| Revision as of 14:24, 14 October 2011 edit193.156.99.179 (talk)No edit summary← Previous edit | Latest revision as of 17:06, 3 December 2024 edit undo2603:8080:13f0:8200:5048:344c:cada:a935 (talk) →Animal studies: Fixed sentence structure and grammar, I love how you can discern the exact parts of these pages written by the disso addictsTags: Mobile edit Mobile web edit | ||
| (33 intermediate revisions by 26 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Other uses|AP-7 (disambiguation)}} | |||
| {{chembox | {{chembox | ||
| | Verifiedfields = changed | |||
| ⚫ | | verifiedrevid = |
||
| | Watchedfields = changed | |||
| ⚫ | |ImageFile=AP-7 2D-Structure.svg | ||
| ⚫ | | verifiedrevid = 477236252 | ||
| ⚫ | |ImageSize=240px | ||
| ⚫ | | ImageFile=AP-7 2D-Structure.svg | ||
| ⚫ | | |
||
| ⚫ | | ImageSize=240px | ||
| ⚫ | |OtherNames= | ||
| | Name = AP-7 | |||
| ⚫ | |Section1= |
||
| ⚫ | | PIN=2-Amino-7-phosphonoheptanoic acid | ||
| ⚫ | | |
||
| ⚫ | | OtherNames= | ||
| ⚫ | |Section1={{Chembox Identifiers | ||
| ⚫ | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 3010 | | ChemSpiderID = 3010 | ||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | | ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| Line 17: | Line 18: | ||
| | StdInChIKey = MYDMWESTDPJANS-UHFFFAOYSA-N | | StdInChIKey = MYDMWESTDPJANS-UHFFFAOYSA-N | ||
| | SMILES1 = O=P(O)(O)CCCCCC(C(=O)O)N | | SMILES1 = O=P(O)(O)CCCCCC(C(=O)O)N | ||
| | CASNo_Ref = {{cascite|correct| |
| CASNo_Ref = {{cascite|correct|CAS}} | ||
| | CASNo=85797-13-3<!--not validated by ]--> | | CASNo=85797-13-3<!--not validated by ]--> | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| ⚫ | | |
||
| | UNII = P34K80CUSM | |||
| ⚫ | | |
||
| ⚫ | | PubChem=3122 | ||
| ⚫ | | |
||
| ⚫ | | SMILES=O=P(O)(O)CCCCCC(N)C(=O)O | ||
| ⚫ | | |
||
| ⚫ | | InChI=1/C7H16NO5P/c8-6(7(9)10)4-2-1-3-5-14(11,12)13/h6H,1-5,8H2,(H,9,10)(H2,11,12,13) | ||
| ⚫ | |||
| ⚫ | | MeSHName= | ||
| ⚫ | |Section2= |
||
| ⚫ | }} | ||
| ⚫ | | |
||
| ⚫ | |Section2={{Chembox Properties | ||
| ⚫ | | |
||
| ⚫ | | Formula=C<sub>7</sub>H<sub>16</sub>NO<sub>5</sub>P | ||
| | Appearance= | |||
| ⚫ | | MolarMass=225.179 g/mol | ||
| ⚫ | | |
||
| | |
| Appearance= | ||
| ⚫ | | Density=1.39 g/mL | ||
| | BoilingPt= | |||
| | |
| MeltingPt= | ||
| | BoilingPtC= 480.1 | |||
| ⚫ | |||
| | Solubility= | |||
| ⚫ | |Section3= |
||
| ⚫ | }} | ||
| ⚫ | | |
||
| ⚫ | |Section3={{Chembox Hazards | ||
| ⚫ | | |
||
| ⚫ | | MainHazards= | ||
| | Autoignition= | |||
| ⚫ | | FlashPt= | ||
| ⚫ | |||
| | AutoignitionPt = | |||
| ⚫ | }} | ||
| }} | }} | ||
| ⚫ | '''AP-7''' is a selective ] that ] the glutamate binding site and thus activation of ]. It has ] effects.<ref name="pmid3047315">{{cite journal |vauthors=Meldrum B, Millan M, Patel S, de Sarro G | title = Anti-epileptic effects of focal micro-injection of excitatory amino acid antagonists | journal = J. Neural Transm. | volume = 72 | issue = 3 | pages = 191–200 | year = 1988 | pmid = 3047315 | doi = 10.1007/BF01243419 | s2cid = 6216838 }}</ref> | ||
| AP-7 functions specifically as a NMDA recognition site blocker, in contrast with ], which acts as a glycine site modulation blocker.<ref name="pmid22248144">{{cite journal | author = Guillemin GJ | title = Quinolinic acid, the inescapable neurotoxin | journal = FEBS J. | volume = 279 | issue = 8 | pages = 1356–65 |date=April 2012 | pmid = 22248144 | doi = 10.1111/j.1742-4658.2012.08485.x | s2cid = 205884904 | doi-access = free }}</ref> | |||
| ⚫ | '''AP-7''' is a selective ] that ] the glutamate binding site and thus activation of ]. It has ] effects.<ref>Meldrum B, Millan M, Patel S, de Sarro G |
||
| == Animal studies == | |||
| AP-7 injected directly into the dorsal periaqueductal grey (DPAG) of rats produced an anxiolytic effect, whereas direct injection outside of the DPAG did not elicit anxiolytic effects, suggesting that the anxiolytic effect of NMDA antagonists in rats may depend on their action in the DPAG.<ref name="pmid1672463">{{cite journal |vauthors=Guimarães FS, Carobrez AP, De Aguiar JC, Graeff FG | title = Anxiolytic effect in the elevated plus-maze of the NMDA receptor antagonist AP7 microinjected into the dorsal periaqueductal grey | journal = Psychopharmacology | volume = 103 | issue = 1 | pages = 91–4 | year = 1991 | pmid = 1672463 | doi = 10.1007/BF02244080 | s2cid = 6498237 }}</ref> | |||
| The DPAG of the brain is thought to deal with fear-like defensive behavior via NMDA and glycine B receptors.<ref name="pmid11801295">{{cite journal |vauthors=Carobrez AP, Teixeira KV, Graeff FG | title = Modulation of defensive behavior by periaqueductal gray NMDA/glycine-B receptor | journal = Neurosci Biobehav Rev | volume = 25 | issue = 7–8 | pages = 697–709 |date=December 2001 | pmid = 11801295 | doi = 10.1016/S0149-7634(01)00059-8 | s2cid = 28687622 }}</ref> These excitatory glutamate receptors work with the inhibitory GABA receptors to achieve equilibrium in the DPAG of the brain.<ref name="pmid9512072">{{cite journal |vauthors=Car H, Wiśniewski K | title = The effect of baclofen and AP-7 on selected behavior in rats | journal = Pharmacol. Biochem. Behav. | volume = 59 | issue = 3 | pages = 685–9 |date=March 1998 | pmid = 9512072 | doi = 10.1016/S0091-3057(97)00462-0 | s2cid = 37405373 }}</ref> | |||
| AP-7 has been known to cause muscle rigidity and catalepsy in rats following bilateral microinjections (0.02-0.5 nmol) into the globus pallidus and ventral-posterior portions of the caudate-putamen.<ref name="pmid2159826">{{cite journal |vauthors=Turski L, Klockgether T, Turski WA, Schwarz M, Sontag KH | title = Blockade of excitatory neurotransmission in the globus pallidus induces rigidity and akinesia in the rat: implications for excitatory neurotransmission in pathogenesis of Parkinson's diseases | journal = Brain Res. | volume = 512 | issue = 1 | pages = 125–31 |date=March 1990 | pmid = 2159826 | doi = 10.1016/0006-8993(90)91180-O | s2cid = 37123476 }}</ref> | |||
| The optically pure D-(−)-2-amino-7-phosphonoheptanoic acid , has also been examined. In groups of hypoxia-treated rats, D-AP7 enhanced motility, exhibited anxiogenic-like effect and impaired consolidation in passive avoidance. Both AP-7 and D-AP7 function as potent, specific antagonists of the NMDA receptor.<ref name="pmid14506312">{{cite journal |vauthors=Nadlewska A, Car H, Wiśniewska R, Hoły Z, Wiśniewski K | title = Behavioral effects of D-AP7 in rats subjected to experimental hypoxia | journal = Pol J Pharmacol | volume = 55 | issue = 3 | pages = 337–44 | year = 2003 | pmid = 14506312 | url = http://rabbit.if-pan.krakow.pl/pjp/pdf/2003/3_337.pdf }}</ref> | |||
| ==See also== | ==See also== | ||
| Line 46: | Line 59: | ||
| ==References== | ==References== | ||
| {{reflist}} | |||
| <references/> | |||
| {{Ionotropic glutamate receptor modulators}} | |||
| {{Glutamate_receptor_ligands}} | |||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| {{pharm-stub}} | |||
Latest revision as of 17:06, 3 December 2024
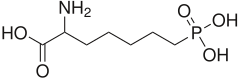
| |
| Names | |
|---|---|
| Preferred IUPAC name 2-Amino-7-phosphonoheptanoic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C7H16NO5P |
| Molar mass | 225.179 g/mol |
| Density | 1.39 g/mL |
| Boiling point | 480.1 °C (896.2 °F; 753.2 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
AP-7 is a selective NMDA receptor (NMDAR) antagonist that competitively inhibits the glutamate binding site and thus activation of NMDAR. It has anticonvulsant effects.
AP-7 functions specifically as a NMDA recognition site blocker, in contrast with 7-chlorokynurenate, which acts as a glycine site modulation blocker.
Animal studies
AP-7 injected directly into the dorsal periaqueductal grey (DPAG) of rats produced an anxiolytic effect, whereas direct injection outside of the DPAG did not elicit anxiolytic effects, suggesting that the anxiolytic effect of NMDA antagonists in rats may depend on their action in the DPAG.
The DPAG of the brain is thought to deal with fear-like defensive behavior via NMDA and glycine B receptors. These excitatory glutamate receptors work with the inhibitory GABA receptors to achieve equilibrium in the DPAG of the brain.
AP-7 has been known to cause muscle rigidity and catalepsy in rats following bilateral microinjections (0.02-0.5 nmol) into the globus pallidus and ventral-posterior portions of the caudate-putamen.
The optically pure D-(−)-2-amino-7-phosphonoheptanoic acid , has also been examined. In groups of hypoxia-treated rats, D-AP7 enhanced motility, exhibited anxiogenic-like effect and impaired consolidation in passive avoidance. Both AP-7 and D-AP7 function as potent, specific antagonists of the NMDA receptor.
See also
References
- Meldrum B, Millan M, Patel S, de Sarro G (1988). "Anti-epileptic effects of focal micro-injection of excitatory amino acid antagonists". J. Neural Transm. 72 (3): 191–200. doi:10.1007/BF01243419. PMID 3047315. S2CID 6216838.
- Guillemin GJ (April 2012). "Quinolinic acid, the inescapable neurotoxin". FEBS J. 279 (8): 1356–65. doi:10.1111/j.1742-4658.2012.08485.x. PMID 22248144. S2CID 205884904.
- Guimarães FS, Carobrez AP, De Aguiar JC, Graeff FG (1991). "Anxiolytic effect in the elevated plus-maze of the NMDA receptor antagonist AP7 microinjected into the dorsal periaqueductal grey". Psychopharmacology. 103 (1): 91–4. doi:10.1007/BF02244080. PMID 1672463. S2CID 6498237.
- Carobrez AP, Teixeira KV, Graeff FG (December 2001). "Modulation of defensive behavior by periaqueductal gray NMDA/glycine-B receptor". Neurosci Biobehav Rev. 25 (7–8): 697–709. doi:10.1016/S0149-7634(01)00059-8. PMID 11801295. S2CID 28687622.
- Car H, Wiśniewski K (March 1998). "The effect of baclofen and AP-7 on selected behavior in rats". Pharmacol. Biochem. Behav. 59 (3): 685–9. doi:10.1016/S0091-3057(97)00462-0. PMID 9512072. S2CID 37405373.
- Turski L, Klockgether T, Turski WA, Schwarz M, Sontag KH (March 1990). "Blockade of excitatory neurotransmission in the globus pallidus induces rigidity and akinesia in the rat: implications for excitatory neurotransmission in pathogenesis of Parkinson's diseases". Brain Res. 512 (1): 125–31. doi:10.1016/0006-8993(90)91180-O. PMID 2159826. S2CID 37123476.
- Nadlewska A, Car H, Wiśniewska R, Hoły Z, Wiśniewski K (2003). "Behavioral effects of D-AP7 in rats subjected to experimental hypoxia" (PDF). Pol J Pharmacol. 55 (3): 337–44. PMID 14506312.