieting.{{Citation needed|date=January 2010}} This can also allow strength athletes or those athletes in sports which have weight classes to increase performance without the risk of being raised into a higher weight class or add mass that may hinder performance.{{Citation needed|date=January 2010}} | |||
| {{Androgen/anabolic steroid dosages for breast cancer}} | |||
| ==Non-medical uses== | |||
| Drostanolone propionate is or has been used for ]s by ] ]s, ]s, and ]s.<ref name="Llewellyn2011" /> | |||
| ==Side effects== | |||
| {{See also|Anabolic steroid#Adverse effects}} | |||
| Drostanolone propionate produces considerably less ] in women compared to equal doses of ].<ref name="Llewellyn2011" /> However, since the given dosage for breast cancer was relatively high (200 mg/twice a week),<ref>{{Cite web|date=2020-01-24|title=Drostanolone propionate (Masteron) administration - The Dose for Treating Breast Cancer|url=https://masterone.online/know-the-masteron-administration.html|url-status=live|access-date=2021-02-23|website=Masterone|language=en-US|archive-url=https://web.archive.org/web/20201001115527/https://masterone.online/know-the-masteron-administration.html |archive-date=2020-10-01 }}</ref> mild virilization including ], ], ], ], and ] could still occur, and marked virilization could manifest with long-term therapy.<ref name="Llewellyn2011" /> The drug has no ]ic activity and hence has no propensity for causing ] (in males) or ].<ref name="Llewellyn2011" /> Drostanolone propionate is not known to pose a risk of ].<ref>{{cite journal | vauthors = Solimini R, Rotolo MC, Mastrobattista L, Mortali C, Minutillo A, Pichini S, Pacifici R, Palmi I | display-authors = 6 | title = Hepatotoxicity associated with illicit use of anabolic androgenic steroids in doping | journal = European Review for Medical and Pharmacological Sciences | volume = 21 | issue = 1 Suppl | pages = 7–16 | date = March 2017 | pmid = 28379599 | url = https://pubmed.ncbi.nlm.nih.gov/28379599/ }}</ref><ref name="Llewellyn2011" /> | |||
| ==Pharmacology== | |||
| ===Pharmacodynamics=== | |||
| {{Relative androgenic to anabolic activity in animals}} | |||
| Drostanolone propionate is a ] of ].<ref name="Llewellyn2011" /> Like other AAS, drostanolone is an ] of the ] (AR).<ref name="Llewellyn2011" /> It is not a substrate for ] and is a poor substrate for ] (3α-HSD), and therefore shows a high ratio of ] to ] activity.<ref name="Llewellyn2011" /> As a DHT derivative, drostanolone is not a ] for ] and hence cannot be aromatized into ]ic ]s.<ref name="Llewellyn2011" /> While no data are available on the ]ic activity of drostanolone, it is thought to have low or no such activity similarly to other DHT derivatives.<ref name="Llewellyn2011" /> Since the drug is not ], it is not known to cause ].<ref name="Llewellyn2011" /> | |||
| Drostanolone propionate, via its active form drostanolone, interacts with the AR and activates a cascade of genetic changes, including increased ] (]) and decreased ] ] (]). It also induces a reduction or inhibition of ] or ]s in the ]s, which is linked to its ] effects.<ref name="INCHEM">{{Cite web|url=http://www.inchem.org/documents/pims/pharm/pim901.htm|title=Drostanolone (PIM 901)|website=www.inchem.org|access-date=2016-03-15}}</ref> | |||
| ===Pharmacokinetics=== | |||
| Drostanolone propionate is not active via the ] route and must be administered via ].<ref name="Llewellyn2011" /> The ] of the drug via this route is approximately 2 days.<ref name="Llewellyn2011" /> It has a much longer elimination half-life via intramuscular injection than drostanolone.<ref name="Llewellyn2011" /> Drostanolone propionate is ] into drostanolone, which is the active form.<ref name="Llewellyn2011" /> | |||
| ==Chemistry== | |||
| {{See also|List of androgens/anabolic steroids|List of androgen esters}} | |||
| Drostanolone propionate, or drostanolone 17β-propionate, is a ] ] ] and a ] of DHT.<ref name="Elks2014">{{cite book| vauthors = Elks J |title=The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies|url=https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA652|date=14 November 2014|publisher=Springer|isbn=978-1-4757-2085-3|pages=652–}}</ref><ref name="IndexNominum2000">{{cite book|title=Index Nominum 2000: International Drug Directory|url=https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA377|date=January 2000|publisher=Taylor & Francis|isbn=978-3-88763-075-1|pages=377–}}</ref><ref name="Llewellyn2011" /> It is the C17β ] (propanoate) ] of ], which itself is 2α-methyl-4,5α-dihydrotestosterone (2α-methyl-DHT) or 2α-methyl-5α-androstan-17β-ol-3-one.<ref name="Elks2014" /><ref name="IndexNominum2000" /><ref name="Llewellyn2011" /> | |||
| {{Structural properties of major anabolic steroid esters}} | |||
| ==History== | |||
| Drostanolone and drostanolone propionate were first described in 1959.<ref name="Llewellyn2011" /><ref name="RingoldBatres1959">{{cite journal| vauthors = Ringold HJ, Batres E, Halpern O, Necoechea E |title=Steroids. CV.12-Methyl and 2-Hydroxymethylene-androstane Derivatives|journal=Journal of the American Chemical Society|volume=81|issue=2|year=1959|pages=427–432|issn=0002-7863|doi=10.1021/ja01511a040|bibcode=1959JAChS..81..427R }}</ref> The related AAS ] and ] (methyldrostanolone) were first described in the same paper as well.<ref name="Llewellyn2011" /> Drostanolone propionate was introduced for medical use in the ] in 1961 and in ] shortly thereafter.<ref name="Publishing2013">{{cite book|author=William Andrew Publishing|title=Pharmaceutical Manufacturing Encyclopedia, 3rd Edition|url=https://books.google.com/books?id=_J2ti4EkYpkC&pg=PA1402|date=22 October 2013|publisher=Elsevier|isbn=978-0-8155-1856-3|pages=1402–}}</ref> | |||
| ==Society and culture== | |||
| ===Generic names=== | |||
| ''Drostanolone propionate'' is the ] of the drug and its {{abbrlink|BANM|British Approved Name}}, while ''dromostanolone propionate'' is the {{abbrlink|USAN|United States Adopted Name}} and {{abbrlink|USP|United States Pharmacopeia}}; there is no {{abbrlink|INN|International Nonproprietary Name}} for this form.<ref name="Elks2014" /><ref name="IndexNominum2000" /><ref name="MortonHall2012">{{cite book| vauthors = Morton IK, Hall JM |title=Concise Dictionary of Pharmacological Agents: Properties and Synonyms|url=https://books.google.com/books?id=tsjrCAAAQBAJ&pg=PA106|date=6 December 2012|publisher=Springer Science & Business Media|isbn=978-94-011-4439-1|pages=106–}}</ref> The generic name of the unesterified form of the drug is ''drostanolone'' or ''dromostanolone'' and the former is its {{abbrlink|INN|International Nonproprietary Name}}, {{abbrlink|BAN|British Approved Name}}, and {{abbrlink|DCF|Dénomination Commune Française}} while there is no {{abbrlink|USAN|United States Adopted Name}}.<ref name="Elks2014" /><ref name="IndexNominum2000" /><ref name="MortonHall2012" /><ref name="Drugs.com" /> | |||
| ===Brand names=== | |||
| Drostanolone propionate was marketed under a variety of brand names including Drolban, Masterid, Masteril, Masteron, Masterone, Mastisol, Metormon, Permastril, and Prometholone.<ref name="Elks2014" /><ref name="IndexNominum2000" /><ref name="Llewellyn2011" /> | |||
| ===Availability=== | |||
| Drostanolone propionate appears to no longer be marketed.<ref name="Llewellyn2011" /><ref name="Drugs.com" /> It was previously available in the ], ], and ].<ref name="IndexNominum2000" /><ref name="Llewellyn2011" /> In Europe, it was specifically marketed in the ], ], ], ], ], ], ], and ].<ref name="IndexNominum2000" /><ref name="Llewellyn2011" /> | |||
| ===Legal status=== | |||
| Drostanolone propionate, along with other AAS, is a ] ] in the ] under the ].<ref name="FFFLM2006">{{cite book| vauthors = Karch SB |title=Drug Abuse Handbook, Second Edition|url=https://books.google.com/books?id=ZjrMBQAAQBAJ&pg=PA30|date=21 December 2006|publisher=CRC Press|isbn=978-1-4200-0346-8|pages=30–}}</ref> | |||
| == References == | == References == | ||
| {{ |
{{Reflist|2}} | ||
| == External links == | |||
| * | |||
| {{Androgens and antiandrogens}} | |||
| {{Anabolic steroids}} | |||
| {{Androgen receptor modulators}} | |||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 07:01, 9 December 2024
Chemical compound Pharmaceutical compound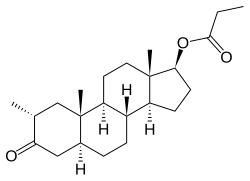 | |
| Clinical data | |
|---|---|
| Trade names | Drolban, Masteril, Masteron, others |
| Other names | Dromostanolone propionate; NSC-12198; Drostanolone 17β-propionate; 2α-Methyl-4,5α-dihydrotestosterone 17β-propionate; 2α-Methyl-DHT propionate; 2α-Methyl-5α-androstan-17β-ol-3-one 17β-propionate |
| Pregnancy category |
|
| Routes of administration | Intramuscular injection |
| Drug class | Androgen; Anabolic steroid; Androgen ester |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | Oral: 0–2% Intramuscular: 100% |
| Protein binding | High |
| Metabolism | Hepatic |
| Elimination half-life | Intramuscular: 2 days |
| Excretion | Urine |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.007.550 |
| Chemical and physical data | |
| Formula | C23H36O3 |
| Molar mass | 360.538 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Drostanolone propionate, or dromostanolone propionate, sold under the brand names Drolban, Masteril, and Masteron among others, is an androgen and anabolic steroid (AAS) medication which was used to treat breast cancer in women but is now no longer marketed. It is given by injection into muscle.
Side effects of drostanolone propionate include symptoms of masculinization like acne, increased hair growth, voice changes, and increased sexual desire. It has no risk of liver damage. The drug is a synthetic androgen and anabolic steroid and hence is an agonist of the androgen receptor (AR), the biological target of androgens like testosterone and dihydrotestosterone (DHT). It has moderate anabolic effects and weak androgenic effects, which give it a mild side effect profile and make it especially suitable for use in women. The drug has no estrogenic effects. Drostanolone propionate is an androgen ester and a long-lasting prodrug of drostanolone in the body.
Drostanolone propionate was first described in 1959 and was introduced for medical use in 1961. In addition to its medical use, drostanolone propionate is used to improve physique and performance. The drug is a controlled substance in many countries and so non-medical use is generally illicit.
Medical uses
The principal clinical indication of drostanolone propionate in the United States as well as international markets was the treatment of advanced inoperable breast cancer in women.
Hormonal treatment is part of the complex therapy for some kind of tumors, particularly the ones associated with hormone-active tissues like breast or prostate cancer. Some types of breast cancer cells, expressing estrogen receptors (called ER+ cancers), use estrogen for their growth and dissemination. That is why drugs that block estrogen receptors or decrease their expression on the cell membrane, antiestrogens, could limit the tumor spread and size. Drostanolone propionate has been FDA approved as an antiestrogenic drug for the treatment of breast cancer. By the time of its release, there were not many alternatives for patients with breast cancer and drostanolone propionate was a revolution for these patients. As it has lower androgenic rate compared to testosterone, the risk of virilization is much lighter. Due to this fact, women, who usually do not respond well to any AAS, were having much greater chance to survive cancer. Drostanolone propionate can also be used for breast tumors that do not respond well to other treatments or also as palliative care for advanced incurable tumors. The effects of the product depend of course on the dose and period of administration. The risk of virilization becomes greater with high doses and continuous administration period.
| Route | Medication | Form | Dosage | |
|---|---|---|---|---|
| Oral | Methyltestosterone | Tablet | 30–200 mg/day | |
| Fluoxymesterone | Tablet | 10–40 mg 3x/day | ||
| Calusterone | Tablet | 40–80 mg 4x/day | ||
| Normethandrone | Tablet | 40 mg/day | ||
| Buccal | Methyltestosterone | Tablet | 25–100 mg/day | |
| Injection (IMTooltip intramuscular injection or SCTooltip subcutaneous injection) | Testosterone propionate | Oil solution | 50–100 mg 3x/week | |
| Testosterone enanthate | Oil solution | 200–400 mg 1x/2–4 weeks | ||
| Testosterone cypionate | Oil solution | 200–400 mg 1x/2–4 weeks | ||
| Mixed testosterone esters | Oil solution | 250 mg 1x/week | ||
| Methandriol | Aqueous suspension | 100 mg 3x/week | ||
| Androstanolone (DHT) | Aqueous suspension | 300 mg 3x/week | ||
| Drostanolone propionate | Oil solution | 100 mg 1–3x/week | ||
| Metenolone enanthate | Oil solution | 400 mg 3x/week | ||
| Nandrolone decanoate | Oil solution | 50–100 mg 1x/1–3 weeks | ||
| Nandrolone phenylpropionate | Oil solution | 50–100 mg/week | ||
| Note: Dosages are not necessarily equivalent. Sources: See template. | ||||
Non-medical uses
Drostanolone propionate is or has been used for physique- and performance-enhancing purposes by competitive athletes, bodybuilders, and powerlifters.
Side effects
See also: Anabolic steroid § Adverse effectsDrostanolone propionate produces considerably less virilization in women compared to equal doses of testosterone propionate. However, since the given dosage for breast cancer was relatively high (200 mg/twice a week), mild virilization including oily skin, acne, voice deepening, hirsutism, and clitoral enlargement could still occur, and marked virilization could manifest with long-term therapy. The drug has no estrogenic activity and hence has no propensity for causing gynecomastia (in males) or fluid retention. Drostanolone propionate is not known to pose a risk of hepatotoxicity.
Pharmacology
Pharmacodynamics
| Medication | Ratio |
|---|---|
| Testosterone | ~1:1 |
| Androstanolone (DHT) | ~1:1 |
| Methyltestosterone | ~1:1 |
| Methandriol | ~1:1 |
| Fluoxymesterone | 1:1–1:15 |
| Metandienone | 1:1–1:8 |
| Drostanolone | 1:3–1:4 |
| Metenolone | 1:2–1:30 |
| Oxymetholone | 1:2–1:9 |
| Oxandrolone | 1:3–1:13 |
| Stanozolol | 1:1–1:30 |
| Nandrolone | 1:3–1:16 |
| Ethylestrenol | 1:2–1:19 |
| Norethandrolone | 1:1–1:20 |
| Notes: In rodents. Footnotes: = Ratio of androgenic to anabolic activity. Sources: See template. | |
Drostanolone propionate is a prodrug of drostanolone. Like other AAS, drostanolone is an agonist of the androgen receptor (AR). It is not a substrate for 5α-reductase and is a poor substrate for 3α-hydroxysteroid dehydrogenase (3α-HSD), and therefore shows a high ratio of anabolic to androgenic activity. As a DHT derivative, drostanolone is not a substrate for aromatase and hence cannot be aromatized into estrogenic metabolites. While no data are available on the progestogenic activity of drostanolone, it is thought to have low or no such activity similarly to other DHT derivatives. Since the drug is not 17α-alkylated, it is not known to cause hepatotoxicity.
Drostanolone propionate, via its active form drostanolone, interacts with the AR and activates a cascade of genetic changes, including increased protein synthesis (anabolism) and decreased amino acid degradation (catabolism). It also induces a reduction or inhibition of prolactin or estrogen receptors in the breasts, which is linked to its antitumor effects.
Pharmacokinetics
Drostanolone propionate is not active via the oral route and must be administered via intramuscular injection. The elimination half-life of the drug via this route is approximately 2 days. It has a much longer elimination half-life via intramuscular injection than drostanolone. Drostanolone propionate is metabolized into drostanolone, which is the active form.
Chemistry
See also: List of androgens/anabolic steroids and List of androgen estersDrostanolone propionate, or drostanolone 17β-propionate, is a synthetic androstane steroid and a derivative of DHT. It is the C17β propionate (propanoate) ester of drostanolone, which itself is 2α-methyl-4,5α-dihydrotestosterone (2α-methyl-DHT) or 2α-methyl-5α-androstan-17β-ol-3-one.
| Anabolic steroid | Structure | Ester | Relative mol. weight |
Relative AAS content |
Duration | ||||
|---|---|---|---|---|---|---|---|---|---|
| Position | Moiety | Type | Length | ||||||
| Boldenone undecylenate | C17β | Undecylenic acid | Straight-chain fatty acid | 11 | 1.58 | 0.63 | Long | ||
| Drostanolone propionate | C17β | Propanoic acid | Straight-chain fatty acid | 3 | 1.18 | 0.84 | Short | ||
| Metenolone acetate | C17β | Ethanoic acid | Straight-chain fatty acid | 2 | 1.14 | 0.88 | Short | ||
| Metenolone enanthate | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.37 | 0.73 | Long | ||
| Nandrolone decanoate | C17β | Decanoic acid | Straight-chain fatty acid | 10 | 1.56 | 0.64 | Long | ||
| Nandrolone phenylpropionate | C17β | Phenylpropanoic acid | Aromatic fatty acid | – (~6–7) | 1.48 | 0.67 | Long | ||
| Trenbolone acetate | C17β | Ethanoic acid | Straight-chain fatty acid | 2 | 1.16 | 0.87 | Short | ||
| Trenbolone enanthate | C17β | Heptanoic acid | Straight-chain fatty acid | 7 | 1.41 | 0.71 | Long | ||
| Footnotes: = Length of ester in carbon atoms for straight-chain fatty acids or approximate length of ester in carbon atoms for aromatic fatty acids. = Relative androgen/anabolic steroid content by weight (i.e., relative androgenic/anabolic potency). = Duration by intramuscular or subcutaneous injection in oil solution. = Never marketed. Sources: See individual articles. | |||||||||
History
Drostanolone and drostanolone propionate were first described in 1959. The related AAS oxymetholone and methasterone (methyldrostanolone) were first described in the same paper as well. Drostanolone propionate was introduced for medical use in the United States in 1961 and in Europe shortly thereafter.
Society and culture
Generic names
Drostanolone propionate is the generic name of the drug and its BANMTooltip British Approved Name, while dromostanolone propionate is the USANTooltip United States Adopted Name and USPTooltip United States Pharmacopeia; there is no INNTooltip International Nonproprietary Name for this form. The generic name of the unesterified form of the drug is drostanolone or dromostanolone and the former is its INNTooltip International Nonproprietary Name, BANTooltip British Approved Name, and DCFTooltip Dénomination Commune Française while there is no USANTooltip United States Adopted Name.
Brand names
Drostanolone propionate was marketed under a variety of brand names including Drolban, Masterid, Masteril, Masteron, Masterone, Mastisol, Metormon, Permastril, and Prometholone.
Availability
Drostanolone propionate appears to no longer be marketed. It was previously available in the United States, Europe, and Japan. In Europe, it was specifically marketed in the United Kingdom, Germany, Belgium, France, Spain, Portugal, Italy, and Bulgaria.
Legal status
Drostanolone propionate, along with other AAS, is a schedule III controlled substance in the United States under the Controlled Substances Act.
References
- ^ Llewellyn W (2011). Anabolics. Molecular Nutrition Llc. pp. 517–. ISBN 978-0-9828280-1-4.
- ^ "Anabolic Agents". Drugs.com.
- Kicman AT (June 2008). "Pharmacology of anabolic steroids". British Journal of Pharmacology. 154 (3): 502–521. doi:10.1038/bjp.2008.165. PMC 2439524. PMID 18500378.
- ^ Ringold HJ, Batres E, Halpern O, Necoechea E (1959). "Steroids. CV.12-Methyl and 2-Hydroxymethylene-androstane Derivatives". Journal of the American Chemical Society. 81 (2): 427–432. Bibcode:1959JAChS..81..427R. doi:10.1021/ja01511a040. ISSN 0002-7863.
- ^ William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia, 3rd Edition. Elsevier. pp. 1402–. ISBN 978-0-8155-1856-3.
- ^ Karch SB (21 December 2006). Drug Abuse Handbook, Second Edition. CRC Press. pp. 30–. ISBN 978-1-4200-0346-8.
- "Orange Book: Approved Drug Products with Therapeutic Equivalence Evaluations". www.accessdata.fda.gov. Retrieved 2016-03-15.
- "Drostanolone propionate (Masteron) administration - The Dose for Treating Breast Cancer". Masterone. 2020-01-24. Archived from the original on 2020-10-01. Retrieved 2021-02-23.
- Solimini R, Rotolo MC, Mastrobattista L, Mortali C, Minutillo A, Pichini S, et al. (March 2017). "Hepatotoxicity associated with illicit use of anabolic androgenic steroids in doping". European Review for Medical and Pharmacological Sciences. 21 (1 Suppl): 7–16. PMID 28379599.
- "Drostanolone (PIM 901)". www.inchem.org. Retrieved 2016-03-15.
- ^ Elks J (14 November 2014). The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. pp. 652–. ISBN 978-1-4757-2085-3.
- ^ Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 377–. ISBN 978-3-88763-075-1.
- ^ Morton IK, Hall JM (6 December 2012). Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. pp. 106–. ISBN 978-94-011-4439-1.
External links
| Androgen receptor modulators | |||||||
|---|---|---|---|---|---|---|---|
| ARTooltip Androgen receptor |
| ||||||
| GPRC6A |
| ||||||