| Revision as of 18:31, 27 September 2011 edit89.169.76.213 (talk) Add picture← Previous edit | Latest revision as of 13:00, 31 August 2024 edit undoCitation bot (talk | contribs)Bots5,420,055 edits Added title. Changed bare reference to CS1/2. | Use this bot. Report bugs. | Suggested by Grimes2 | #UCB_webform 171/648 | ||
| (103 intermediate revisions by 72 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Chembox | {{Chembox | ||
| | Verifiedfields = changed | |||
| ⚫ | | verifiedrevid = |
||
| | Watchedfields = changed | |||
| | ImageFile = brilliantgruen.png | |||
| ⚫ | | verifiedrevid = 434149900 | ||
| | ImageFile = Brilliant green (dye) Structural Formula V1.svg | |||
| | ImageSize = 150px | | ImageSize = 150px | ||
| | Name = Brilliant green | |||
| | IUPACName = |
| IUPACName = | ||
| | OtherNames = Malachite green G, Emerald green, Solid green JJO, Diamond green G, Aniline green, Benzaldehyde green, Fast green J | | OtherNames = Malachite green G, Emerald green, Solid green JJO, Diamond green G, Aniline green, Benzaldehyde green, Fast green J | ||
| | |
|Section1={{Chembox Identifiers | ||
| | |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 11941 | | ChemSpiderID = 11941 | ||
| | ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| | ChEMBL = 1181633 | |||
| | ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| | ChEBI = 88173 | |||
| | UNII_Ref = {{fdacite|correct|FDA}} | | UNII_Ref = {{fdacite|correct|FDA}} | ||
| | UNII = G0L543D370 | | UNII = G0L543D370 | ||
| Line 17: | Line 24: | ||
| | StdInChIKey = NNBFNNNWANBMTI-UHFFFAOYSA-M | | StdInChIKey = NNBFNNNWANBMTI-UHFFFAOYSA-M | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | CASNo = |
| CASNo = 633-03-4 | ||
| | |
| PubChem = 12449 | ||
| | |
| SMILES = S(=O)(=O)O.(=C/1\C=C/C(C=C\1)=C(/c2ccccc2)c3ccc(N(CC)CC)cc3)(\CC)CC | ||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| | |
| Formula = ]<sub>27</sub>]<sub>33</sub>]<sub>2</sub>.H]<sub>4</sub>] | ||
| | |
| MolarMass = 482.64 g/mol | ||
| | |
| Appearance = | ||
| | |
| Density = | ||
| | |
| MeltingPtC = 210 | ||
| | MeltingPt_notes = (decomposes) | |||
| | BoilingPt = | |||
| | Solubility = 100 g/L a 20 °C | | BoilingPt = | ||
| | Solubility = 100 g/L a 20 °C | |||
| ⚫ | |||
| ⚫ | | |
||
| ⚫ | | |
||
| ⚫ | | |
||
| | Autoignition = | |||
| }} | }} | ||
| ⚫ | |Section3={{Chembox Hazards | ||
| ⚫ | | MainHazards = | ||
| ⚫ | | FlashPt = | ||
| | AutoignitionPt = | |||
| ⚫ | }} | ||
| }} | }} | ||
| '''Brilliant |
'''Brilliant green''' (also known as {{transl|ru|zelyonka}} or {{transl|uk|zelenka}}) is one of the ]s. It is closely related to ].<ref name=gessner2002>{{citation | last1=Gessner | first1=T. | last2=Mayer | first2=U. | year=2002 | contribution= Triarylmethane and Diarylmethane Dyes | title= Ullmann's Encyclopedia of Industrial Chemistry 6th Edition | publisher=Wiley-VCH | place=Weinheim | doi=10.1002/14356007.a27_179 | isbn=3527306730 }}</ref> | ||
| ==Uses== | ==Uses== | ||
| ] | ] | ||
| Brilliant |
Brilliant green has been used to color ] and ]. | ||
| It is indicated for disinfection of fresh postoperative and post-traumatic scars, ] of newborns, abrasions, cuts, and other violations of the integrity of the skin, in the treatment of purulent-inflammatory processes of the skin - hordeolum ("barley"), meibomite, ], ], local ], carbunculosis, ].<ref>{{cite web | url=https://pubchem.ncbi.nlm.nih.gov/compound/Malachite-Green | title=Malachite Green }}</ref> It is applied externally, the drug is applied to the damaged surface, capturing the surrounding healthy tissue.{{Citation needed|date=August 2020}} | |||
| In ] and ] (and formerly the ]) the dilute alcoholic solution of Brilliant Green is sold as a topical ], also known under a Latin name ''Viridis nitentis spirituosa'' and a Russian name ''зелёнка'' .<ref>M. Balabanova, L. Popova, R. Tchipeva, ''Disease-a-Month'', 50(6), '''2004''', 270-279. </ref> | |||
| In ] and ] (and much of the rest of the former ]), the dilute alcoholic solution of brilliant green is sold as a topical ], also known under a Latin name {{lang|la|solutio viridis nitentis spirituosa}} and the colloquial Russian name of {{transl|ru|zelyonka}} ({{wikt-lang|ru|зелёнка}}, {{literally|green stuff}} in Russian),<ref>{{cite journal | doi = 10.1016/j.disamonth.2004.05.002| title = Dyes in dermatology| journal = Disease-a-Month| volume = 50| issue = 6| pages = 270| year = 2004| last1 = Balabanova| first1 = Maria| last2 = Popova| first2 = Liudmila| last3 = Tchipeva| first3 = Rositsa}}</ref>{{Failed verification|date=July 2019}} which is {{transl|uk|zelenka}} ({{wikt-lang|uk|зеленка}}) in Ukrainian. | |||
| Brilliant Green is effective against ]. The main advantage of Brilliant Green over the more common antiseptics such as ] is that it does not irritate mucous membranes. Therefore it is often used to treat infections of the eye, tongue sores and sinus infections. Brilliant green induces vomiting when swallowed and is toxic when ingested.<ref>Joseph K. Narat, Brilliant Green: A Clinical Study of its Value as a Local Antiseptic" Annals of Surgery 1931 December; 94(6): 1007–1012. </ref> | |||
| A 1% solution in 60% alcohol can be used for treatment of skin. 0.5% solution is used for mucous membranes or for infants.<ref name="Narat1931"/> | |||
| ⚫ | ==References== | ||
| ⚫ | <references/> | ||
| Brilliant green is a visible light-activated ] in organic synthesis.<ref>{{Cite journal|last1=Rogers|first1=David A.|last2=Bensalah|first2=Adam T.|last3=Espinosa|first3=Alvaro Tomas|last4=Hoerr|first4=John L.|last5=Refai|first5=Fares H.|last6=Pitzel|first6=Amy K.|last7=Alvarado|first7=Juan J.|last8=Lamar|first8=Angus A.|date=2019-06-07|title=Amplification of Trichloroisocyanuric Acid (TCCA) Reactivity for Chlorination of Arenes and Heteroarenes via Catalytic Organic Dye Activation|journal=Organic Letters|language=en|volume=21|issue=11|pages=4229–4233|doi=10.1021/acs.orglett.9b01414|pmid=31140821|s2cid=169034253 }}</ref> | |||
| ==Other reading== | |||
| * (in Portuguese) | |||
| ==Safety and toxicity== | |||
| Brilliant green is effective against ].<ref>{{Cite journal|last=|first=|date=June 1984|title=Martindale: The extra pharmacopeia, 28th Ed. Edited By James E. F. Reynolds and Anne B. Prasad. The Pharmaceopeial Press, 1 Lamberth High Street, London, SE1 7JN. Distributed in the U.S. by Rittenhouse Book Distributors, Inc., King of Prussia, PA 19406. 1982.|url=http://dx.doi.org/10.1002/jps.2600730653|journal=Journal of Pharmaceutical Sciences|volume=73|issue=6|pages=862|doi=10.1002/jps.2600730653|issn=0022-3549|via=}}</ref> The main advantage of brilliant green over the more common antiseptics such as ] is that it does not irritate mucous membranes as harshly on accidental contact. Soviet medical doctrine deemed it "not for use on ]" and cautions that it can cause eye damage and ophthalmic chemical burns and burns to an eye, at least in the typical formulations produced for medical use.{{Citation needed|date=July 2019}} | |||
| Brilliant green induces vomiting when swallowed and is toxic when ingested.<ref name="Narat1931">{{cite journal | pmc = 1391517| year = 1931| last1 = Narat| first1 = J. K.| title = Brilliant Green: A Clinical Study of Its Value As a Local Antiseptic| journal = Annals of Surgery| volume = 94| issue = 6| pages = 1007–1012| doi=10.1097/00000658-193112000-00003| pmid=17866691}}</ref> The compound may lead to serious injuries if it comes in contact with an eye, even resulting in bilateral ] due to ].<ref>{{PubChem|12449}}</ref> | |||
| == Politics == | |||
| {{main|Zelyonka attack}} | |||
| In ] and sometimes in ], {{transl|ru|zelyonka}} has been used to physically attack political opponents.<ref>{{cite web|url=https://intpolicydigest.org/2017/05/06/how-the-soviet-era-antiseptic-zelyonka-became-a-political-weapon-in-russia-and-ukraine|title=How the Soviet-Era Antiseptic "Zelyonka" Became a Political Weapon in Russia and Ukraine|date=6 May 2017|publisher=|access-date=26 September 2017|archive-date=23 November 2020|archive-url=https://web.archive.org/web/20201123093230/https://intpolicydigest.org/2017/05/06/how-the-soviet-era-antiseptic-zelyonka-became-a-political-weapon-in-russia-and-ukraine/|url-status=dead}}</ref> Since 2016, many opponents of the Russian government have been splashed with {{transl|ru|zelyonka}}, including ], ], liberal activists, ], ], ], ] and ].<ref>{{cite news | url = https://www.economist.com/blogs/economist-explains/2017/05/economist-explains-7 | title = Why are Russian opposition leaders' faces turning green? | newspaper = ] | date = May 10, 2017 | access-date = May 11, 2017}}</ref> | |||
| ⚫ | ==References== | ||
| ⚫ | <references /> | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | ] | ||
| ] | |||
| ] | |||
| {{antimicrobial-stub}} | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 13:00, 31 August 2024
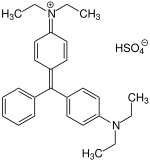
| |
| Names | |
|---|---|
| Other names Malachite green G, Emerald green, Solid green JJO, Diamond green G, Aniline green, Benzaldehyde green, Fast green J | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.010.174 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C27H33N2.HO4S |
| Molar mass | 482.64 g/mol |
| Melting point | 210 °C (410 °F; 483 K) (decomposes) |
| Solubility in water | 100 g/L a 20 °C |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Brilliant green (also known as zelyonka or zelenka) is one of the triarylmethane dyes. It is closely related to malachite green.
Uses

Brilliant green has been used to color silk and wool.
It is indicated for disinfection of fresh postoperative and post-traumatic scars, umbilical cord of newborns, abrasions, cuts, and other violations of the integrity of the skin, in the treatment of purulent-inflammatory processes of the skin - hordeolum ("barley"), meibomite, blepharitis, pyoderma, local furunculosis, carbunculosis, staphylococcal infection. It is applied externally, the drug is applied to the damaged surface, capturing the surrounding healthy tissue.
In Russia and Ukraine (and much of the rest of the former Soviet Union), the dilute alcoholic solution of brilliant green is sold as a topical antiseptic, also known under a Latin name solutio viridis nitentis spirituosa and the colloquial Russian name of zelyonka (зелёнка, lit. 'green stuff' in Russian), which is zelenka (зеленка) in Ukrainian.
A 1% solution in 60% alcohol can be used for treatment of skin. 0.5% solution is used for mucous membranes or for infants.
Brilliant green is a visible light-activated photocatalyst in organic synthesis.
Safety and toxicity
Brilliant green is effective against Gram-positive bacteria. The main advantage of brilliant green over the more common antiseptics such as iodine is that it does not irritate mucous membranes as harshly on accidental contact. Soviet medical doctrine deemed it "not for use on mucosa" and cautions that it can cause eye damage and ophthalmic chemical burns and burns to an eye, at least in the typical formulations produced for medical use.
Brilliant green induces vomiting when swallowed and is toxic when ingested. The compound may lead to serious injuries if it comes in contact with an eye, even resulting in bilateral blindness due to corneal opacification.
Politics
Main article: Zelyonka attackIn Russia and sometimes in Ukraine, zelyonka has been used to physically attack political opponents. Since 2016, many opponents of the Russian government have been splashed with zelyonka, including Alexei Navalny, Igor Kalyapin, liberal activists, Nadya Tolokonnikova, Maria Alekhina, Lyudmila Ulitskaya, Ilya Varlamov and Mikhail Kasyanov.
References
- Gessner, T.; Mayer, U. (2002), "Triarylmethane and Diarylmethane Dyes", Ullmann's Encyclopedia of Industrial Chemistry 6th Edition, Weinheim: Wiley-VCH, doi:10.1002/14356007.a27_179, ISBN 3527306730
- "Malachite Green".
- Balabanova, Maria; Popova, Liudmila; Tchipeva, Rositsa (2004). "Dyes in dermatology". Disease-a-Month. 50 (6): 270. doi:10.1016/j.disamonth.2004.05.002.
- ^ Narat, J. K. (1931). "Brilliant Green: A Clinical Study of Its Value As a Local Antiseptic". Annals of Surgery. 94 (6): 1007–1012. doi:10.1097/00000658-193112000-00003. PMC 1391517. PMID 17866691.
- Rogers, David A.; Bensalah, Adam T.; Espinosa, Alvaro Tomas; Hoerr, John L.; Refai, Fares H.; Pitzel, Amy K.; Alvarado, Juan J.; Lamar, Angus A. (2019-06-07). "Amplification of Trichloroisocyanuric Acid (TCCA) Reactivity for Chlorination of Arenes and Heteroarenes via Catalytic Organic Dye Activation". Organic Letters. 21 (11): 4229–4233. doi:10.1021/acs.orglett.9b01414. PMID 31140821. S2CID 169034253.
- "Martindale: The extra pharmacopeia, 28th Ed. Edited By James E. F. Reynolds and Anne B. Prasad. The Pharmaceopeial Press, 1 Lamberth High Street, London, SE1 7JN. Distributed in the U.S. by Rittenhouse Book Distributors, Inc., King of Prussia, PA 19406. 1982". Journal of Pharmaceutical Sciences. 73 (6): 862. June 1984. doi:10.1002/jps.2600730653. ISSN 0022-3549.
- CID 12449 from PubChem
- "How the Soviet-Era Antiseptic "Zelyonka" Became a Political Weapon in Russia and Ukraine". 6 May 2017. Archived from the original on 23 November 2020. Retrieved 26 September 2017.
- "Why are Russian opposition leaders' faces turning green?". The Economist. May 10, 2017. Retrieved May 11, 2017.