| Revision as of 17:56, 20 September 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref', 'ChEBI_Ref') per Chem/Drugbox validation (report errors or [[use← Previous edit | Latest revision as of 19:14, 24 October 2024 edit undoSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,993 edits →Production: diisobutene | ||
| (87 intermediate revisions by 56 users not shown) | |||
| Line 1: | Line 1: | ||
| {{short description|1=Unsaturated hydrocarbon compound (H2C=C(CH3)2)}} | |||
| {{chembox | {{chembox | ||
| |Verifiedfields = changed | |||
| | verifiedrevid = 422526251 | |||
| |Watchedfields = changed | |||
| | ImageFileL1 = Isobutylene-2D-skeletal.png | |||
| |verifiedrevid = 451537760 | |||
| | ImageSizeL1 = 110px | |||
| |ImageFile1 = Isobutylene.svg | |||
| | ImageNameL1 = Skeletal formula | |||
| |ImageName1 = Skeletal formula of isobutylene | |||
| | ImageFileR1 = Isobutylene-3D-vdW.png | |||
| | |
|ImageSize1 = 120px | ||
| |ImageFileL2 = Isobutylene-3D-balls.png | |||
| | ImageNameR1 = Space-filling model | |||
| |ImageNameL2 = Ball-and-stick model of isobutylene | |||
| | ImageFile2 = Isobutylene-3D-balls.png | |||
| |ImageFileR2 = Isobutylene-3D-vdW.png | |||
| | ImageSize2 = 180px | |||
| | |
|ImageNameR2 = Space-filling model of isobutylene | ||
| |PIN = 2-Methylprop-1-ene | |||
| | IUPACName = 2-Methylpropene | |||
| | |
|OtherNames = 2-Methylpropene<br />Isobutene<br/>γ-Butylene<br/>2-Methylpropylene<br />Methylpropene | ||
| | |
|Section1={{Chembox Identifiers | ||
| | |
|StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | |
|StdInChI = 1S/C4H8/c1-4(2)3/h1H2,2-3H3 | ||
| | |
|StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | |
|StdInChIKey = VQTUBCCKSQIDNK-UHFFFAOYSA-N | ||
| | |
|CASNo = 115-11-7 | ||
| | |
|CASNo_Ref = {{cascite|correct|CAS}} | ||
| | |
|UNNumber = ]<br/>''In ]: ''] | ||
| | |
|EINECS = 204-066-3 | ||
| | |
|ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | |
|ChemSpiderID = 7957 | ||
| | |
|PubChem = 8255 | ||
| |ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| | SMILES = CC(=C)C | |||
| |ChEBI = 43907 | |||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| |SMILES = CC(=C)C | |||
| | UNII = QA2LMR467H | |||
| |UNII_Ref = {{fdacite|correct|FDA}} | |||
| | InChI = 1/C4H8/c1-4(2)3/h1H2,2-3H3 | |||
| |UNII = QA2LMR467H | |||
| | RTECS = UD0890000 | |||
| |InChI = 1/C4H8/c1-4(2)3/h1H2,2-3H3 | |||
| }} | |||
| |RTECS = UD0890000 | |||
| | Section2 = {{Chembox Properties | |||
| | Reference = <ref>{{Merck11th|5024}}.</ref> | |||
| | C=4|H=8 | |||
| | MolarMass = 56.106 g/mol | |||
| | Appearance = Colorless gas | |||
| | Density = 0.5879 g/cm<sup>3</sup>, liquid | |||
| | MeltingPt = −140.3 ºC | |||
| | BoilingPtC = -6.9 | |||
| | Boiling_notes = | |||
| | Solubility = Insoluble | |||
| | SolubleOther = | |||
| | Solvent = | |||
| }} | |||
| | Section7 = {{Chembox Hazards | |||
| | Reference = <ref name="ICSC">{{ICSC-ref|1027|name=Isobutene|date=April 2000}}</ref> | |||
| | EUIndex = 601-012-00-4 | |||
| | EUClass = Extremely flammable ('''F+''') | |||
| | RPhrases = {{R12}} | |||
| | SPhrases = {{S2}}, {{S9}}, {{S16}}, {{S33}} | |||
| | GHSPictograms = {{GHS02|Flam. Gas 1}}{{GHS04|Press. Gas}} | |||
| | GHSSignalWord = DANGER | |||
| | HPhrases = {{H-phrases|220}} | |||
| | PPhrases = {{P-phrases|210|377|381|403}} | |||
| | NFPA-H = 1 | |||
| | NFPA-F = 4 | |||
| | NFPA-R = 0 | |||
| | NFPA-O = | |||
| | FlashPt = flammable gas | |||
| | Autoignition = 465 °C (869 ºF) | |||
| | ExploLimits = 1.8–9.6% | |||
| }} | |||
| | Section8 = {{Chembox Related | |||
| | OtherFunctn = ]<br/>]<br/>] | |||
| | Function = ]s | |||
| | OtherCpds = ] | |||
| }} | |||
| }} | }} | ||
| |Section2={{Chembox Properties | |||
| |Properties_ref = <ref>{{Merck11th|5024}}.</ref> | |||
| |C=4 | H=8 | |||
| |MolarMass = 56.106 g/mol | |||
| |Appearance = Colorless gas | |||
| |Density = 0.5879 g/cm<sup>3</sup>, liquid | |||
| |MeltingPtC = −140.3 | |||
| |BoilingPtC = -6.9 | |||
| |MagSus = -44.4·10<sup>−6</sup> cm<sup>3</sup>/mol }} | |||
| |Section7={{Chembox Hazards | |||
| |Hazards_ref = <ref name="ICSC">{{ICSC-ref|1027|name=Isobutene|date=April 2000}}</ref> | |||
| |GHSPictograms = {{GHS02|Flam. Gas 1}}{{GHS exclamation mark|Press. Gas}} | |||
| |GHSSignalWord = DANGER | |||
| |HPhrases = {{H-phrases|220}} | |||
| |PPhrases = {{P-phrases|210|377|381|403}} | |||
| |NFPA-H = 1 | |||
| |NFPA-F = 4 | |||
| |NFPA-R = 0 | |||
| |FlashPt = flammable gas | |||
| |AutoignitionPtC = 465 | |||
| |ExploLimits = 1.8–9.6% | |||
| }} | |||
| |Section8={{Chembox Related | |||
| |OtherFunction = ]<br/>]<br/>] | |||
| |OtherFunction_label = ]s | |||
| |OtherCompounds = ] | |||
| }} | |||
| }} | |||
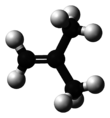
| '''Isobutylene''' (or '''2-methylpropene''') is a ] with the ] {{chem2|(CH3)2C\dCH2}}. It is a four-carbon branched ] (olefin), one of the four ]. It is a colorless flammable gas, and is of considerable industrial value.<ref>{{Ullmann|last1=Geilen|first1=Frank M.A.|last2=Stochniol|first2=Guido|last3=Peitz|first3=Stephan|last4=Schulte-Koerne|first4=Ekkehard|title=Butenes|year=2014|doi=10.1002/14356007.a04_483.pub3}}</ref> | |||
| ==Production== | |||
| Polymer and chemical grade isobutylene is typically obtained by dehydrating ] (TBA) or ] ] of ] (Catofin or similar processes).<ref>{{citation | title = Hydrocarbon Chemistry | first1 = George A. | last1 = Olah | author-link1 = George Andrew Olah | first2 = Árpád | last2 = Molnár | date = May 2003 | publisher = Wiley-Interscience | isbn = 978-0-471-41782-8}}.</ref> Gasoline additives ] (MTBE) and ] (ETBE), respectively, are produced by reacting ] or ] with isobutylene contained in butene streams from olefin steam crackers or refineries, or with isobutylene from dehydrated TBA. Isobutylene is not isolated from the olefin or refinery butene stream before the reaction, as separating the ethers from the remaining butenes is simpler. Isobutylene can also be produced in high purities by "back-cracking" MTBE or ETBE at high temperatures and then separating the isobutylene by distillation from methanol. | |||
| Isobutylene is a byproduct in the ] of ] to prepare ]:<ref name=KO>{{cite encyclopedia|title=Metathesis|encyclopedia=Kirk-Othmer Encyclopedia of Chemical Technology|author=Lionel Delaude |author2=Alfred F. Noels |publisher=Wiley}}</ref> | |||
| :(CH<sub>3</sub>)<sub>3</sub>C-CH=C(CH<sub>3</sub>)<sub>2</sub> + {{red|1=CH<sub>2</sub>=CH<sub>2</sub>}} → (CH<sub>3</sub>)<sub>3</sub>C-CH={{red|CH<sub>2</sub>}} + (CH<sub>3</sub>)<sub>2</sub>C={{red|CH<sub>2</sub>}} | |||
| '''Isobutylene''' (or 2-methylpropene) is a ] of significant industrial importance. It is a four-carbon branched ] (olefin), one of the four ]. At standard temperature and pressure it is a colorless flammable gas. | |||
| ==Uses== | ==Uses== | ||
| Isobutylene is used |
Isobutylene is used in the production of a variety of products. It is alkylated with butane to produce ] or dimerized to diisobutylene (DIB) and then hydrogenated to make ], a fuel additive. Isobutylene is also used in the production of ]. ] of isobutylene produces ] (polyisobutylene or PIB). Antioxidants such as ] (BHT) and ] (BHA) are produced by ] of ]s with isobutylene. | ||
| ] is produced commercially by amination of isobutylene using ] ]s:<ref name=Ullmann>{{Ullmann|doi=10.1002/14356007.a02_001|title=Amines, Aliphatic|year=2000|last1= Eller|first1=Karsten|last2=Henkes|first2=Erhard|last3=Rossbacher|first3=Roland|last4=Höke|first4=Hartmut|isbn=3527306730}}</ref> | |||
| ==Manufacture== | |||
| :{{chem2 | NH3 + CH2\dC(CH3)2 -> H2NC(CH3)3 }} | |||
| Isobutylene can be isolated from refinery streams by reaction with ], but the most common industrial method for its production is by ] ] of ].<ref>{{citation | title = Hydrocarbon Chemistry | first1 = George A. | last1 = Olah | authorlink1 = George Andrew Olah | first2 = Árpád | last2 = Molnár | publisher = Wiley-Interscience | isbn = 978-0471417828}}.</ref> In the 1990s, the production of isobutylene increased dramatically as the demand for oxygenates such as MTBE grew. Key manufacturers of this product are Texas Petrochemicals(TPC Group) and Lyondell in North America. | |||
| Applications are found in the calibration of ]s. | |||
| ==Safety== | ==Safety== | ||
| Isobutylene is a highly flammable gas. | |||
| Isobutylene is a highly flammable gas and presents an explosion danger. Usually stored as a compressed gas, if released it may produce an oxygen-deficient atmosphere that presents an asphyxiation hazard.<ref name="ICSC"/> | |||
| ==See also== | ==See also== | ||
| *] | *] | ||
| *] | |||
| *] | *] | ||
| *] | |||
| ==References== | ==References== | ||
| Line 90: | Line 94: | ||
| *{{SIDS|id=115117|name=Isobutylene|date=November 2003}} | *{{SIDS|id=115117|name=Isobutylene|date=November 2003}} | ||
| {{Hydrocarbons}} | |||
| ] | |||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 19:14, 24 October 2024
Unsaturated hydrocarbon compound (H2C=C(CH3)2)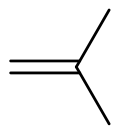 | |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 2-Methylprop-1-ene | |||
| Other names
2-Methylpropene Isobutene γ-Butylene 2-Methylpropylene Methylpropene | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.697 | ||
| EC Number |
| ||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
| UN number | 1055 In Liquefied petroleum gas: 1075 | ||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C4H8 | ||
| Molar mass | 56.106 g/mol | ||
| Appearance | Colorless gas | ||
| Density | 0.5879 g/cm, liquid | ||
| Melting point | −140.3 °C (−220.5 °F; 132.8 K) | ||
| Boiling point | −6.9 °C (19.6 °F; 266.2 K) | ||
| Magnetic susceptibility (χ) | -44.4·10 cm/mol | ||
| Hazards | |||
| GHS labelling: | |||
| Pictograms |  
| ||
| Signal word | Danger | ||
| Hazard statements | H220 | ||
| Precautionary statements | P210, P377, P381, P403 | ||
| NFPA 704 (fire diamond) |
 | ||
| Flash point | flammable gas | ||
| Autoignition temperature |
465 °C (869 °F; 738 K) | ||
| Explosive limits | 1.8–9.6% | ||
| Related compounds | |||
| Related butenes | 1-Butene cis-2-Butene trans-2-Butene | ||
| Related compounds | Isobutane | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Isobutylene (or 2-methylpropene) is a hydrocarbon with the chemical formula (CH3)2C=CH2. It is a four-carbon branched alkene (olefin), one of the four isomers of butylene. It is a colorless flammable gas, and is of considerable industrial value.
Production
Polymer and chemical grade isobutylene is typically obtained by dehydrating tertiary butyl alcohol (TBA) or catalytic dehydrogenation of isobutane (Catofin or similar processes). Gasoline additives methyl tert-butyl ether (MTBE) and ethyl tert-butyl ether (ETBE), respectively, are produced by reacting methanol or ethanol with isobutylene contained in butene streams from olefin steam crackers or refineries, or with isobutylene from dehydrated TBA. Isobutylene is not isolated from the olefin or refinery butene stream before the reaction, as separating the ethers from the remaining butenes is simpler. Isobutylene can also be produced in high purities by "back-cracking" MTBE or ETBE at high temperatures and then separating the isobutylene by distillation from methanol.
Isobutylene is a byproduct in the ethenolysis of diisobutene to prepare neohexene:
- (CH3)3C-CH=C(CH3)2 + CH2=CH2 → (CH3)3C-CH=CH2 + (CH3)2C=CH2
Uses
Isobutylene is used in the production of a variety of products. It is alkylated with butane to produce isooctane or dimerized to diisobutylene (DIB) and then hydrogenated to make isooctane, a fuel additive. Isobutylene is also used in the production of methacrolein. Polymerization of isobutylene produces butyl rubber (polyisobutylene or PIB). Antioxidants such as butylated hydroxytoluene (BHT) and butylated hydroxyanisole (BHA) are produced by Friedel-Crafts alkylation of phenols with isobutylene.
tert-Butylamine is produced commercially by amination of isobutylene using zeolite catalysts:
- NH3 + CH2=C(CH3)2 → H2NC(CH3)3
Applications are found in the calibration of photoionization detectors.
Safety
Isobutylene is a highly flammable gas.
See also
References
- The Merck Index: An Encyclopedia of Chemicals, Drugs, and Biologicals (11th ed.). Merck. 1989. ISBN 091191028X., 5024.
- Isobutene, International Chemical Safety Card 1027, Geneva: International Programme on Chemical Safety, April 2000
- Geilen, Frank M.A.; Stochniol, Guido; Peitz, Stephan; Schulte-Koerne, Ekkehard (2014). "Butenes". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a04_483.pub3. ISBN 978-3-527-30673-2.
- Olah, George A.; Molnár, Árpád (May 2003), Hydrocarbon Chemistry, Wiley-Interscience, ISBN 978-0-471-41782-8.
- Lionel Delaude; Alfred F. Noels. "Metathesis". Kirk-Othmer Encyclopedia of Chemical Technology. Wiley.
- Eller, Karsten; Henkes, Erhard; Rossbacher, Roland; Höke, Hartmut (2000). "Amines, Aliphatic". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a02_001. ISBN 3527306730.
External links
- International Chemical Safety Card 1027
- SIDS Initial Assessment Report for Isobutylene from the Organisation for Economic Co-operation and Development (OECD)
| Hydrocarbons | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Saturated aliphatic hydrocarbons |
| ||||||||||||||||||||||||||||||||||
| Unsaturated aliphatic hydrocarbons |
| ||||||||||||||||||||||||||||||||||
| Aromatic hydrocarbons |
| ||||||||||||||||||||||||||||||||||
| Other | |||||||||||||||||||||||||||||||||||