| Revision as of 20:49, 2 September 2011 editBogBot (talk | contribs)Bots53,132 edits populated new fields in {{drugbox}} and reordered per bot approval. Report errors and suggestions to User_talk:BogBot← Previous edit | Latest revision as of 10:41, 21 October 2024 edit undoJWBE (talk | contribs)Extended confirmed users10,126 edits removed Category:Phenols; added Category:Hydroxyarenes using HotCat | ||
| (18 intermediate revisions by 16 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Chemical compound}} | |||
| {{Drugbox | {{Drugbox | ||
| | verifiedrevid = |
| verifiedrevid = 477236127 | ||
| | IUPAC_name = (6aR,10aR)-3-(1-hexylcyclopropyl)-6,6,9-trimethyl-6a,7,10,10a-tetrahydrobenzochromen-1-ol | | IUPAC_name = (6aR,10aR)-3-(1-hexylcyclopropyl)-6,6,9-trimethyl-6a,7,10,10a-tetrahydrobenzochromen-1-ol | ||
| | image = AMG-41.svg | | image = AMG-41.svg | ||
| Line 8: | Line 9: | ||
| | tradename = | | tradename = | ||
| | legal_status = | | legal_status = | ||
| | routes_of_administration = |
| routes_of_administration = | ||
| <!--Pharmacokinetic data--> | <!--Pharmacokinetic data--> | ||
| | metabolism = | | metabolism = | ||
| | elimination_half-life = | | elimination_half-life = | ||
| | excretion = |
| excretion = | ||
| <!--Identifiers--> | <!--Identifiers--> | ||
| | CAS_number = | | CAS_number = 499237-35-3 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | UNII = 3062P60MH9 | |||
| | PubChem = 10361702 | | PubChem = 10361702 | ||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| Line 25: | Line 28: | ||
| <!--Chemical data--> | <!--Chemical data--> | ||
| | C=25 | H=36 | O=2 | | C=25 | H=36 | O=2 | ||
| | molecular_weight = 368.551 g/mol | |||
| | smiles = C=3CC2C(C)(C)Oc(cc(cc1O)C4(CC4)CCCCCC)c1C2CC=3C | | smiles = C=3CC2C(C)(C)Oc(cc(cc1O)C4(CC4)CCCCCC)c1C2CC=3C | ||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| Line 33: | Line 35: | ||
| }} | }} | ||
| '''AMG-41''' is an ] drug which is a ] ]. It is a derivative of |
'''AMG-41''' (part of the ]) is an ] drug which is a ] ]. It is a derivative of Δ<sup>8</sup>-] substituted with a ] on the C1'-position of the C3-alkyl side chain. AMG-41 is a potent agonist at both ] and ], with a ] of 0.44 nM at CB<sub>1</sub> vs 0.86 nM at CB<sub>2</sub>.<ref>{{cite journal | vauthors = Papahatjis DP, Nikas SP, Andreou T, Makriyannis A | title = Novel 1',1'-chain substituted Delta(8)-tetrahydrocannabinols | journal = Bioorganic & Medicinal Chemistry Letters | volume = 12 | issue = 24 | pages = 3583–6 | date = December 2002 | pmid = 12443781 | doi = 10.1016/s0960-894x(02)00785-0 }}</ref><ref>{{cite journal | vauthors = Papahatjis DP, Nikas SP, Kourouli T, Chari R, Xu W, Pertwee RG, Makriyannis A | title = Pharmacophoric requirements for the cannabinoid side chain. Probing the cannabinoid receptor subsite at C1' | journal = Journal of Medicinal Chemistry | volume = 46 | issue = 15 | pages = 3221–9 | date = July 2003 | pmid = 12852753 | doi = 10.1021/jm020558c }}</ref><ref>{{cite journal | vauthors = Papahatjis DP, Nahmias VR, Nikas SP, Andreou T, Alapafuja SO, Tsotinis A, Guo J, Fan P, Makriyannis A | display-authors = 6 | title = C1'-cycloalkyl side chain pharmacophore in tetrahydrocannabinols | journal = Journal of Medicinal Chemistry | volume = 50 | issue = 17 | pages = 4048–60 | date = August 2007 | pmid = 17672444 | doi = 10.1021/jm070121a }}</ref> | ||
| == See also == | |||
| * ] | |||
| * ] | |||
| ==References== | == References == | ||
| {{reflist}} | |||
| <references/> | |||
| {{cannabinoids}} | {{cannabinoids}} | ||
| ⚫ | {{cannabinoid-stub}} | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ⚫ | {{cannabinoid-stub}} | ||
Latest revision as of 10:41, 21 October 2024
Chemical compound Pharmaceutical compound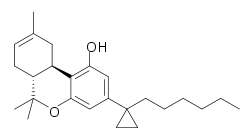 | |
| Identifiers | |
|---|---|
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C25H36O2 |
| Molar mass | 368.561 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
AMG-41 (part of the AM cannabinoid series) is an analgesic drug which is a cannabinoid agonist. It is a derivative of Δ-THC substituted with a cyclopropyl group on the C1'-position of the C3-alkyl side chain. AMG-41 is a potent agonist at both CB1 and CB2, with a Ki of 0.44 nM at CB1 vs 0.86 nM at CB2.
See also
References
- Papahatjis DP, Nikas SP, Andreou T, Makriyannis A (December 2002). "Novel 1',1'-chain substituted Delta(8)-tetrahydrocannabinols". Bioorganic & Medicinal Chemistry Letters. 12 (24): 3583–6. doi:10.1016/s0960-894x(02)00785-0. PMID 12443781.
- Papahatjis DP, Nikas SP, Kourouli T, Chari R, Xu W, Pertwee RG, Makriyannis A (July 2003). "Pharmacophoric requirements for the cannabinoid side chain. Probing the cannabinoid receptor subsite at C1'". Journal of Medicinal Chemistry. 46 (15): 3221–9. doi:10.1021/jm020558c. PMID 12852753.
- Papahatjis DP, Nahmias VR, Nikas SP, Andreou T, Alapafuja SO, Tsotinis A, et al. (August 2007). "C1'-cycloalkyl side chain pharmacophore in tetrahydrocannabinols". Journal of Medicinal Chemistry. 50 (17): 4048–60. doi:10.1021/jm070121a. PMID 17672444.
This cannabinoid related article is a stub. You can help Misplaced Pages by expanding it. |