| Revision as of 01:37, 13 October 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (changes to watched fields - added verified revid - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEMBL_Ref', 'ChEBI_Ref', 'KEGG_Ref', 'ChEBI_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:WikiProject_Chem...← Previous edit | Latest revision as of 18:18, 18 October 2024 edit undoCitation bot (talk | contribs)Bots5,424,422 edits Added bibcode. | Use this bot. Report bugs. | Suggested by Spinixster | Category:Chemicals using indexlabels | #UCB_Category 261/831 | ||
| (70 intermediate revisions by 44 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Use dmy dates|date=August 2022}} | |||
| {{chembox | {{chembox | ||
| | |
| Verifiedfields = changed | ||
| | Watchedfields = changed | |||
| | verifiedrevid = |
| verifiedrevid = 455304200 | ||
| ⚫ | | ImageFile = Ferric amm sulfate.jpg | ||
| | Name = | |||
| | ImageFile1 = Ferric Ammonium Sulfate Dodecahydrate formula.png | |||
| ⚫ | | ImageFile = Ferric amm sulfate.jpg | ||
| ⚫ | | ImageSize1 = 250px | ||
| | |
| ImageFile1 = Ferric Ammonium Sulfate Dodecahydrate formula.png | ||
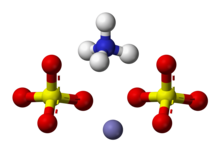
| | ImageFile2 = Ammonium-iron(III)-sulfate-3D-balls-ionic.png | |||
| ⚫ | | OtherNames = Ferric ammonium sulfate<br/> |
||
| ⚫ | | ImageSize1 = 250px | ||
| ⚫ | | Section1 = {{Chembox Identifiers | ||
| | IUPACName = Ammonium iron(III) sulfate | |||
| ⚫ | | OtherNames = Ferric ammonium sulfate<br />Ferric alum | ||
| | SystematicName = | |||
| ⚫ | | Section1 = {{Chembox Identifiers | ||
| | index_label = anhydride | |||
| | index1_label = dodecahydrate | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 55405 | | ChemSpiderID = 55405 | ||
| Line 16: | Line 23: | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = XGGLLRJQCZROSE-UHFFFAOYSA-K | | StdInChIKey = XGGLLRJQCZROSE-UHFFFAOYSA-K | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | CASNo = 10138-04-2 | | CASNo = 10138-04-2 | ||
| | CASNo1_Ref = {{cascite|correct|CAS}} | |||
| | CASOther = <br/>7783-83-7 (dodecahydrate) | |||
| | |
| CASNo1 = 7783-83-7 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | UNII = LUX2X1H1IC | |||
| | UNII1_Ref = {{fdacite|correct|FDA}} | |||
| | UNII1 = 65390568Z5 | |||
| | EINECS = 233-382-4 | |||
| | PubChem1 = 61485 | |||
| | SMILES = .S(=O)(=O).S()(=O)=O. | | SMILES = .S(=O)(=O).S()(=O)=O. | ||
| }} | }} | ||
| | Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| | Formula = FeNH<sub>4</sub>(SO<sub>4</sub>)<sub>2</sub> | | Formula = FeNH<sub>4</sub>(SO<sub>4</sub>)<sub>2</sub>•12H<sub>2</sub>O | ||
| | MolarMass = 482.25 g/mol (dodecahydrate) | | MolarMass = 482.25 g/mol (dodecahydrate) | ||
| | Appearance = Pale violet octahedral crystals | | Appearance = Pale violet octahedral crystals | ||
| | Odor = weak ammonia-like | |||
| | Density = 1.71 g/cm<sup>3</sup> | | Density = 1.71 g/cm<sup>3</sup> | ||
| | |
| MeltingPtC = 39 to 41 | ||
| | MeltingPt_notes = | |||
| | BoilingPt = | | BoilingPt = | ||
| | Solubility = 1240 g/L | | Solubility = 1240 g/L | ||
| }} | }} | ||
| | Section3 = {{Chembox Hazards | | Section3 = {{Chembox Hazards | ||
| | MainHazards = Irritant |
| MainHazards = Irritant | ||
| | |
| GHSPictograms = | ||
| | |
| GHSSignalWord = | ||
| | HPhrases = {{HPhrases|}} | |||
| | PPhrases = {{PPhrases|}} | |||
| | GHS_ref = | |||
| | FlashPt = | | FlashPt = | ||
| | |
| ExternalSDS = | ||
| | NFPA-H = 1 | |||
| | NFPA-F = 0 | |||
| | NFPA-R = 0 | |||
| | NFPA_ref =<ref>{{cite web|title=Material Safety Data Sheet. Iron (III) Ammonium Sulfate Dodecahydrate | website=fscimage.fishersci.com | url=https://fscimage.fishersci.com/msds/09713.htm | access-date=8 June 2023}}</ref> | |||
| }} | }} | ||
| | Section4 = {{Chembox Related | | Section4 = {{Chembox Related | ||
| | OtherAnions = ]<br>] | | OtherAnions = ]<br />] | ||
| | OtherCations = ]<br>] | | OtherCations = ]<br />] | ||
| | |
| OtherFunction = | ||
| | |
| OtherFunction_label = | ||
| | |
| OtherCompounds= ] | ||
| }} | }} | ||
| | Section5 = | |||
| | Section6 = | |||
| }} | }} | ||
| '''Ammonium iron(III) sulfate''', |
'''Ammonium iron(III) sulfate''', NH<sub>4</sub>Fe(SO<sub>4</sub>)<sub>2</sub>·12 H<sub>2</sub>O, or NH<sub>4</sub>(SO<sub>4</sub>)<sub>2</sub>·6 H<sub>2</sub>O, also known as '''ferric ammonium sulfate''' ('''FAS''') or '''iron alum''', is a ] in the class of ]s, which consists of compounds with the general formula AB(SO<sub>4</sub>)<sub>2</sub> · 12 H<sub>2</sub>O.<ref>Considine, Douglas M: ''Chemical and process technology encyclopedia'', McGraw-Hill, New York, 1974, p. 993</ref> It has the appearance of weakly violet, octahedrical ]s. There has been some discussion regarding the origin of the crystals' color, with some ascribing it to impurities in the compound,<ref>{{cite journal | last1 = Christensen | first1 = Odin T | title = On the Cause of the Amethyst Color of Ferric Alum and of Mixed Crystals of Ferric and Manganic Alum | journal = Chem. Lab. Roy. Vet. Agr. Hochschule, KGL. Danske Vidsk. Selsk. Forh. | volume = 1906 | pages = 173–95 }}</ref> and others claiming it to be a property of the crystal itself.<ref>{{cite journal | last1 = Bonnell | first1 = Jane | last2 = Philip Perman | first2 = Edgar | year = 1921 | title = CCXXIX.—The colour of iron alum | url = https://zenodo.org/record/1609699| journal = J. Chem. Soc., Trans. | volume = 119 | pages = 1994–1997 | doi = 10.1039/CT9211901994 }}</ref> | ||
| FAS is ]<ref>Cooke |
FAS is ],<ref>{{cite journal | last1 = Cooke | first1 = Meyer | last2 = Wolf | year = 1956| title = The Specific Heats of Three Paramagnetic salts at Very Low Temperatures | journal = Proceedings of the Royal Society of London. Series A, Mathematical and Physical Sciences | volume = 237 | issue = 1210| pages = 395–403 | doi=10.1098/rspa.1956.0185| bibcode = 1956RSPSA.237..395C | s2cid = 97076961 }}</ref> ] and ] towards ]s.<ref>{{cite journal | last1 = Wang | first1 = Fei | display-authors = etal | year = 2008| title = Microcalorimetric investigation of the toxic action of ammonium ferric(III)sulfate on the metabolic activity of pure microbes | journal = Environmental Toxicology and Pharmacology| volume = 25| issue = 3| pages = 351–357| doi = 10.1016/j.etap.2007.11.004 | pmid = 21783873 | bibcode = 2008EnvTP..25..351W }}</ref> It is a weak oxidizing agent, capable of being reduced to ], ferrous ammonium sulfate. | ||
| ==Preparation== | ==Preparation== | ||
| FAS can be prepared by crystallization from a solution of ] and ]. Iron(II) in ferrous sulfate is oxidized to |
FAS can be prepared by crystallization from a solution of ] and ]. Iron(II) in ferrous sulfate is oxidized to ferric sulfate by addition of ] and ]. Upon addition of ammonium sulfate to the solution and damping in of the solution, ferric ammonium sulfate crystals precipitate. Equations for these conversions ignore the ] of the material. | ||
| :Oxidation: {{chem2 | 6 FeSO4 + 2 HNO3 + 3 H2SO4 -> 3 Fe2(SO4)3 + 2 NO + 4 H2O }} | |||
| :Synthesis: {{chem2 | Fe2(SO4)3 + (NH4)2SO4 -> 2 NH4Fe(SO4)2 }} | |||
| '''Synthesis''': Fe<sub>2</sub>(SO<sub>4</sub>)<sub>3</sub> + (NH<sub>4</sub>)<sub>2</sub>SO<sub>4</sub> = 2 NH<sub>4</sub>Fe(SO<sub>4</sub>)<sub>2</sub> | |||
| '''Procedure<ref>Hecht, Horstmar: “Prãparative Anorganische Chemie”, ''Springer-verlag, Berlin'', 1951. p. 127</ref>''': | |||
| The nitric and sulfuric acid is reacted with the ferrous sulfate to make ferric sulfate, ], and water. The ferric sulfate is mixed with ] and crystallized to get ammonium iron(III) sulfate. The solution is normally tested to ensure that no more iron(II) is left. | |||
| ⚫ | |||
| ==Uses== | ==Uses== | ||
| ⚫ | Areas of use for FAS include ] treatment,<ref name="ReferenceA">''Wiley Encyclopedia of inorganic chemistry'': Volume 4, p. 1704:</ref> ],<ref name="ReferenceA" /> production of ]stuffs,<ref name="ReferenceA" /> and as an ] agent in the production of ].<ref>Chen et al.: ''United States Patent 5518131'' – "Etching molydbenum with ferric sulfate and ferric ammonium sulfate"</ref> It has been used in a wide area of applications, including adiabatic ] equipment,<ref>Grant W. Wilson, Peter T. Timbie: "Construction techniques for adiabatic demagnetization refrigerators using ferric ammonium alum". ''Cryogenics'', Volume 39, Number 4, (1999), pp. 319–322</ref> biochemical ],<ref>J. C. Whitehorn: "A system of blood analysis. Supplement II. Simplified method for the determination of chlorides in blood or plasma". ''Journal of Biological Chemistry'' (1921), 45 p. 449–60.</ref> and ].<ref>{{cite journal | last1 = Yu | first1 = Shanxin | display-authors = etal | year = 2005 | title = Application of ammonium ferric sulfate dodecahydrate in organic synthesis | journal = General Review | volume = 17 | issue = 1| pages = 27–30 }}</ref> | ||
| ==Gallery== | |||
| ⚫ | Areas of use for FAS include ] treatment<ref name="ReferenceA">''Wiley Encyclopedia of inorganic chemistry'': Volume 4, p. 1704:</ref> |
||
| <gallery> | |||
| ⚫ | Ammoniumeisenalaun.jpg|Crystals of ferric ammonium sulfate | ||
| Ammonium iron(III) sulfate dodecahydrate in moist air, 2015-10-22 (1).jpg|Crystals of ammonium iron(III) sulfate after 16 days in the air | |||
| </gallery> | |||
| ==References== | ==References== | ||
| {{Reflist}} | {{Reflist}} | ||
| {{Ammonium salts}} | |||
| {{iron compounds}} | |||
| {{sulfates}} | |||
| {{DEFAULTSORT:Ammonium Iron(Iii) Sulfate}} | {{DEFAULTSORT:Ammonium Iron(Iii) Sulfate}} | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 18:18, 18 October 2024

| |

| |
| |
| Names | |
|---|---|
| IUPAC name Ammonium iron(III) sulfate | |
| Other names
Ferric ammonium sulfate Ferric alum | |
| Identifiers | |
| CAS Number |
|
| 3D model (JSmol) |
|
| ChemSpider |
|
| ECHA InfoCard | 100.030.335 |
| EC Number |
|
| PubChem CID |
|
| UNII |
|
| CompTox Dashboard (EPA) |
|
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | FeNH4(SO4)2•12H2O |
| Molar mass | 482.25 g/mol (dodecahydrate) |
| Appearance | Pale violet octahedral crystals |
| Odor | weak ammonia-like |
| Density | 1.71 g/cm |
| Melting point | 39 to 41 °C (102 to 106 °F; 312 to 314 K) |
| Solubility in water | 1240 g/L |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Irritant |
| NFPA 704 (fire diamond) |
 |
| Related compounds | |
| Other anions | Ammonium iron(III) citrate Ammonium chloride |
| Other cations | Ammonium aluminium sulfate potassium aluminium sulfate |
| Related compounds | Ammonium iron(II) sulfate |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Ammonium iron(III) sulfate, NH4Fe(SO4)2·12 H2O, or NH4(SO4)2·6 H2O, also known as ferric ammonium sulfate (FAS) or iron alum, is a double salt in the class of alums, which consists of compounds with the general formula AB(SO4)2 · 12 H2O. It has the appearance of weakly violet, octahedrical crystals. There has been some discussion regarding the origin of the crystals' color, with some ascribing it to impurities in the compound, and others claiming it to be a property of the crystal itself.
FAS is paramagnetic, acidic and toxic towards microorganisms. It is a weak oxidizing agent, capable of being reduced to Mohr's salt, ferrous ammonium sulfate.
Preparation
FAS can be prepared by crystallization from a solution of ferric sulfate and ammonium sulfate. Iron(II) in ferrous sulfate is oxidized to ferric sulfate by addition of sulfuric and nitric acid. Upon addition of ammonium sulfate to the solution and damping in of the solution, ferric ammonium sulfate crystals precipitate. Equations for these conversions ignore the degree of hydration of the material.
- Oxidation: 6 FeSO4 + 2 HNO3 + 3 H2SO4 → 3 Fe2(SO4)3 + 2 NO + 4 H2O
- Synthesis: Fe2(SO4)3 + (NH4)2SO4 → 2 NH4Fe(SO4)2
Uses
Areas of use for FAS include waste water treatment, tanning, production of dyestuffs, and as an etching agent in the production of electronic components. It has been used in a wide area of applications, including adiabatic refrigeration equipment, biochemical analysis, and organic synthesis.
Gallery
References
- "Material Safety Data Sheet. Iron (III) Ammonium Sulfate Dodecahydrate". fscimage.fishersci.com. Retrieved 8 June 2023.
- Considine, Douglas M: Chemical and process technology encyclopedia, McGraw-Hill, New York, 1974, p. 993
- Christensen, Odin T. "On the Cause of the Amethyst Color of Ferric Alum and of Mixed Crystals of Ferric and Manganic Alum". Chem. Lab. Roy. Vet. Agr. Hochschule, KGL. Danske Vidsk. Selsk. Forh. 1906: 173–95.
- Bonnell, Jane; Philip Perman, Edgar (1921). "CCXXIX.—The colour of iron alum". J. Chem. Soc., Trans. 119: 1994–1997. doi:10.1039/CT9211901994.
- Cooke, Meyer; Wolf (1956). "The Specific Heats of Three Paramagnetic salts at Very Low Temperatures". Proceedings of the Royal Society of London. Series A, Mathematical and Physical Sciences. 237 (1210): 395–403. Bibcode:1956RSPSA.237..395C. doi:10.1098/rspa.1956.0185. S2CID 97076961.
- Wang, Fei; et al. (2008). "Microcalorimetric investigation of the toxic action of ammonium ferric(III)sulfate on the metabolic activity of pure microbes". Environmental Toxicology and Pharmacology. 25 (3): 351–357. Bibcode:2008EnvTP..25..351W. doi:10.1016/j.etap.2007.11.004. PMID 21783873.
- ^ Wiley Encyclopedia of inorganic chemistry: Volume 4, p. 1704:
- Chen et al.: United States Patent 5518131 – "Etching molydbenum with ferric sulfate and ferric ammonium sulfate"
- Grant W. Wilson, Peter T. Timbie: "Construction techniques for adiabatic demagnetization refrigerators using ferric ammonium alum". Cryogenics, Volume 39, Number 4, (1999), pp. 319–322
- J. C. Whitehorn: "A system of blood analysis. Supplement II. Simplified method for the determination of chlorides in blood or plasma". Journal of Biological Chemistry (1921), 45 p. 449–60.
- Yu, Shanxin; et al. (2005). "Application of ammonium ferric sulfate dodecahydrate in organic synthesis". General Review. 17 (1): 27–30.
| Iron compounds | |||
|---|---|---|---|
| Fe(−II) | |||
| Fe(0) | |||
| Fe(I) |
| ||
| Fe(0,II) | |||
| Fe(II) |
| ||
| Fe(0,III) | |||
| Fe(II,III) | |||
| Fe(III) |
| ||
| Fe(IV) | |||
| Fe(VI) | |||
| Purported | |||
| sort | |||
| Compounds containing the sulfate group (SO2−4) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||

