| Revision as of 10:41, 27 October 2011 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'DrugBank').← Previous edit | Latest revision as of 22:34, 4 January 2025 edit undoՍահակ (talk | contribs)Extended confirmed users6,079 edits →Further reading | ||
| Line 1: | Line 1: | ||
| {{Short description|Phytocannabinoid discovered in 1940}} | |||
| {{Drugbox | |||
| {{Distinguish|cannabinol|cannabinodiol}} | |||
| | Verifiedfields = changed | |||
| {{Use mdy dates|date=August 2024}} | |||
| | Watchedfields = changed | |||
| {{cs1 config|name-list-style=vanc|display-authors=6}} | |||
| | verifiedrevid = 399704031 | |||
| {{Infobox drug | |||
| | IUPAC_name = 2--5-pentylbenzene-1,3-diol | |||
| | Verifiedfields = verified | |||
| | image = Cannabidiol.png | |||
| | Watchedfields = verified | |||
| | width = 230px | |||
| | verifiedrevid = 457636058 | |||
| | image = Cannabidiol.svg | |||
| | width = 225 | |||
| | alt = | |||
| | caption = | |||
| | image2 = CBD-3D-balls.png | | image2 = CBD-3D-balls.png | ||
| | width2 = |
| width2 = 250 | ||
| <!--Clinical data--> | <!-- Clinical data -->| pronounce = {{IPAc-en|k|æ|.|n|ə|.|b|ə|.|ˈ|d|aɪ|.|əl}} | ||
| | tradename = | | tradename = Epidiolex, Epidyolex | ||
| | Drugs.com = {{drugs.com| |
| Drugs.com = {{drugs.com|monograph|cannabidiol}} | ||
| | MedlinePlus = a618051 | |||
| | legal_status = Schedule II (Can)<br>Unscheduled (USA) | |||
| | DailyMedID = Cannabidiol | |||
| | pregnancy_AU = B2 | |||
| | pregnancy_AU_comment = <ref name="Epidyolex TGA">{{cite web | title=Epidyolex | website=Therapeutic Goods Administration (TGA) | date=September 29, 2020 | url=https://www.tga.gov.au/apm-summary/epidyolex | access-date=September 30, 2020 | archive-date=October 30, 2021 | archive-url=https://web.archive.org/web/20211030203454/https://www.tga.gov.au/apm-summary/epidyolex | url-status=live }}</ref> | |||
| | addiction_liability = None <ref name="fedreg-v">{{cite web |title=Schedules of Controlled Substances: Placement in Schedule V of Certain FDA-Approved Drugs Containing Cannabidiol; Corresponding Change to Permit Requirements|publisher=Federal Register, US Federal Government|url=https://www.federalregister.gov/documents/2018/09/28/2018-21121/schedules-of-controlled-substances-placement-in-schedule-v-of-certain-fda-approved-drugs-containing |date=September 28, 2018 |access-date=March 10, 2024}}</ref> | |||
| | routes_of_administration = ],<ref name="Epidiolex FDA label">{{cite web | title=Epidiolex – cannabidiol solution | website=DailyMed | date=August 26, 2020 | url=https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8bf27097-4870-43fb-94f0-f3d0871d1eec | access-date=September 11, 2020 | archive-date=February 25, 2021 | archive-url=https://web.archive.org/web/20210225194817/https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=8bf27097-4870-43fb-94f0-f3d0871d1eec | url-status=live }}</ref> ], ],<ref name="SativexLabel">{{cite web|url=https://omr.bayer.ca/omr/online/sativex-pm-en.pdf|title=Sativex (Cannabidiol/Tetrahydrocannabinol) Bayer Label|website=bayer.ca|access-date=June 28, 2018|archive-date=January 16, 2021|archive-url=https://web.archive.org/web/20210116114657/https://omr.bayer.ca/omr/online/sativex-pm-en.pdf|url-status=live}}</ref>] | |||
| | class = ] | |||
| | ATC_prefix = N03 | |||
| | ATC_suffix = AX24 | |||
| <!-- Legal status -->| legal_AU = S4 | |||
| <!--Identifiers--> | |||
| | legal_AU_comment = | |||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | legal_BR = Synthetic: ]; Oil with <0,2% THC: ]; Oil <30mg/ml THC and <30mg/ml CBD: ]. | |||
| | CAS_number_Ref = {{cascite|correct|??}} | |||
| | legal_BR_comment = <ref>{{Cite web |author=Anvisa |author-link=Brazilian Health Regulatory Agency |date=July 24, 2023 |title=RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial |trans-title=Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control|url=https://www.in.gov.br/en/web/dou/-/resolucao-rdc-n-804-de-24-de-julho-de-2023-498447451 |url-status=live |archive-url=https://web.archive.org/web/20230827163149/https://www.in.gov.br/en/web/dou/-/resolucao-rdc-n-804-de-24-de-julho-de-2023-498447451 |archive-date=August 27, 2023 |access-date=August 27, 2023 |publisher=] |language=pt-BR |publication-date=July 25, 2023}}</ref> | |||
| | legal_CA = Unscheduled | |||
| | legal_CA_comment = | |||
| | legal_DE = Anlage III <!-- Anlage I, II, III or Unscheduled --> | |||
| | legal_DE_comment = | |||
| | legal_NZ = Unscheduled <!-- Class A, B, C --> | |||
| | legal_NZ_comment = | |||
| | legal_UK = GSL | |||
| | legal_UK_comment = <ref name="Home Office"/> | |||
| | legal_US = Rx-only | |||
| | legal_US_comment = as Epidiolex.<ref name="Epidiolex FDA label"/> Unscheduled if derived from hemp with less than 0.3% Δ<sup>9</sup>-THC, otherwise ].<ref>{{cite web | title=DEA announces steps necessary to improve access to marijuana research | website=DEA | date=26 August 2019 | url=https://www.dea.gov/press-releases/2019/08/26/dea-announces-steps-necessary-improve-access-marijuana-research | access-date=7 September 2024}}</ref><ref>{{cite web | title=7 U.S. Code § 1639o | website=LII / Legal Information Institute | url=https://www.law.cornell.edu/uscode/text/7/1639o | access-date=7 September 2024}}</ref><ref>{{cite web | last=Laurence | first=Emily | title=Your Guide To CBD Legalization By State | website=Forbes Health | date=31 May 2022 | url=https://www.forbes.com/health/cbd/cbd-legalization-by-state/ | access-date=7 September 2024}}</ref> | |||
| | legal_EU = Rx-only | |||
| | legal_EU_comment = <ref name="Epidyolex EPAR" /> and OTC in some member states | |||
| | legal_UN = Unscheduled<!-- N I, II, III, IV / P I, II, III, IV --> | |||
| | legal_UN_comment = {{cn|date=August 2024}} | |||
| | legal_status = Rx-only{{cn|date=August 2024}} | |||
| <!-- Pharmacokinetic data -->| bioavailability = {{ubl|]: 6% (fasted); 36–57% (fed-state)<ref name = Mechoulam2002>{{cite journal | vauthors = Perucca E, Bialer M | title = Critical Aspects Affecting Cannabidiol Oral Bioavailability and Metabolic Elimination, and Related Clinical Implications | journal = CNS Drugs | volume = 34 | issue = 8 | pages = 795–800 | date = June 5, 2020 | pmid = 32504461 | doi = 10.1007/s40263-020-00741-5 | s2cid = 219313952 }}</ref>|]: 31% (range 11–45%)<ref name=Scuderi2009>{{cite journal | vauthors = Scuderi C, Filippis DD, Iuvone T, Blasio A, Steardo A, Esposito G | title = Cannabidiol in medicine: a review of its therapeutic potential in CNS disorders | journal = Phytotherapy Research | volume = 23 | issue = 5 | pages = 597–602 | date = May 2009 | pmid = 18844286 | doi = 10.1002/ptr.2625 | url = https://zenodo.org/record/1067705 | type = Review | s2cid = 21836765 | access-date = May 22, 2020 | archive-date = April 11, 2021 | archive-url = https://web.archive.org/web/20210411045636/https://zenodo.org/record/1067705 | url-status = live }}</ref>}} ]: 12–35%<ref>{{Cite journal |last=Hossain |first=Khondker |date=25 September 2023 |title=Current Challenges and Opportunities for Improved Cannabidiol Solubility |journal=International Journal of Molecular Sciences|volume=24 |issue=19 |page=14514 |doi=10.3390/ijms241914514 |doi-access=free |pmid=37833962 |pmc=10572536 }}</ref> | |||
| | protein_bound = | |||
| | metabolism = | |||
| | metabolites = | |||
| | onset = | |||
| | elimination_half-life = 18–32 hours<ref name=devinsky/> | |||
| | duration_of_action = | |||
| | excretion = <!-- Identifiers --> | |||
| | CAS_number_Ref = {{cascite|correct|CAS}} | |||
| | CAS_number = 13956-29-1 | | CAS_number = 13956-29-1 | ||
| | ATC_prefix = no | |||
| | ATC_suffix = entry | |||
| | PubChem = 644019 | | PubChem = 644019 | ||
| | IUPHAR_ligand = 4150 | |||
| | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| | DrugBank = |
| DrugBank = DB09061 | ||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = |
| ChemSpiderID = 559095 | ||
| | UNII_Ref = {{fdacite| |
| UNII_Ref = {{fdacite|correct|FDA}} | ||
| | UNII = 19GBJ60SN5 | | UNII = 19GBJ60SN5 | ||
| | KEGG_Ref = | |||
| | KEGG = D10915 | |||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | ChEBI = 69478 | |||
| | ChEMBL_Ref = | |||
| | ChEMBL = | |||
| | NIAID_ChemDB = | |||
| | PDB_ligand = P0T | |||
| | synonyms = CBD, cannabidiolum, (−)-cannabidiol<ref>{{cite web|url=https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:69478|title=cannabidiol (CHEBI:69478)|website=ebi.ac.uk|access-date=February 12, 2019|archive-date=May 12, 2021|archive-url=https://web.archive.org/web/20210512151607/https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:69478|url-status=live}}</ref> | |||
| <!-- Chemical data -->| IUPAC_name = 2--5-pentylbenzene-1,3-diol | |||
| <!--Chemical data--> | |||
| | C |
| C = 21 | ||
| | H = 30 | |||
| | molecular_weight = 314.46 | |||
| | O = 2 | |||
| | smiles = Oc1c(c(O)cc(c1)CCCCC)2\C=C(/CC2\C(=C)C)C | |||
| | SMILES = Oc1c(c(O)cc(c1)CCCCC)2\C=C(/CC2\C(=C)C)C | |||
| | InChI = 1/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | |||
| | StdInChI_Ref = {PubChem} | |||
| | InChIKey = QHMBSVQNZZTUGM-ZWKOTPCHBJ | |||
| | StdInChI = 1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h11-13,17-18,22-23H,2,5-10H2,1,3-4H3/t17-,18+/m0/s1 | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChI_comment = | |||
| | StdInChI = 1S/C21H30O2/c1-5-6-7-8-16-12-19(22)21(20(23)13-16)18-11-15(4)9-10-17(18)14(2)3/h9,12-13,17-18,22-23H,2,5-8,10-11H2,1,3-4H3/t17-,18+/m0/s1 | |||
| | StdInChIKey_Ref = { |
| StdInChIKey_Ref = {PubChem} | ||
| | StdInChIKey = |
| StdInChIKey = QHMBSVQNZZTUGM-ZWKOTPCHSA-N | ||
| <!-- Physical data -->| density = | |||
| | density_notes = | |||
| | melting_point = 66 | | melting_point = 66 | ||
| | |
| melting_high = | ||
| | melting_notes = | |||
| | boiling_notes = <br>(Range: 160°C-180°C) <ref>McPartland JM, Russo EB. (2001). . ''Journal of Cannabis Therapeutics''. 1(3/4):103-132.</ref> | |||
| | boiling_point = 160-180 | |||
| | boiling_notes = <ref name="projectcbd">{{cite web |url=https://www.projectcbd.org/sites/projectcbd/files/downloads/cannabinoid-boiling-points-thc-cbd.pdf |title=Phytocannabinoid Boiling Points |website=Project CBD |access-date=August 20, 2021 |url-status=live|archive-url=https://web.archive.org/web/20190408144226/https://www.projectcbd.org/sites/projectcbd/files/downloads/cannabinoid-boiling-points-thc-cbd.pdf |archive-date=April 8, 2019 }}</ref>{{Unreliable medical source|date=July 2024}} | |||
| | solubility = Insoluble | |||
| | sol_units = | |||
| | specific_rotation = | |||
| }} | }} | ||
| {{Cannabis sidebar}} | |||
| <!-- Definition and medical uses/non-uses --> | |||
| '''Cannabidiol''' ('''CBD''') is a ], one of 113 identified ]s in ] plants, along with ] (THC), and accounts for up to 40% of the plant's extract.<ref name="pmid23108553" /> Medically, it is an ] used to treat multiple forms of ].<ref name="Epidiolex FDA label" /> It was discovered in 1940 and, as of 2024 clinical research on CBD included studies related to the treatment of ], ], ], ], and ], but there is insufficient ] that CBD is effective for these conditions.<ref name="kirkland">{{cite journal |vauthors=Kirkland AE, Fadus MC, Gruber SA, Gray KM, Wilens TE, Squeglia LM |title=A scoping review of the use of cannabidiol in psychiatric disorders |journal=Psychiatry Research |volume=308 |issue= |pages=114347 |date=February 2022 |pmid=34952255 |pmc=8799523 |doi=10.1016/j.psychres.2021.114347}}</ref><ref name="black">{{cite journal | vauthors = Black N, Stockings E, Campbell G, Tran LT, Zagic D, Hall WD, Farrell M, Degenhardt L | title = Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis | journal = The Lancet. Psychiatry | volume = 6 | issue = 12 | pages = 995–1010 | date = December 2019 | pmid = 31672337 | pmc = 6949116 | doi = 10.1016/S2215-0366(19)30401-8 }}</ref><ref name="mayo">{{cite journal | vauthors = VanDolah HJ, Bauer BA, Mauck KF | title = Clinicians' Guide to Cannabidiol and Hemp Oils | journal = Mayo Clinic Proceedings | volume = 94 | issue = 9 | pages = 1840–1851 | date = September 2019 | pmid = 31447137 | doi = 10.1016/j.mayocp.2019.01.003 | doi-access = free }}</ref><ref>{{cite journal | vauthors = Prud'homme M, Cata R, Jutras-Aswad D | title = Cannabidiol as an Intervention for Addictive Behaviors: A Systematic Review of the Evidence | journal = Substance Abuse | volume = 9 | pages = 33–38 | date = January 2015 | pmid = 26056464 | pmc = 4444130 | doi = 10.4137/SART.S25081 }}</ref> CBD is sold as an herbal ] and promoted with yet unproven claims of particular therapeutic effects.<ref name=sbm/> | |||
| <!-- Administration and effects --> | |||
| '''Cannabidiol''' ('''CBD''') is a ] found in '']''. It is a major constituent of the plant, representing up to 40% in its extracts.<ref name="Grlie_1976">{{cite journal | |||
| Cannabidiol can be ] in multiple ways, including by ] cannabis ] or ], ], and through use of an ] into the ].<ref name="lipo">{{cite journal | vauthors = Itin C, Barasch D, Domb AJ, Hoffman A | title = Prolonged oral transmucosal delivery of highly lipophilic drug cannabidiol | journal = International Journal of Pharmaceutics | volume = 581 | pages = 119276 | date = May 2020 | pmid = 32243971 | doi = 10.1016/j.ijpharm.2020.119276 | s2cid = 214785913 }}</ref><ref name="MetaOpinion">{{cite journal | vauthors = Itin C, Domb AJ, Hoffman A | title = A meta-opinion: cannabinoids delivered to oral mucosa by a spray for systemic absorption are rather ingested into gastro-intestinal tract: the influences of fed / fasting states | journal = Expert Opinion on Drug Delivery | volume = 16 | issue = 10 | pages = 1031–1035 | date = October 2019 | pmid = 31393180 | doi = 10.1080/17425247.2019.1653852 | s2cid = 199505274 }}</ref> It may be supplied as CBD oil containing only CBD as the active ingredient (excluding THC or ]s), CBD-dominant ] ] oil, capsules, dried cannabis, or prescription liquid ].<ref name="Epidiolex FDA label" /><ref name=mayo/> CBD does not have the same ] as ],<ref name="pmid28232276" /><ref name="Iseger2015">{{cite journal | vauthors = Iseger TA, Bossong MG | title = A systematic review of the antipsychotic properties of cannabidiol in humans | journal = Schizophrenia Research | volume = 162 | issue = 1–3 | pages = 153–161 | date = March 2015 | pmid = 25667194 | doi = 10.1016/j.schres.2015.01.033 | s2cid = 3745655 }}</ref> and can modulate the psychoactive effects of THC on the body if both are present.<ref name="pmid23108553">{{cite journal | vauthors = Campos AC, Moreira FA, Gomes FV, Del Bel EA, Guimarães FS | title = Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders | journal = Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences | volume = 367 | issue = 1607 | pages = 3364–3378 | date = December 2012 | pmid = 23108553 | pmc = 3481531 | doi = 10.1098/rstb.2011.0389 | type = Review }}</ref><ref name="pmid28232276">{{cite journal | vauthors = Pisanti S, Malfitano AM, Ciaglia E, Lamberti A, Ranieri R, Cuomo G, Abate M, Faggiana G, Proto MC, Fiore D, Laezza C, Bifulco M | title = Cannabidiol: State of the art and new challenges for therapeutic applications | journal = Pharmacology & Therapeutics | volume = 175 | pages = 133–150 | date = July 2017 | pmid = 28232276 | doi = 10.1016/j.pharmthera.2017.02.041 }}</ref><ref name="Boggs">{{cite journal | vauthors = Boggs DL, Nguyen JD, Morgenson D, Taffe MA, Ranganathan M | title = Clinical and Preclinical Evidence for Functional Interactions of Cannabidiol and Δ{{sup|9}}-Tetrahydrocannabinol | journal = Neuropsychopharmacology | volume = 43 | issue = 1 | pages = 142–154 | date = January 2018 | pmid = 28875990 | pmc = 5719112 | doi = 10.1038/npp.2017.209 }}</ref><ref name="pmid26836472">{{cite journal | vauthors = Aizpurua-Olaizola O, Soydaner U, Öztürk E, Schibano D, Simsir Y, Navarro P, Etxebarria N, Usobiaga A | title = Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes | journal = Journal of Natural Products | volume = 79 | issue = 2 | pages = 324–331 | date = February 2016 | pmid = 26836472 | doi = 10.1021/acs.jnatprod.5b00949 | hdl = 1874/350973 | url = https://figshare.com/articles/journal_contribution/5028338 | access-date = November 27, 2022 | archive-date = January 5, 2023 | archive-url = https://web.archive.org/web/20230105025827/https://figshare.com/articles/journal_contribution/Evolution_of_the_Cannabinoid_and_Terpene_Content_during_the_Growth_of_Cannabis_sativa_Plants_from_Different_Chemotypes/5028338 | url-status = live }}</ref> ] can occur when CBD is heated to temperatures between 250–300 °C, potentially leading to its partial transformation into THC.<ref name = "Czégény_2021">{{cite journal | vauthors = Czégény Z, Nagy G, Babinszki B, Bajtel Á, Sebestyén Z, Kiss T, Csupor-Löffler B, Tóth B, Csupor D | title = CBD, a precursor of THC in e-cigarettes | journal = Scientific Reports | volume = 11 | issue = 1 | pages = 8951 | date = April 2021 | pmid = 33903673 | pmc = 8076212 | doi = 10.1038/s41598-021-88389-z | bibcode = 2021NatSR..11.8951C }}</ref> | |||
| | last =Grlie | |||
| | first = L | |||
| | authorlink = | |||
| | coauthors = | |||
| | title =A comparative study on some chemical and biological characteristics of various samples of cannabis resin | |||
| | journal =Bulletin on Narcotics | |||
| | volume =14 | |||
| | issue = | |||
| | pages =37–46 | |||
| | publisher = | |||
| | year = 1976 | |||
| | url = | |||
| | doi = | |||
| | id = | |||
| | accessdate = }}</ref> | |||
| <!-- Regulation, control, pharmaceutical --> | |||
| It has displayed ] effects in animal tests.<ref name="pmid6269680">{{cite journal | |||
| In the United States, the cannabidiol drug '''Epidiolex''' was approved by the ] (FDA) in 2018 for the treatment of two ].<ref name="Epidiolex FDA label" /> While the ] removed hemp and hemp extracts (including CBD) from the ], the marketing and sale of CBD formulations for medical use or as an ingredient in dietary supplements or manufactured foods remains illegal under FDA regulation, {{as of|2024|lc=y}}.<ref name="Mead 2019">{{cite journal | vauthors = Mead A | title = Legal and Regulatory Issues Governing Cannabis and Cannabis-Derived Products in the United States | journal = Frontiers in Plant Science | volume = 10 | pages = 697 | date = June 14, 2019 | pmid = 31263468 | pmc = 6590107 | doi = 10.3389/fpls.2019.00697 | doi-access = free }}</ref><ref name="fdareg-hemp">{{cite web |title=FDA Regulation of Cannabis and Cannabis-Derived Products, Including Cannabidiol (CBD). #2. How does the 2018 Farm Bill define hemp? What does it mean for FDA-regulated products? |url=https://www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-including-cannabidiol-cbd |publisher=US ] (FDA) |access-date=February 6, 2024 |date=February 6, 2024}}</ref> | |||
| | author=Pickens JT | |||
| {{TOC limit}} | |||
| | title=Sedative activity of cannabis in relation to its delta'-trans-tetrahydrocannabinol and cannabidiol content | |||
| | journal=Br. J. Pharmacol. | |||
| | volume=72 | |||
| | issue=4 | |||
| | pages=649–56 | |||
| | year=1981 | |||
| | pmid=6269680 | |||
| | doi= | |||
| | pmc=2071638 | |||
| }}</ref> Some research, however, indicates that CBD can increase alertness.<ref>{{cite journal | |||
| | last = Nicholson | |||
| | first = AN | |||
| | authorlink = | |||
| | coauthors = C Turner, BM Stone, and PJ Robson | |||
| | year = 2004 | |||
| | month = June | |||
| | title = Effect of Delta-9-tetrahydrocannabinol and cannabidiol on nocturnal sleep and early-morning behavior in young adults | |||
| | journal = J Clin Psychopharmacol | |||
| | volume = 24 | |||
| | issue = 3 | |||
| | pages = 305–13 | |||
| | issn = 0271-0749 | |||
| | pmid = 15118485 | |||
| | url = http://meta.wkhealth.com/pt/pt-core/template-journal/lwwgateway/media/landingpage.htm?issn=0271-0749&volume=24&issue=3&spage=305 | |||
| | format = fee required | |||
| | accessdate = 2007-05-03 | |||
| | doi = 10.1097/01.jcp.0000125688.05091.8f }}</ref> | |||
| It may decrease the rate of THC clearance from the body, perhaps by interfering with the ] of THC in the ]. | |||
| ==Medical uses== | |||
| Medically, it has been shown to relieve ], ], ], and ], as well as inhibit ] growth.<ref name="recentadvances">{{cite journal | |||
| | last = Mechoulam | |||
| | first = R. | |||
| | authorlink = | |||
| | coauthors = M. Peters, Murillo-Rodriguez | |||
| | title = Cannabidiol - recent advances | |||
| | journal = Chemistry & Biodiversity | |||
| | volume = 4 | |||
| | issue = 8 | |||
| | pages = 1678–1692 | |||
| | publisher = | |||
| | date = 21 Aug 2007 | |||
| | url = http://www3.interscience.wiley.com/journal/115806131/abstract | |||
| | pmid = 17712814 | |||
| | doi =10.1002/cbdv.200790147 | |||
| }}</ref> Recent studies have shown cannabidiol to be as effective as ] in treating ].<ref name="braz">{{cite journal | |||
| | last = Zuardi | |||
| | first = A.W | |||
| | coauthors = J.A.S. Crippa, J.E.C. Hallak, F.A. Moreira, F.S. Guimarães | |||
| | title = Cannabidiol, a ''Cannabis sativa'' constituent, as an antipsychotic drug | |||
| | journal = Braz. J. Med. Biol. Res. | |||
| | year = 2006 | |||
| | volume = 39 | |||
| | pages = 421–429 | |||
| | url = http://www.scielo.br/pdf/bjmbr/v39n4/6164.pdf | |||
| | format = PDF | |||
| | pmid = 16612464 | |||
| | doi = 10.1590/S0100-879X2006000400001 | |||
| | issue = 4 | |||
| }}</ref> Studies have also shown that it may relieve symptoms of ].<ref name=pmid3793381>{{cite pmid|3793381}}</ref><ref name=Snider1985>Snider, Stuart R. and Consroe, Paul. (1985). "". ''Neurology''. (Suppl 1) p. 201.</ref> | |||
| ===Epilepsy=== | |||
| In November 2007, it was reported that CBD reduces growth of aggressive human ] cells '']'' and reduces their invasiveness. | |||
| {{See also|Charlotte's Web (cannabis)}} | |||
| In the United States, the FDA has ] only one brand of prescription cannabidiol called Epidiolex for the treatment of seizures associated with ], ], or ] in people one year of age and older.<ref name="Epidiolex FDA label" /><ref name="Office of the Commissioner_2020" /><ref name=stockings>{{cite journal | vauthors = Stockings E, Zagic D, Campbell G, Weier M, Hall WD, Nielsen S, Herkes GK, Farrell M, Degenhardt L | title = Evidence for cannabis and cannabinoids for epilepsy: a systematic review of controlled and observational evidence | journal = Journal of Neurology, Neurosurgery, and Psychiatry | volume = 89 | issue = 7 | pages = 741–753 | date = July 2018 | pmid = 29511052 | doi = 10.1136/jnnp-2017-317168 | doi-access = free | hdl = 1959.4/unsworks_50076 | hdl-access = free }}</ref><ref name="Elliott">{{cite journal | vauthors = Elliott J, DeJean D, Clifford T, Coyle D, Potter BK, Skidmore B, Alexander C, Repetski AE, Shukla V, McCoy B, Wells GA | title = Cannabis-based products for pediatric epilepsy: An updated systematic review | journal = Seizure | volume = 75 | pages = 18–22 | date = February 2020 | pmid = 31865133 | doi = 10.1016/j.seizure.2019.12.006 | s2cid = 208878465 | doi-access = free }}</ref> While Epidiolex treatment is generally well tolerated, it is associated with minor adverse effects, such as ], ], ], ], and poor sleep quality.<ref name="Epidiolex FDA label"/><ref name=stockings/><ref>{{Cite journal |date=June 26, 2018 |title=Cannabis derivative may reduce seizures in some severe drug-resistant epilepsies, but adverse events increase |url=https://evidence.nihr.ac.uk/alert/cannabis-derivative-may-reduce-seizures-in-some-severe-drug-resistant-epilepsies-but-adverse-events-increase |journal=NIHR Evidence |type=Plain English summary |doi=10.3310/signal-000606 |s2cid=242083755 |access-date=March 15, 2022 |archive-date=July 24, 2021 |archive-url=https://web.archive.org/web/20210724033322/https://evidence.nihr.ac.uk/alert/cannabis-derivative-may-reduce-seizures-in-some-severe-drug-resistant-epilepsies-but-adverse-events-increase/ |url-status=live }}</ref><ref name=Elliott/> | |||
| A 2008 study published in the ] showed significant differences in ] scores between three groups: The first consisted of non-cannabis users, the second consisted of users with ] detected, and the third consisted of users with both Δ<sup>9</sup>-THC and CBD detected. The Δ<sup>9</sup>-THC only group scored significantly higher for unusual experiences than the Δ<sup>9</sup>-THC and CBD group, whereas the Δ<sup>9</sup>-THC and CBD group had significantly lower ] scores than the Δ<sup>9</sup>-THC only group and non-cannabis user group. This research indicates that CBD acts as an anti-psychotic and may counteract the potential effects of THC on individuals with latent schizophrenia.<ref>Celia J. A. Morgan, PhD and H. Valerie Curran, PhD, DClinPsy </ref> | |||
| In the European Union, '''Epidyolex''' is indicated for use as adjunctive therapy of seizures associated with Lennox–Gastaut syndrome or Dravet syndrome, in conjunction with ], for people two years of age and older.<ref name="Epidyolex EPAR">{{cite web | title=Epidyolex EPAR | website=] (EMA) | date=June 24, 2019 | url=https://www.ema.europa.eu/en/medicines/human/EPAR/epidyolex | access-date=September 11, 2020 | archive-date=August 9, 2021 | archive-url=https://web.archive.org/web/20210809152037/https://www.ema.europa.eu/en/medicines/human/EPAR/epidyolex | url-status=live }} Text was copied from this source which is copyrighted by the European Medicines Agency. Reproduction is authorized provided the source is acknowledged.</ref> In 2020, the label for Epidiolex in the US was expanded to include seizures associated with tuberous sclerosis complex. Epidiolex/Epidyolex is the first prescription formulation of plant-derived cannabidiol approved by regulatory bodies in the US and Europe.<ref>{{Cite web|author=Office of the Commissioner|date=March 27, 2020|title=FDA Approves First Drug {{sic|Comprised |hide=y|of}} an Active Ingredient Derived from Marijuana to Treat Rare, Severe Forms of Epilepsy|url=https://www.fda.gov/news-events/press-announcements/fda-approves-first-drug-comprised-active-ingredient-derived-marijuana-treat-rare-severe-forms|access-date=May 28, 2021|website=FDA|language=en|archive-date=April 23, 2019|archive-url=https://web.archive.org/web/20190423071605/https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm611046.htm|url-status=live}}</ref> | |||
| == Medicinal use == | |||
| Cannabidiol is shown to decrease activity of the ]<ref>{{cite journal |author= José Alexandre de Souza Crippa, Antonio Waldo Zuardi, Griselda E J Garrido, Lauro Wichert-Ana, Ricardo Guarnieri, Lucas Ferrari, Paulo M Azevedo-Marques, Jaime Eduardo Cecílio Hallak, Philip K McGuire and Geraldo Filho Busatto |title= Effects of Cannabidiol (CBD) on Regional Cerebral Blood Flow. |journal=Neuropsychopharmacology |volume=29 |issue= 2|pages=417–426 |year=2003 |month=October |pmid=14583744|doi=10.1038/sj.npp.1300340|url=}}</ref> and to decrease social isolation induced by ].<ref>{{cite journal |author= Daniel Thomas Malone, Dennis Jongejana and David Alan Taylora |title= Cannabidiol reverses the reduction in social interaction produced by low dose Δ9-tetrahydrocannabinol in rats |journal=Pharmacology Biochemistry and Behavior |volume=93|issue=2 |pages=91–96 |year=2009 |month=August |pmid=19393686|doi=10.1016/j.pbb.2009.04.010|url=}}</ref> | |||
| It's also shown that Cannabidiol reduces anxiety in ]. | |||
| <ref>{{cite journal |author= Mateus M Bergamaschi, Regina Helena Costa Queiroz, Marcos Hortes Nisihara Chagas, Danielle Chaves Gomes de Oliveira, Bruno Spinosa De Martinis, Flávio Kapczinski, João Quevedo, Rafael Roesler, Nadja Schröder, Antonio E Nardi, Rocio Martín-Santos, Jaime Eduardo Cecílio |title=Cannabidiol Reduces the Anxiety Induced by Simulated Public Speaking in Treatment-Naïve Social Phobia Patients |journal=Neuropsychopharmacology |volume=36 |pages=1219–1226 |year=2011 |month=may |doi=10.1038/npp.2011.6|url=http://www.nature.com/npp/journal/v36/n6/full/npp20116a.html}}</ref> | |||
| <ref>{{cite journal |author= Crippa JA, Derenusson GN, Ferrari TB, Wichert-Ana L, Duran FL, Martin-Santos R, Simões MV, Bhattacharyya S, Fusar-Poli P, Atakan Z, Santos Filho A, Freitas-Ferrari MC, McGuire PK, Zuardi AW, Busatto GF, Hallak JE. |title= Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. |journal=J Psychopharmacol. |volume=25 |issue= 1|pages=121–130 |year=2011 |month=January|doi=10.1177/0269881110379283 |url=http://jop.sagepub.com/content/25/1/121.long}}</ref> | |||
| In April 2005, Canadian authorities approved the marketing of ], a mouth spray for ] to alleviate pain. Sativex contains tetrahydrocannabinol together with cannabidiol. It is marketed in ] by GW Pharmaceuticals. | |||
| ===Other uses=== | |||
| Studies have shown that CBD may reduce ] symptoms in patients, likely due to their apparent ability to stabilize disrupted or disabled ] receptor pathways in the brain, which are shared and sometimes contested by ] and ].<ref name="braz" /><ref>http://www.nature.com/npp/journal/v31/n4/abs/1300838a.html</ref> Leweke ''et al.'' performed a double blind, 4 week, explorative controlled clinical trial to compare the effects of purified cannabidiol and the atypical antipsychotic ] on improving the symptoms of schizophrenia in 42 patients with acute paranoid schizophrenia. Both treatments were associated with a significant decrease of psychotic symptoms after 2 and 4 weeks as assessed by ] and ]. While there was no statistical difference between the two treatment groups, cannabidiol induced significantly less side effects (], increase in prolactin, weight gain) when compared to amisulpride.<ref name="Leweke 2009 s207">{{cite journal | |||
| Research on other uses for cannabidiol includes several ], but the findings have not been confirmed to establish such uses in clinical practice.<ref name="devinsky">{{cite journal | vauthors = Devinsky O, Cilio MR, Cross H, Fernandez-Ruiz J, French J, Hill C, Katz R, Di Marzo V, Jutras-Aswad D, Notcutt WG, Martinez-Orgado J, Robson PJ, Rohrback BG, Thiele E, Whalley B, Friedman D | title = Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders | journal = Epilepsia | volume = 55 | issue = 6 | pages = 791–802 | date = June 2014 | pmid = 24854329 | pmc = 4707667 | doi = 10.1111/epi.12631 }}</ref><ref name=black/><ref name=mayo/><ref name="pmid28232276" /><ref name="prud">{{cite journal | vauthors = Prud'homme M, Cata R, Jutras-Aswad D | title = Cannabidiol as an Intervention for Addictive Behaviors: A Systematic Review of the Evidence | journal = Substance Abuse | volume = 9 | pages = 33–38 | year = 2015 | pmid = 26056464 | pmc = 4444130 | doi = 10.4137/SART.S25081 }}</ref><ref>{{cite journal | vauthors = Silva TB, Balbino CQ, Weiber AF | title = The relationship between cannabidiol and psychosis: A review | journal = Annals of Clinical Psychiatry | volume = 27 | issue = 2 | pages = 134–141 | date = May 2015 | pmid = 25954940 }}</ref><ref name="Blessing_2015">{{cite journal | vauthors = Blessing EM, Steenkamp MM, Manzanares J, Marmar CR | title = Cannabidiol as a Potential Treatment for Anxiety Disorders | journal = Neurotherapeutics | volume = 12 | issue = 4 | pages = 825–836 | date = October 2015 | pmid = 26341731 | pmc = 4604171 | doi = 10.1007/s13311-015-0387-1 }}</ref> In October 2019, the FDA issued an advisory warning that the effects of CBD during pregnancy or ] are unknown, indicating that the safety, doses, interactions with other drugs or foods, and ]s of CBD are not ], and may pose a risk to the mother and infant.<ref>{{cite web |title=What You Should Know About Using Cannabis, Including CBD, When Pregnant or Breastfeeding |url=https://www.fda.gov/consumers/consumer-updates/what-you-should-know-about-using-cannabis-including-cbd-when-pregnant-or-breastfeeding |publisher=US ] (FDA) |access-date=October 17, 2019 |date=October 16, 2019 |archive-date=October 17, 2019 |archive-url=https://web.archive.org/web/20191017212718/https://www.fda.gov/consumers/consumer-updates/what-you-should-know-about-using-cannabis-including-cbd-when-pregnant-or-breastfeeding |url-status=live }}</ref> | |||
| | last = Leweke | |||
| | first = FM | |||
| | coauthors = Koethe D, Pahlisch F, Schreiber D, Gerth1 CW, Nolden1 BM, Klosterkötter J, Hellmich M and Piomelli D. | |||
| | title = Antipsychotic effects of cannabidiol | |||
| | journal = European Psychiatry 17th EPA Congress | |||
| |year=2009 | |||
| | volume = 24 | |||
| | pages = s207 | |||
| | doi = 10.1016/S0924-9338(09)70440-7 | |||
| |format=PDF | |||
| | issue=1}}</ref> | |||
| Many claims are made for the therapeutic benefit of cannabidiol that are not backed by sound evidence. Some claims, such as treatment of ], are ].<ref name=sbm>{{cite web |publisher=] |vauthors=Novella S |author-link=Steven Novella |title=Where Are We With CBD? |url=https://sciencebasedmedicine.org/where-are-we-with-cbd/ |date=September 30, 2020 |access-date=October 1, 2020 |archive-date=August 12, 2021 |archive-url=https://web.archive.org/web/20210812120451/https://sciencebasedmedicine.org/where-are-we-with-cbd/ |url-status=live }}</ref> | |||
| Cannabidiol has also been shown as being effective treating an often drug-induced set of neurological movement disorders known as ].<ref name=Snider1985/> In one study, five out of five participants showed noted improvement in their dystonic symptoms by 20-50%.<ref name=pmid3793381/> CBD also appears to protect against 'binge' alcohol induced neurodegeneration.<ref>http://dx.doi.org/10.1124/jpet.105.085779 Comparison of Cannabidiol, Antioxidants, and Diuretics in Reversing Binge Ethanol-Induced Neurotoxicity</ref><ref>http://www.ncbi.nlm.nih.gov/pubmed/19631736 White matter integrity in adolescents with histories of marijuana use and binge drinking.</ref> | |||
| In 2020, the label for Epidiolex in the US was expanded to include treatment of seizures associated with ].<ref name="Office of the Commissioner_2020">{{Cite web|author=Office of the Commissioner|date=July 31, 2020|title=FDA Approves New Indication for Drug Containing an Active Ingredient Derived from Cannabis to Treat Seizures in Rare Genetic Disease|url=https://www.fda.gov/news-events/press-announcements/fda-approves-new-indication-drug-containing-active-ingredient-derived-cannabis-treat-seizures-rare|access-date=November 25, 2020|website=FDA|language=en|archive-date=September 29, 2021|archive-url=https://web.archive.org/web/20210929230838/https://www.fda.gov/news-events/press-announcements/fda-approves-new-indication-drug-containing-active-ingredient-derived-cannabis-treat-seizures-rare|url-status=live}}</ref> | |||
| Cannabidiol may block ]'s interference with memory.<ref>http://www.nature.com/news/2010/101001/full/news.2010.508.html</ref> | |||
| Acclaimed for relieving ], some researchers conclude that the evidence is insufficient to determine the effectiveness of CBD in pain relief, primarily due to the challenging access to pure CBD.<ref>{{cite journal | vauthors = Villanueva MR, Joshaghani N, Villa N, Badla O, Goit R, Saddik SE, Dawood SN, Rabih AM, Niaj A, Raman A, Uprety M, Calero M, Khan S | title = Efficacy, Safety, and Regulation of Cannabidiol on Chronic Pain: A Systematic Review | journal = Cureus | volume = 14 | issue = 7 | pages = e26913 | date = July 2022 | pmid = 35860716 | pmc = 9288157 | doi = 10.7759/cureus.26913 | doi-access = free }}</ref> | |||
| == Pharmacology == | |||
| CBD oil is used for massage therapy as a substitute for body oil and for its health benefits.<ref>{{Cite web |date=2020-02-28 |title=Experience best massage - CBD Oil - body health benefits |url=https://thailotusbodywork.com/articles/massage-with-cbd-oil/ |access-date=2024-10-22 |language=en-US}}</ref> | |||
| Cannabidiol has no affinity for ] and ] receptors but acts as an indirect antagonist of cannabinoid agonists.<ref name="recentadvances" /> | |||
| Recently it was found to be an antagonist at the putative new cannabinoid receptor, ], a ] expressed in the ] and ].<ref>{{cite journal | |||
| | author=Ryberg E, Larsson N, Sjögren S, ''et al.'' | |||
| | title=The orphan receptor GPR55 is a novel cannabinoid receptor | |||
| | journal= British Journal of Pharmacology | |||
| | volume= 152 | |||
| | issue=7 | |||
| | pages= 1092 | |||
| | year=2007 | |||
| | pmid=17876302 | |||
| | doi=10.1038/sj.bjp.0707460 | |||
| | pmc=2095107}}</ref> Cannabidiol has also been shown to act as a ] agonist,<ref name="pmid16258853">{{cite journal | author = Russo EB, Burnett A, Hall B, Parker KK | title = Agonistic properties of cannabidiol at 5-HT1a receptors | journal = Neurochemical Research | volume = 30 | issue = 8 | pages = 1037–43 | year = 2005 | month = August | isbn = 1106400569781 | pmid = 16258853 | doi = 10.1007/s11064-005-6978-1}}</ref> an action which is involved in its ],<ref name="pmid20002102">{{cite journal | unused_data = DUPLICATE DATA: doi = 10.1111/j.1476-5381.2009.00521.x | author = Zanelati T, Biojone C, Moreira F, Guimarães F, Joca S | title = Antidepressant-like effects of cannabidiol in mice: possible involvement of 5-HT receptors | journal = British Journal of Pharmacology | volume = 159| issue = 1| pages = 122–8| year = 2009 | month = December | pmid = 20002102 | doi = 10.1111/j.1476-5381.2009.00521.x | pmc = 2823358}}</ref><ref name="pmid19133999">{{cite journal | unused_data = DUPLICATE DATA: doi = 10.1111/j.1476-5381.2008.00046.x | author = Resstel LB, Tavares RF, Lisboa SF, Joca SR, Corrêa FM, Guimarães FS | title = 5-HT1A receptors are involved in the cannabidiol-induced attenuation of behavioural and cardiovascular responses to acute restraint stress in rats | journal = British Journal of Pharmacology | volume = 156 | issue = 1 | pages = 181–8 | year = 2009 | month = January | pmid = 19133999 | pmc = 2697769 | doi = 10.1111/j.1476-5381.2008.00046.x}}</ref> ],<ref name="pmid19133999"/><ref name="pmid18446323">{{cite journal | author = Campos AC, Guimarães FS | title = Involvement of 5HT1A receptors in the anxiolytic-like effects of cannabidiol injected into the dorsolateral periaqueductal gray of rats | journal = Psychopharmacology | volume = 199 | issue = 2 | pages = 223–30 | year = 2008 | month = August | isbn = 2130081168 | pmid = 18446323 | doi = 10.1007/s00213-008-1168-x}}</ref> and ]<ref name="pmid15845890">{{cite journal | author = Mishima K, Hayakawa K, Abe K, ''et al.'' | title = Cannabidiol prevents cerebral infarction via a serotonergic 5-hydroxytryptamine1A receptor-dependent mechanism | journal = Stroke; a Journal of Cerebral Circulation | volume = 36 | issue = 5 | pages = 1077–82 | year = 2005 | month = May | pmid = 15845890 | doi = 10.1161/01.STR.0000163083.59201.34 | url = http://stroke.ahajournals.org/cgi/pmidlookup?view=long&pmid=15845890}}</ref><ref name="pmid17320118">{{cite journal | author = Hayakawa K, Mishima K, Nozako M, ''et al.'' | title = Repeated treatment with cannabidiol but not Delta9-tetrahydrocannabinol has a neuroprotective effect without the development of tolerance | journal = Neuropharmacology | volume = 52 | issue = 4 | pages = 1079–87 | year = 2007 | month = March | pmid = 17320118 | doi = 10.1016/j.neuropharm.2006.11.005 | url = http://linkinghub.elsevier.com/retrieve/pii/S0028-3908(06)00392-3}}</ref> effects. | |||
| ===Non-intoxicating effects=== | |||
| Cannabidiol has also been shown to inhibit cancer cell growth with low potency in non-cancer cells. Although the inhibitory mechanism is not yet fully understood, Ligresti et al. suggest that "cannabidiol exerts its effects on these cells through a combination of mechanisms that include either direct or indirect activation of CB<sub>2</sub> and ] receptors, and induction of ], all contributing to induce ]."<ref name="pmid16728591">{{cite journal | |||
| Cannabidiol does not appear to have any ] effects<ref>{{cite journal | vauthors = Kicman A, Toczek M | title = The Effects of Cannabidiol, a Non-Intoxicating Compound of Cannabis, on the Cardiovascular System in Health and Disease | journal = International Journal of Molecular Sciences | volume = 21 | issue = 18 | page = 6740 | date = September 2020 | pmid = 32937917 | pmc = 7554803 | doi = 10.3390/ijms21186740 | doi-access = free }}</ref> such as those caused by ∆{{sup|9}}-THC in cannabis, but it is under preliminary research for its possible ] and ] effects.<ref name=black/><ref name=mayo/><ref name=Iseger2015/> As the legal landscape and understanding about the differences in medical cannabinoids unfolds, experts are working to distinguish "medical cannabis" (with varying degrees of psychotropic effects and deficits in executive function) from "medical CBD therapies", which would commonly present as having a reduced or non-psychoactive side-effect profile.<ref name=mayo/><ref name="Iseger2015" /><ref>{{cite journal | vauthors = Sachs J, McGlade E, Yurgelun-Todd D | title = Safety and Toxicology of Cannabinoids | journal = Neurotherapeutics | volume = 12 | issue = 4 | pages = 735–746 | date = October 2015 | pmid = 26269228 | pmc = 4604177 | doi = 10.1007/s13311-015-0380-8 }}</ref> | |||
| | author=Ligresti A, Moriello AS, Starowicz K, ''et al.'' | |||
| | title=Antitumor activity of plant cannabinoids with emphasis on the effect of cannabidiol on human breast carcinoma | |||
| | journal=J. Pharmacol. Exp. Ther. | |||
| | volume=318 | |||
| | issue=3 | |||
| | pages=1375–87 | |||
| | year=2006 | |||
| | pmid=16728591 | |||
| | doi=10.1124/jpet.106.105247 | |||
| }}</ref> | |||
| In November 2007, researchers at the ] reported that CBD shows promise for controlling the spread of ] ]. '']'' CBD downregulates the activity of the gene ] which is responsible for tumor metastasis.<ref name="pmid18025276">{{cite journal | |||
| | author=McAllister SD, Christian RT, Horowitz MP, Garcia A, Desprez PY | |||
| | title=Cannabidiol as a novel inhibitor of Id-1 gene expression in aggressive breast cancer cells | |||
| | journal=Mol. Cancer Ther. | |||
| | volume=6 | |||
| | issue=11 | |||
| | pages=2921–7 | |||
| | year=2007 | |||
| | pmid=18025276 | |||
| | doi=10.1158/1535-7163.MCT-07-0371 | |||
| }}</ref> | |||
| Various strains of "medical cannabis" are found to have a significant variation in the ratios of CBD-to-THC and are known to contain other non-psychotropic cannabinoids.<ref name="pmid19729208">{{cite journal | vauthors = Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R | title = Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb | journal = Trends in Pharmacological Sciences | volume = 30 | issue = 10 | pages = 515–527 | date = October 2009 | pmid = 19729208 | doi = 10.1016/j.tips.2009.07.006 }}</ref> Any psychoactive cannabis, regardless of its CBD content, is derived from the flower (or bud) of the genus ''Cannabis''. As defined by US federal law, non-psychoactive hemp (also commonly termed "]"), regardless of its CBD content, is any part of the cannabis plant, whether growing or not, containing a ∆{{sup|9}}-] concentration of no more than 0.3% on a dry-weight basis.<ref name="col">{{cite web|date=2018|title=Industrial hemp|url=https://www.colorado.gov/pacific/agplants/industrial-hemp|access-date=September 14, 2018|publisher=Department of Agriculture, State of Colorado|archive-date=August 26, 2018|archive-url=https://web.archive.org/web/20180826025810/https://www.colorado.gov/pacific/agplants/industrial-hemp|url-status=live}}</ref> In the United States, certain standards are required for legal growing, cultivating, and producing the hemp plant, but there are no federal standards for quality being enforced in the hemp industry. Certain state regulations are in place, but vary state to state.<ref>{{Cite web|title=Cannabinoid Clinical {{!}} Cannabinoids Research, Effects, and Uses|url=https://www.cannabinoidclinical.com/fda-regulation-cannabinoids|access-date=October 27, 2020|website=CannabinoidClinical.com|language=en|archive-date=June 26, 2021|archive-url=https://web.archive.org/web/20210626065243/https://www.cannabinoidclinical.com/fda-regulation-cannabinoids|url-status=live}}</ref> For instance, the Colorado Industrial Hemp Program registers growers of industrial hemp and samples crops to verify that the dry-weight THC concentration does not exceed 0.3%.<ref name=col/> | |||
| == Chemistry == | |||
| ===Available forms=== | |||
| Cannabidiol is insoluble in water but soluble in organic solvents. At room temperature it is a colorless crystalline solid.<ref>{{cite journal | |||
| CBD is present as an active constituent of ], which is used both recreationally and medically. | |||
| | author=Jones PG, Falvello L, Kennard O, Sheldrick GM Mechoulam R | |||
| | title=Cannabidiol | |||
| ] (brand name Sativex), an oromucosal spray made of a complex botanical mixture containing cannabidiol (CBD), delta-9-tetrahydrocannabinol (THC), and additional cannabinoid and non-cannabinoid constituents from cannabis sativa plants, was approved by Health Canada in 2005, to treat central ] in ], and in 2007, for cancer-related pain.<ref name="ReferenceA">{{cite journal | vauthors = Russo EB | title = Cannabinoids in the management of difficult to treat pain | journal = Therapeutics and Clinical Risk Management| volume = 4 | issue = 1 | pages = 245–259 | date = February 2008 | pmid = 18728714 | pmc = 2503660 | doi = 10.2147/TCRM.S1928 | doi-access = free }}</ref> In New Zealand, Sativex is "approved for use as an add-on treatment for symptom improvement in people with moderate to severe spasticity due to multiple sclerosis who have not responded adequately to other anti-spasticity medication."<ref>{{cite web|url=https://medsafe.govt.nz/profs/RIss/Sativex/Sativex.asp|title=Sativex Oromucosal Spray|publisher=Medsafe, New Zealand Medicines and Medical Devices Safety Authority|date=December 19, 2018|access-date=April 3, 2019|archive-date=July 27, 2020|archive-url=https://web.archive.org/web/20200727071731/https://medsafe.govt.nz/profs/RIss/Sativex/Sativex.asp}}</ref> | |||
| | journal= Acta Cryst. | |||
| | volume= B33 | |||
| Epidiolex (''Epidyolex'' in Europe) is an orally administered cannabidiol solution.<ref name="Epidiolex FDA label"/><ref name="Epidyolex EPAR"/> It was approved in 2018 for treatment of two rare forms of childhood epilepsy, ] and ], and seizures associated with tuberous sclerosis complex.<ref name="Epidiolex FDA label"/><ref name="Epidyolex EPAR"/> In the US, it is approved in these indications for people one year of age and older.<ref name="Epidiolex FDA label"/> | |||
| | pages= 3211–3214 | |||
| | year=1977 | |||
| ==Side effects== | |||
| | doi=10.1107/S0567740877010577}}</ref> In strongly basic medium and the presence of air it is oxidized to a ].<ref>{{cite journal | |||
| Research indicates that cannabidiol may reduce adverse effects of THC, particularly those causing ] and ], but only at high doses.<ref name="fischer">{{cite journal | vauthors = Fischer B, Russell C, Sabioni P, van den Brink W, Le Foll B, Hall W, Rehm J, Room R | title = Lower-Risk Cannabis Use Guidelines: A Comprehensive Update of Evidence and Recommendations | journal = American Journal of Public Health | volume = 107 | issue = 8 | pages = e1–e12 | date = August 2017 | pmid = 28644037 | pmc = 5508136 | doi = 10.2105/AJPH.2017.303818 |doi-access=free}}</ref> Safety studies of cannabidiol showed it is well tolerated, but may cause ], ], sedation, ], or changes in appetite as common adverse effects, with the most common being somnolence and sedation. Side effects of CBD are dose related.<ref name="Huestis Solimini Pichini Pacifici pp. 974–989">{{cite journal | vauthors = Huestis MA, Solimini R, Pichini S, Pacifici R, Carlier J, Busardò FP | title = Cannabidiol Adverse Effects and Toxicity | journal = Current Neuropharmacology | volume = 17 | issue = 10 | pages = 974–989 | date = September 13, 2019 | pmid = 31161980 | doi = 10.2174/1570159x17666190603171901 | publisher = Bentham Science Publishers Ltd. | pmc = 7052834 }}</ref> Epidiolex documentation lists sleepiness, ] and poor quality sleep, decreased appetite, diarrhea, and fatigue.<ref name="Epidiolex FDA label" /><ref>{{Cite web|title=Cannabidiol (CBD): MedlinePlus Supplements|url=https://medlineplus.gov/druginfo/natural/1439.html|access-date=January 15, 2021|website=medlineplus.gov|language=en|archive-date=October 20, 2021|archive-url=https://web.archive.org/web/20211020055709/https://medlineplus.gov/druginfo/natural/1439.html|url-status=live}}</ref> | |||
| | author=Mechoulam R, Ben-Zvi Z | |||
| | title=Hashish—XIII On the nature of the beam test | |||
| In November 2019, the FDA issued concerns about the safety of cannabidiol, stating that CBD use has potential to cause ], interfere with the mechanisms of ]s, produce ]s, or affect ] and ].<ref name="fda11-26-19" /> Over 2020–23, the FDA updated its safety concerns about CBD,<ref name="fdareg">{{cite web |title=FDA Regulation of Cannabis and Cannabis-Derived Products, Including Cannabidiol (CBD)|url=https://www.fda.gov/news-events/public-health-focus/fda-regulation-cannabis-and-cannabis-derived-products-including-cannabidiol-cbd |publisher=US ] (FDA) |access-date=October 29, 2023 |date=September 28, 2023}}</ref> acknowledging the unknown effects of protracted use, how it affects the developing brain, fetus, or infants during breastfeeding, whether it interacts with ]s or ]s, whether ] is affected, and its possible ]s, such as ].<ref name="hahn">{{cite web |vauthors=Hahn SM |author-link1=Stephen Hahn (oncologist) |title=FDA Advances Work Related to Cannabidiol Products with Focus on Protecting Public Health, Providing Market Clarity |url=https://www.fda.gov/news-events/press-announcements/fda-advances-work-related-cannabidiol-products-focus-protecting-public-health-providing-market |publisher=US Food and Drug Administration |access-date=March 6, 2020 |date=March 5, 2020 |archive-date=September 29, 2021 |archive-url=https://web.archive.org/web/20210929230626/https://www.fda.gov/news-events/press-announcements/fda-advances-work-related-cannabidiol-products-focus-protecting-public-health-providing-market |url-status=live }}</ref> | |||
| | journal= Tetrahedron | |||
| | volume= 24 | |||
| {{As of|September 2019}}, 1,085 people contacted US ]s about CBD-induced illnesses, doubling the number of cases over the 2018 rate and increasing by 9 times the case numbers of 2017.<ref name="pcc">{{cite web|date=September 30, 2019|title=Cannabidiol (CBD)|url=https://aapcc.org/CBD-Alert|access-date=October 17, 2019|publisher=American Association of Poison Control Centers|archive-date=October 17, 2019|archive-url=https://web.archive.org/web/20191017161650/https://aapcc.org/CBD-Alert|url-status=live}}</ref> Of cases reported in 2019, more than 33% received medical attention and 46 people were admitted to a hospital ], possibly due to exposure to other products, or drug interactions with CBD.<ref name="dawn" /> | |||
| | issue=16 | |||
| | pages= 5615–5624 | |||
| In 2022, the FDA stated that "scientific studies show possible harm to the male reproductive system, including testicular atrophy, harm to the liver, and interactions with certain medications. The FDA has not found adequate information showing how much CBD can be consumed, and for how long, before causing harm. This is particularly true for vulnerable populations like children and those who are pregnant."<ref name=fda11-22/> | |||
| | year=1968 | |||
| | doi=10.1016/0040-4020(68)88159-1}}</ref> Under acidic conditions it cyclizes to ].<ref>{{cite journal | |||
| ==Interactions== | |||
| | author=Gaoni Y, Mechoulam R | |||
| Laboratory evidence indicated that cannabidiol may reduce THC ], increasing plasma concentrations which may raise THC availability to ] and enhance its effect in a ].<ref name="pmid7493549">{{cite journal | vauthors = Bornheim LM, Kim KY, Li J, Perotti BY, Benet LZ | title = Effect of cannabidiol pretreatment on the kinetics of tetrahydrocannabinol metabolites in mouse brain | journal = Drug Metabolism and Disposition | volume = 23 | issue = 8 | pages = 825–831 | date = August 1995 | pmid = 7493549 }}</ref><ref name="pmid21667074">{{cite journal | vauthors = Klein C, Karanges E, Spiro A, Wong A, Spencer J, Huynh T, Gunasekaran N, Karl T, Long LE, Huang XF, Liu K, Arnold JC, McGregor IS | title = Cannabidiol potentiates Δ<sup>9</sup>-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats | journal = Psychopharmacology | volume = 218 | issue = 2 | pages = 443–457 | date = November 2011 | pmid = 21667074 | doi = 10.1007/s00213-011-2342-0 | s2cid = 6240926 }}</ref> '']'', cannabidiol inhibited the activity of ] ] and ] channels, which may affect neural activity.<ref name="Ghovanloo_2018">{{cite journal | vauthors = Ghovanloo MR, Shuart NG, Mezeyova J, Dean RA, Ruben PC, Goodchild SJ | title = Inhibitory effects of cannabidiol on voltage-dependent sodium currents | journal = The Journal of Biological Chemistry | volume = 293 | issue = 43 | pages = 16546–16558 | date = October 2018 | pmid = 30219789 | pmc = 6204917 | doi = 10.1074/jbc.RA118.004929 | doi-access = free }}</ref> A recent study using X-ray crystallography showed that CBD binds inside the sodium channel pore at a novel site at the interface of the fenestrations and the central hydrophobic cavity of the channel. Binding at this site blocks the transmembrane-spanning sodium ion translocation pathway, providing a molecular mechanism for channel inhibition, which could contribute to a reduced excitability.<ref name="Sait_2020">{{cite journal | vauthors = Sait LG, Sula A, Ghovanloo MR, Hollingworth D, Ruben PC, Wallace BA | title = Cannabidiol interactions with voltage-gated sodium channels | journal = eLife | volume = 9 | date = October 2020 | pmid = 33089780 | pmc = 7641581 | doi = 10.7554/eLife.58593 | doi-access = free }}</ref> A small ] reported that CBD partially inhibited the ]-catalyzed ] of THC to ].<ref name="Nadulski_2005">{{cite journal | vauthors = Nadulski T, Pragst F, Weinberg G, Roser P, Schnelle M, Fronk EM, Stadelmann AM | title = Randomized, double-blind, placebo-controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of Delta9-tetrahydrocannabinol (THC) after oral application of THC verses standardized cannabis extract | journal = Therapeutic Drug Monitoring | volume = 27 | issue = 6 | pages = 799–810 | date = December 2005 | pmid = 16306858 | doi = 10.1097/01.ftd.0000177223.19294.5c | s2cid = 12979224 }}</ref> Little is known about potential drug interactions, but CBD mediates a decrease in ] metabolism.<ref name="Lucas_2018">{{cite journal | vauthors = Lucas CJ, Galettis P, Schneider J | title = The pharmacokinetics and the pharmacodynamics of cannabinoids | journal = British Journal of Clinical Pharmacology | volume = 84 | issue = 11 | pages = 2477–2482 | date = November 2018 | pmid = 30001569 | pmc = 6177698 | doi = 10.1111/bcp.13710 }}</ref> Work with human liver microsomes shows that cannabidiol inhibits ] and ] to some degree.<ref>{{cite journal | vauthors = Yamaori S, Ebisawa J, Okushima Y, Yamamoto I, Watanabe K | title = Potent inhibition of human cytochrome P450 3A isoforms by cannabidiol: role of phenolic hydroxyl groups in the resorcinol moiety | journal = Life Sciences | volume = 88 | issue = 15–16 | pages = 730–736 | date = April 2011 | pmid = 21356216 | doi = 10.1016/j.lfs.2011.02.017 }}</ref> | |||
| | title=Hashish—VII The isomerization of cannabidiol to tetrahydrocannabinols | |||
| | journal= Tetrahedron | |||
| ==Pharmacology== | |||
| | volume= 22 | |||
| | issue=4 | |||
| ===Pharmacodynamics=== | |||
| | pages= 1481–1488 | |||
| '']'', cannabidiol has low ] for, and acts as a ] of the ] ]<ref>{{Cite journal |title=Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor |date=2015 |pmc=4621983 |journal=British Journal of Pharmacology |volume=172 |issue=20 |pages=4790–4805 |doi=10.1111/bph.13250 |pmid=26218440 | vauthors = Laprairie RB, Bagher AM, Kelly ME, Denovan-Wright EM }}</ref><ref>{{Cite journal |title=Allosteric Modulation: An Alternate Approach Targeting the Cannabinoid CB1 Receptor |journal= Medicinal Research Reviews|date=2016 |pmc=5397374 |volume=37 |issue=3 |pages=441–474 |doi=10.1002/med.21418 |pmid=27879006 | vauthors = Nguyen T, Li JX, Thomas BF, Wiley JL, Kenakin TP, Zhang Y }}</ref> | |||
| | year=1966 | |||
| | doi=10.1016/S0040-4020(01)99446-3}}</ref> The synthesis of cannabidiol has been accomplished by several research groups.<ref>{{cite journal | |||
| Cannabidiol may be an antagonist of ], a ] and putative non-homologous CB<sub>3</sub> cannabinoid receptor shown by ''in vitro'' studies to be widely distributed in the brain.<ref name=schouten/><ref name="drugs">{{cite web |date=July 14, 2023 |title=Cannabidiol |url=https://www.drugs.com/npp/cannabidiol.html |access-date=February 12, 2024 |publisher=Drugs.com}}</ref><ref>{{cite journal | vauthors = Gray RA, Whalley BJ | title = The proposed mechanisms of action of CBD in epilepsy | journal = Epileptic Disorders | volume = 22 | issue = S1 | pages = 10–15 | date = January 2020 | doi = 10.1684/epd.2020.1135 | pmid = 32053110| doi-access = free }}</ref> Cannabidiol may interact with various ]s, such as ], ], and ].<ref name=schouten/><ref name=drugs/><ref name="PMID33384591">{{cite journal | vauthors = Martínez-Aguirre C, et al | title = Cannabidiol Acts at 5-HT1A Receptors in the Human Brain: Relevance for Treating Temporal Lobe Epilepsy | journal = Front. Behav. Neurosci. | volume = 14 | date = December 2020 | page = 611278 | pmid = 33384591 | doi = 10.3389/fnbeh.2020.611278 | pmc = 7770178 | doi-access = free }}</ref> | |||
| | author=Petrzilka T, Haefliger W, Sikemeier C, Ohloff G, Eschenmoser A | |||
| | title=Synthese und Chiralität des (-)-Cannabidiols | |||
| As of 2024, the cellular effects and mechanisms of cannabidiol '']'' are unknown,<ref name="Epidiolex FDA label"/><ref name="Epidyolex EPAR"/> as research to date has been inconclusive and based on laboratory studies.<ref name="schouten">{{cite journal |vauthors=Schouten M, Dalle S, Mantini D, Koppo K |title=Cannabidiol and brain function: current knowledge and future perspectives |journal=Frontiers in Pharmacology |volume=14 |issue= |pages=1328885 |date=2023 |pmid=38288087 |pmc=10823027 |doi=10.3389/fphar.2023.1328885|doi-access=free}}</ref> The ] effects provided by cannabidiol (Epidiolex) in people with certain forms of ] do not appear to involve ]s.<ref name="Epidiolex FDA label"/> A possible mechanism for the effects of cannabidiol on seizures is by affecting the ] movement of ] in brain structures involved in the excessive electrical activity of ].<ref name="Epidyolex EPAR"/> | |||
| | journal= Helv. Chim. Acta | |||
| | volume= 50 | |||
| ===Pharmacokinetics=== | |||
| | issue=2 | |||
| The ] ] of cannabidiol is approximately 6% in fasting state and 36.5—57.3% in fed-state<ref name="Kolli_2024" /> in humans, while its bioavailability via ] is 11 to 45% (mean 31%).<ref name="Mechoulam2002" /><ref name="Scuderi2009" /><ref name="Kolli_2024">{{cite journal | vauthors = Kolli AR, Hoeng J | title = Cannabidiol Bioavailability Is Nonmonotonic with a Long Terminal Elimination Half-Life: A Pharmacokinetic Modeling-Based Analysis | journal = Cannabis and Cannabinoid Research | date = April 2024 | pmid = 38624257 | doi = 10.1089/can.2023.0214 }}</ref><ref name="O'Sullivan_2024">{{cite journal | vauthors = O'Sullivan SE, Jensen SS, Kolli AR, Nikolajsen GN, Bruun HZ, Hoeng J | title = Strategies to Improve Cannabidiol Bioavailability and Drug Delivery | journal = Pharmaceuticals | volume = 17 | issue = 2 | pages = 244 | date = February 2024 | pmid = 38399459 | pmc = 10892205 | doi = 10.3390/ph17020244 | publisher = MDPI | doi-access = free }}</ref> The oral bioavailability of cannabidiol varies based on several factors such as formulation,<ref name="O'Sullivan_2024" /> dose, and food intake.<ref name="Kolli_2024" /> The ] bioavailability of cannabidiol is approximately 12 to 35%.<ref>{{Cite journal |last=Hossain |first=Khondker |date=25 September 2023 |title=Current Challenges and Opportunities for Improved Cannabidiol Solubility |journal=International Journal of Molecular Sciences|volume=24 |issue=19 |page=14514 |doi=10.3390/ijms241914514 |doi-access=free |pmid=37833962 |pmc=10572536 }}</ref> The ] of cannabidiol in blood is 56 to 61 hours after oral doses twice per day over 7 days.<ref name="Epidiolex FDA label"/> Based on the pharmacokinetic analysis of long-term dosing of cannabidiol in humans, the terminal elimination half-life is estimated to be >134 h.<ref name="Kolli_2024" /> Cannabidiol is ] in the ] as well as in the ]s by ] enzymes.<ref name="Epidiolex FDA label"/><ref name=drugs/> | |||
| | pages= 719–723 | |||
| | year=1967 | |||
| ==Chemistry== | |||
| | doi=10.1002/hlca.19670500235 | |||
| {{See also|Conversion of CBD to THC}} | |||
| | pmid=5587099}}</ref><ref>{{cite journal | |||
| | author=Gaoni Y, Mechoulam R | |||
| At room temperature, cannabidiol is a colorless crystalline solid.<ref>{{cite journal | vauthors = Jones PG, Falvello L, Kennard O, Sheldrick GM, Mechoulam R | title = Cannabidiol | journal = Acta Crystallogr. B | volume = 33 | pages = 3211–3214 | year = 1977 | doi = 10.1107/S0567740877010577 | issue = 10 | bibcode = 1977AcCrB..33.3211J | doi-access = free }}</ref> In strongly basic media and the presence of air, it is oxidized to ] (CBND) and a ] called ].<ref>{{cite journal | vauthors = Mechoulam R, Ben-Zvi Z, Gaoni Y | title = Hashish – 13. On the nature of the Beam test | journal = Tetrahedron | volume = 24 | issue = 16 | pages = 5615–5624 | date = August 1968 | pmid = 5732891 | doi = 10.1016/0040-4020(68)88159-1 }}</ref> Under acidic conditions it ] to THC,<ref>{{cite journal |vauthors=Gaoni Y, Mechoulam R | title = Hashish – VII The isomerization of cannabidiol to tetrahydrocannabinols | journal = Tetrahedron | volume = 22 | issue = 4 | pages = 1481–1488 | year = 1966 | doi = 10.1016/S0040-4020(01)99446-3 }}</ref> which also occurs during ],<ref>{{cite journal | vauthors = Küppers FJ, Bercht CA, Salemink CA, Lousberg RC, Terlouw JK, Heerma W | title=Cannabis – XV: Pyrolysis of cannabidiol. Structure elucidation of four pyrolytic products | journal=Tetrahedron| volume=31| issue=13–14| pages=1513–1516| doi=10.1016/0040-4020(75)87002-5| year=1975 }}</ref> and during smoking.<ref name = "Czégény_2021" /><ref>{{cite journal | vauthors = Quarles W, Ellman G, Jones R | title = Toxicology of marijuana: conditions for conversion of cannabidiol to THC upon smoking | journal = Clinical Toxicology | volume = 6 | issue = 2 | pages = 211–216 | date = 1973 | pmid = 4715204 | doi = 10.3109/15563657308990520 }}</ref> The synthesis of cannabidiol has been accomplished by several research groups.<ref>{{cite journal | vauthors = Petrzilka T, Haefliger W, Sikemeier C, Ohloff G, Eschenmoser A | title = | journal = Helvetica Chimica Acta | volume = 50 | issue = 2 | pages = 719–723 | date = March 1967 | pmid = 5587099 | doi = 10.1002/hlca.19670500235 }}</ref><ref>{{cite journal |vauthors=Gaoni Y, Mechoulam R | title = Boron trifluoride etherate on alumuna – a modified Lewis acid reagent. An improved synthesis of cannabidiol | journal = Tetrahedron Letters | volume = 26 | issue = 8 | pages = 1083–1086 | year = 1985 | doi = 10.1016/S0040-4039(00)98518-6 }}</ref><ref>{{cite journal | vauthors = Kobayashi Y, Takeuchi A, Wang YG | title = Synthesis of cannabidiols via alkenylation of cyclohexenyl monoacetate | journal = Organic Letters | volume = 8 | issue = 13 | pages = 2699–2702 | date = June 2006 | pmid = 16774235 | doi = 10.1021/ol060692h }}</ref> | |||
| | title=Boron trifluoride etherate on alumuna - a modified Lewis acid reagent. An improved synthesis of cannabidiol | |||
| | journal= Tetrahedron Letters | |||
| ] | |||
| | volume= 26 | |||
| | issue=8 | |||
| ===Overview=== | |||
| | pages= 1083–1086 | |||
| | year=1985 | |||
| {| class="wikitable" | |||
| | doi=10.1016/S0040-4039(00)98518-6}}</ref><ref>{{cite journal | |||
| |- | |||
| | author=Kobayashi Y, Takeuchi A, Wang YG | |||
| ! Category !! Compound !! Description | |||
| | title=Synthesis of cannabidiols via alkenylation of cyclohexenyl monoacetate | |||
| |- | |||
| | journal=Org. Lett. | |||
| | Analog || ] || fluorinated cannabidiol derivative of CBD | |||
| | volume=8 | |||
| |- | |||
| | issue=13 | |||
| | Analog || ] (CBDB) || an analogue to tetrahydrocannabutol (THCB) | |||
| | pages=2699–2702 |year=2006 |pmid=16774235 | |||
| |- | |||
| | doi=10.1021/ol060692h}}</ref> | |||
| | Analog || ] || a semi-synthetic derivative of CBD | |||
| |- | |||
| | Analog || ] (CBDD) || an analog of CBD | |||
| |- | |||
| | Analog || ] || a structural analog of CBD | |||
| |- | |||
| | Analog || ] || a structural analog of CBD | |||
| |- | |||
| | Analog || ] || a structural analog of CBD | |||
| |- | |||
| | Homologue || ] (H2CBD) || synthetic derivative of CBD | |||
| |- | |||
| | Homologue || ] (CBD-C1) || a homologue of CBD | |||
| |- | |||
| | Homologue || ] (CBDP) || the heptyl-homologue of CBD | |||
| |- | |||
| | Homologue || ] (CBDV) || a homologue of CBD | |||
| |- | |||
| | Homologue || ] || synthetic homologue of CBD | |||
| |- | |||
| | Homologue || ] || a positional isomer of CBD | |||
| |- | |||
| | Isomer || ] || a synthetic regioisomer of CBD | |||
| |- | |||
| | Metabolite || ] (7-OH-CBD) || an active metabolite of CBD | |||
| |- | |||
| | Precursor || ] (CBDA) || precursor to CBD | |||
| |- | |||
| |} | |||
| ===Biosynthesis=== | ===Biosynthesis=== | ||
| ] | |||
| '']'', produces CBD-] through the same ] as THC, until the last step, where CBDA synthase performs catalysis instead of THCA synthase.<ref>{{cite doi|10.1093/jxb/erp210}}</ref> | |||
| '']'' produces CBD through the same ] as THC, until the next to last step, where ] performs catalysis instead of ].<ref>{{cite journal | vauthors = Marks MD, Tian L, Wenger JP, Omburo SN, Soto-Fuentes W, He J, Gang DR, Weiblen GD, Dixon RA | title = Identification of candidate genes affecting Delta9-tetrahydrocannabinol biosynthesis in Cannabis sativa | journal = Journal of Experimental Botany | volume = 60 | issue = 13 | pages = 3715–3726 | year = 2009 | pmid = 19581347 | pmc = 2736886 | doi = 10.1093/jxb/erp210 }}</ref> | |||
| == Legal Status == | |||
| ===Isomerism=== | |||
| In Canada Cannabidiol is a Schedule 2 Drug, a category that encompasses quantities of cannabis less than 30 grams, and various related synthetic derivatives and preparations.<ref>http://laws.justice.gc.ca/en/showdoc/cs/C-38.8//20090606/en?command=search&caller=SI&fragment=schedule%201&search_type=all&day=6&month=6&year=2009&search_domain=cs&showall=L&statuteyear=all&lengthannual=50&length=50&offset=2</ref> In the United States it is Schedule I. <ref>http://www.justice.gov/dea/pubs/scheduling.html</ref> | |||
| {{See also|Tetrahydrocannabinol#Isomerism|Abnormal cannabidiol}} | |||
| ] | |||
| {| class="wikitable sortable mw-collapsible mw-collapsed" style="<!--font-size:small;--> clear:left; width:50%;" | |||
| == See also == | |||
| |+ class="nowrap" |Cannabidiol's 7 double bond isomers and their 30 stereoisomers | |||
| |- | |||
| ! colspan="3" | Formal numbering !! colspan="2" | Terpenoid numbering !! rowspan="2" | Number of stereoisomers !! rowspan="2" | Natural occurrence !! rowspan="2" |] Schedule !! rowspan="2" | Structure | |||
| |- | |||
| ! style="width:90px;" | Short name !! Chiral centers !! Full name !! style="width:90px;" | Short name !! Chiral centers | |||
| |- | |||
| | Δ{{sup|5}}-Cannabidiol || 1 and 3 || 2-(6-isopropenyl-3-methyl-5-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol || Δ{{sup|4}}-Cannabidiol || 1 and 3 || 4 || No || Unscheduled ||] | |||
| |- | |||
| | Δ{{sup|4}}-Cannabidiol || 1, 3 and 6 || 2-(6-isopropenyl-3-methyl-4-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol || Δ{{sup|5}}-Cannabidiol || 1, 3 and 4 || 8 || No || Unscheduled ||] | |||
| |- | |||
| | ] || 1 and 6 || 2-(6-isopropenyl-3-methyl-3-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol || Δ{{sup|6}}-Cannabidiol || 3 and 4 || 4 || Yes || Unscheduled ||] | |||
| |- | |||
| | Δ{{sup|3,7}}-Cannabidiol || 1 and 6 || 2-(6-isopropenyl-3-methylenecyclohex-1-yl)-5-pentyl-1,3-benzenediol || Δ{{sup|1,7}}-Cannabidiol || 3 and 4 || 4 || No || Unscheduled ||] | |||
| |- | |||
| |'''Δ{{sup|2}}-Cannabidiol'''||'''1 and 6'''||'''2-(6-isopropenyl-3-methyl-2-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol'''||'''Δ{{sup|1}}-Cannabidiol'''||'''3 and 4'''||'''4'''||'''Yes'''||'''Unscheduled'''||] | |||
| |- | |||
| | Δ{{sup|1}}-Cannabidiol || 3 and 6 || 2-(6-isopropenyl-3-methyl-1-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol || Δ{{sup|2}}-Cannabidiol || 1 and 4 || 4 || No || Unscheduled ||] | |||
| |- | |||
| | Δ{{sup|6}}-Cannabidiol || 3 || 2-(6-isopropenyl-3-methyl-6-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol || Δ{{sup|3}}-Cannabidiol ||1 || 2 || No || Unscheduled ||] | |||
| |} | |||
| ===Pyrolysis=== | |||
| * ] | |||
| In the typical operating temperature range of e-cigarettes ({{cvt|250–400|C}}), 25–52% of CBD is transformed into other chemical substances: ], Δ8-THC, ] and ] as predominant pyrolysates. From a chemical point of view, ] can be considered a precursor of THC.<ref name = "Czégény_2021" /> | |||
| * ]s | |||
| * ] | |||
| * ] | |||
| * ] | |||
| ===Synthetic derivatives=== | |||
| == References == | |||
| Numerous synthetic derivatives of CBD are known, and have been researched for generally similar applications as CBD itself.<ref name = "Nelson2020">{{cite journal | vauthors = Nelson KM, Bisson J, Singh G, Graham JG, Chen SN, Friesen JB, Dahlin JL, Niemitz M, Walters MA, Pauli GF | title = The Essential Medicinal Chemistry of Cannabidiol (CBD) | journal = Journal of Medicinal Chemistry | volume = 63 | issue = 21 | pages = 12137–12155 | date = November 2020 | pmid = 32804502 | doi = 10.1021/acs.jmedchem.0c00724 | pmc = 7666069 }}</ref> | |||
| {{reflist|2}} | |||
| ==History== | |||
| == External links == | |||
| Efforts to isolate the active ingredients in cannabis were made in the 19th century.<ref name="Adams" /> Cannabidiol was studied in 1940 from ] wild hemp<ref name="Adams">{{cite journal | vauthors = Adams R, Hunt M, Clark JH | title=Structure of cannabidiol, a product isolated from the marihuana extract of Minnesota wild hemp.| journal=Journal of the American Chemical Society | volume=62 | issue=1 | year=1940 | issn=0002-7863 | doi=10.1021/ja01858a058 | pages=196–200| bibcode=1940JAChS..62..196A}}</ref> and ]ian ''Cannabis indica'' resin.<ref name="Jacob">{{cite journal | vauthors = Jacob A, Todd AR | title=Cannabidiol and cannabol, constituents of ''Cannabis indica'' resin | journal=Nature | volume=145 | issue=3670 | year=1940 | issn=0028-0836 | doi=10.1038/145350a0 | pages=350| bibcode=1940Natur.145..350J | s2cid=4106662 | doi-access=free }}</ref><ref>{{cite journal | vauthors = Work TS, Bergel F, Todd AR | title = The active principles of Cannabis indica resin. I | journal = The Biochemical Journal | volume = 33 | issue = 1 | pages = 123–127 | date = January 1939 | pmid = 16746878 | pmc = 1264344 | doi = 10.1042/bj0330123 }}</ref> The chemical formula of CBD was proposed from a method for isolating it from wild hemp.<ref name="Adams" /> Its structure and ] were determined in 1963.<ref name="Mechoulam">{{cite journal | vauthors = Mechoulam R, Shvo Y | title = Hashish. I. The structure of cannabidiol | journal = Tetrahedron | volume = 19 | issue = 12 | pages = 2073–2078 | date = December 1963 | pmid = 5879214 | doi = 10.1016/0040-4020(63)85022-x }}</ref> | |||
| * Compounds found in ''Cannabis sativa'' | |||
| ===Plant breeding=== | |||
| {{Antidepressants}} | |||
| Selective breeding of cannabis plants has expanded and diversified as commercial and therapeutic markets develop. Some growers in the US succeeded in lowering the proportion of CBD-to-THC to accommodate customers who preferred varietals that were more mind-altering due to the higher THC and lower CBD content.<ref name="Romney_2012">{{cite news|vauthors=Romney L|date=September 13, 2012|title=On the frontier of medical pot to treat boy's epilepsy|work=Los Angeles Times|url=https://www.latimes.com/health/la-xpm-2012-sep-13-la-me-customized-marijuana-20120914-story.html|access-date=April 16, 2020|archive-date=October 21, 2018|archive-url=https://web.archive.org/web/20181021190630/http://articles.latimes.com/2012/sep/13/local/la-me-customized-marijuana-20120914|url-status=live}}</ref> In the US, hemp is classified by the federal government as cannabis containing no more than 0.3% THC by dry weight. This classification was established in the 2018 Farm Bill and was refined to include hemp-sourced extracts, cannabinoids, and derivatives in the definition of hemp.<ref name="cornell">{{Cite web|title=7 U.S. Code § 5940 – Legitimacy of industrial hemp research|url=https://www.law.cornell.edu/uscode/text/7/5940|access-date=November 27, 2018|website=LII / Legal Information Institute|archive-date=December 22, 2020|archive-url=https://web.archive.org/web/20201222033600/https://www.law.cornell.edu/uscode/text/7/5940|url-status=live}}</ref> | |||
| {{Anxiolytics}} | |||
| {{Antipsychotics}} | |||
| ==Society and culture== | |||
| {{Serotonergics}} | |||
| ===Names=== | |||
| ''Cannabidiol'' is the ] of the drug and its {{Abbrlink|INN|International Nonproprietary Name}}.<ref>{{cite journal|year=2016|title=International Nonproprietary Names for Pharmaceutical Substances (INN)|url=https://cdn.who.int/media/docs/default-source/international-nonproprietary-names-(inn)/pl115.pdf|journal=WHO Drug Information|volume=30|issue=2|page=241|access-date=February 18, 2023|archive-date=February 5, 2018|archive-url=https://web.archive.org/web/20180205073351/http://www.who.int/medicines/publications/druginformation/innlists/PL115.pdf|url-status=live}}</ref> | |||
| ===Foods and beverages=== | |||
| ] | |||
| Food and beverage products containing cannabidiol were widely marketed in the United States as early as 2017.<ref>{{Cite news|url=https://www.wlwt.com/article/billboard-featuring-hemp-leaf-raises-questions-about-new-beverage-for-sale-in-cincinnati/12662040|title=Billboard featuring hemp leaf raises questions about new beverage for sale in Cincinnati {{!}} WLWT5|date=September 29, 2017|work=WLWT5|access-date=September 29, 2017|archive-date=December 26, 2020|archive-url=https://web.archive.org/web/20201226180044/https://www.wlwt.com/article/billboard-featuring-hemp-leaf-raises-questions-about-new-beverage-for-sale-in-cincinnati/12662040|url-status=live}}</ref> Hemp seed ingredients which do not naturally contain THC or CBD (but which may be contaminated with trace amounts on the outside during harvesting) were declared by the US ] as ] (GRAS) in December 2018. CBD itself has not been declared GRAS, and under US federal law is illegal to sell as a food, dietary supplement, or animal feed.<ref name="fdareg" /> State laws vary considerably as non-medical cannabis and derived products have been legalized in various jurisdictions.<ref name=creswell/> Despite once having a promising market, the industry for CBD stalled out during the ] beginning in 2020, and, by 2024, it collapsed due to withdrawal of investors, the absence of a FDA ruling on efficacy and safety, inconsistent state-by-state legislation, and consumer ambivalence.<ref name="creswell">{{cite news |author1=Creswell, Julie |title=Companies were big on CBD. Not anymore |url=https://www.nytimes.com/2024/02/28/business/cbd-companies-regulations.html |access-date=March 25, 2024 |work=The New York Times |date=February 28, 2024}}</ref> | |||
| Similar to ] and ] which may contain vitamin or herbal additives, food and beverage items can be infused with CBD as an alternative means of ingesting the substance.<ref name=creswell/> In the United States, numerous products are marketed as containing CBD, but in reality contain little or none.<ref name="fdareg" /><ref>{{cite web |title = Warning Letters and Test Results for Cannabidiol-Related Products |url = https://www.fda.gov/newsevents/publichealthfocus/ucm484109.htm |publisher = US ] (FDA) |access-date = January 2, 2018 |date = November 2, 2017 |archive-date = January 26, 2018 |archive-url = https://web.archive.org/web/20180126014518/https://www.fda.gov/NewsEvents/PublicHealthFocus/ucm484109.htm |url-status = live }}</ref> Some companies marketing CBD-infused food products with claims that are similar to the effects of ]s have received ] from the FDA for making unsubstantiated ]s.<ref name="fdareg" /><ref>{{cite web | url = https://www.lexology.com/library/detail.aspx?g=fe616692-9e03-43bc-9149-8aa1a35a9d9e | title = Companies Marketing CBD Products Be Warned: FDA Is Watching | work = Lexology | vauthors = Fox A, Ravitz JR, Leongini EM, Malkin BJ | date = November 20, 2017 | access-date = December 14, 2017 | archive-date = August 3, 2020 | archive-url = https://web.archive.org/web/20200803230044/https://www.lexology.com/library/detail.aspx?g=fe616692-9e03-43bc-9149-8aa1a35a9d9e | url-status = live }}</ref> In February 2019, the ] Department of Health announced plans to ] restaurants that sell food or drinks containing CBD, beginning in October 2019.<ref>{{Cite news|url=https://www.cnbc.com/2019/02/15/new-york-may-start-fining-restaurants-for-using-weed-related-cbd-.html|title=New York City plans to fine restaurants using CBD in food and drinks|vauthors=La Vito A, Franck T|date=February 15, 2019|newspaper=CNBC|access-date=February 19, 2019|archive-date=November 7, 2020|archive-url=https://web.archive.org/web/20201107233204/https://www.cnbc.com/2019/02/15/new-york-may-start-fining-restaurants-for-using-weed-related-cbd-.html|url-status=live}}</ref> | |||
| ===Labeling and advertising=== | |||
| Studies conducted by the FDA from 2014 through 2019 have determined that a majority of CBD products are not accurately labeled with the amount of CBD they contain.<ref name=FDAReport>{{cite web | title = Sampling Study of the Current Cannabidiol Marketplace to Determine the Extent That Products are Mislabeled or Adulterated Report in Response to Further Consolidated Appropriations Act, 2020 | publisher = United States Food and Drug Administration | date = July 2020 | url = https://hempindustrydaily.com/wp-content/uploads/2020/07/CBD-Marketplace-Sampling_RTC_FY20_Final.pdf | access-date = January 30, 2021 | archive-date = June 23, 2021 | archive-url = https://web.archive.org/web/20210623193320/https://hempindustrydaily.com/wp-content/uploads/2020/07/CBD-Marketplace-Sampling_RTC_FY20_Final.pdf | url-status = live }}</ref> For example, a 2017 analysis of cannabidiol content in oil, ], or liquid ] products purchased online in the United States showed that 69% were mislabeled, with 43% having higher and 26% having lower content than stated on ]s.<ref name="Bonn">{{cite journal | vauthors = Bonn-Miller MO, Loflin MJ, Thomas BF, Marcu JP, Hyke T, Vandrey R | title = Labeling Accuracy of Cannabidiol Extracts Sold Online | journal = JAMA | volume = 318 | issue = 17 | pages = 1708–1709 | date = November 2017 | pmid = 29114823 | pmc = 5818782 | doi = 10.1001/jama.2017.11909 }}</ref><ref name="dawn">{{cite news|vauthors=MacKeen D|date=October 16, 2019|title=Scam or Not: What Are the Benefits of CBD?|work=The New York Times|url=https://www.nytimes.com/2019/10/16/style/self-care/cbd-oil-benefits.html|access-date=October 17, 2019 |archive-date=October 16, 2019|archive-url=https://web.archive.org/web/20191016221458/https://www.nytimes.com/2019/10/16/style/self-care/cbd-oil-benefits.html|url-status=live}}</ref> In 2020, the FDA conducted a study of 147 CBD products and found that half contained THC.<ref name=FDAReport/><ref>{{cite journal | vauthors = Evans DG | title = Medical Fraud, Mislabeling, Contamination: All Common in CBD Products | journal = Missouri Medicine | volume = 117 | issue = 5 | pages = 394–399 | year = 2020 | pmid = 33311737 | pmc = 7723146 }}</ref> | |||
| From 2015 to November 2022, the FDA issued dozens of ] to American manufacturers of CBD products for false advertising and illegal interstate marketing of CBD as an unapproved ] to treat diseases, such as cancer, ], symptoms of ], Alzheimer's disease, and ] disorders.<ref name="fda11-22">{{cite web |title=FDA Warns Companies for Illegally Selling Food and Beverage Products that Contain CBD |url=https://www.fda.gov/food/cfsan-constituent-updates/fda-warns-companies-illegally-selling-food-and-beverage-products-contain-cbd |publisher=US Food and Drug Administration |access-date=November 23, 2022 |date=November 21, 2022 |quote=These companies are selling CBD containing products that people may confuse for traditional foods or beverages which may result in unintentional consumption or overconsumption of CBD. CBD-containing products in forms that are appealing to children, such as gummies, hard candies and cookies, are especially concerning. |archive-date=November 23, 2022 |archive-url=https://web.archive.org/web/20221123224059/https://www.fda.gov/food/cfsan-constituent-updates/fda-warns-companies-illegally-selling-food-and-beverage-products-contain-cbd |url-status=live }}</ref> Chemical analysis of CBD products found that many did not contain the levels of CBD claimed in advertising.<ref name=fda11-22/> | |||
| In December 2020, the ] initiated a law enforcement crackdown on American companies marketing CBD products as unapproved drugs.<ref name="ftc">{{cite web|title=FTC Announces Crackdown on Deceptively Marketed CBD Products|url=https://www.ftc.gov/news-events/press-releases/2020/12/ftc-announces-crackdown-deceptively-marketed-cbd-products|publisher=US ] (FTC)|access-date=January 12, 2021|date=December 17, 2020|archive-date=June 22, 2021|archive-url=https://web.archive.org/web/20210622223827/https://www.ftc.gov/news-events/press-releases/2020/12/ftc-announces-crackdown-deceptively-marketed-cbd-products|url-status=live}}</ref><ref name="rooted">{{cite web |vauthors=Ashley DD, Engle MK |title=Warning letter: Rooted Apothecary LLC |url=https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/rooted-apothecary-llc-585312-10102019 |publisher=Office of Compliance, Center for Drug Evaluation and Research, US Food and Drug Administration; US Federal Trade Commission |access-date=October 23, 2019 |date=October 22, 2019 |archive-date=December 12, 2019 |archive-url=https://web.archive.org/web/20191212105951/https://www.fda.gov/inspections-compliance-enforcement-and-criminal-investigations/warning-letters/rooted-apothecary-llc-585312-10102019 |url-status=live }}</ref> The warning also applied to hemp CBD ] and oil that were being marketed illegally while not adhering to the federal definition of a ].<ref name="rooted" /> | |||
| ===Sports=== | |||
| {{See also|Cannabis and sports}} | |||
| Cannabidiol has been used by professional and amateur athletes across disciplines and countries, with the ] removing CBD from its ] list. The ] and United Kingdom-Anti-Doping Agency do not have anti-CBD policies, with the latter stating that, "CBD is not currently listed on the World Anti-Doping Agency Prohibited List. As a result, it is permitted to use in sport, though the intended benefits are unclear and not backed by clinical evidence. All other cannabinoids (including but not limited to cannabis, hashish, marijuana, and THC) are prohibited in-competition. The intention of the regulations is to prohibit cannabinoids that activate the same receptors in the brain as activated by THC."<ref>{{Cite web|url=https://www.ukad.org.uk/athlete-advisory-note-cannabidiol-cbd|title=Athlete Advisory Note: Cannabidiol (CBD)|publisher=United Kingdom Anti-Doping Agency|access-date=October 9, 2019|archive-date=October 9, 2019|archive-url=https://web.archive.org/web/20191009110419/https://www.ukad.org.uk/athlete-advisory-note-cannabidiol-cbd|url-status=live}}</ref><ref name="usada">{{cite web |title=Athletes: 6 things to know about cannabidiol |url=https://www.usada.org/spirit-of-sport/education/six-things-know-about-cannabidiol/ |publisher=US Anti-Doping Agency |access-date=February 17, 2020 |date=October 23, 2018 |archive-date=October 11, 2021 |archive-url=https://web.archive.org/web/20211011032929/https://www.usada.org/spirit-of-sport/education/six-things-know-about-cannabidiol/ |url-status=live }}</ref> | |||
| In 2019, the cannabis manufacturer ] acquired majority ownership of ], which is developing CBD products under endorsement by numerous professional athletes.<ref name="spencer">{{cite news |vauthors=Donna S |title=Canopy cannabis company buys ex-NHL player's sports nutrition business |url=https://www.ctvnews.ca/business/canopy-cannabis-company-buys-ex-nhl-player-s-sports-nutrition-business-1.4620013 |access-date=February 17, 2020 |work=CTV Business |publisher=The Canadian Press |date=October 2, 2019 |quote=BioSteel's brand ambassadors also include well-known athletes across major sports leagues in North America, which could be beneficial as the company's attempt to push regulated CBD nutrition products into the mainstream health and wellness segments |archive-date=November 26, 2020 |archive-url=https://web.archive.org/web/20201126082519/https://www.ctvnews.ca/business/canopy-cannabis-company-buys-ex-nhl-player-s-sports-nutrition-business-1.4620013 |url-status=live }}</ref> The ] Alumni Association began a project with Canopy Growth to determine if CBD or other cannabis products might improve neurological symptoms and quality of life in head-injured players.<ref name=spencer/> Some sports leagues have announced sponsorships with CBD companies, such as ] (Charlotte's Web) and ] (Love Hemp).<ref>{{cite press release |date=October 12, 2022 |title=Major League Baseball, pioneering CBD brand Charlotte's Web strike groundbreaking deal |url=https://www.mlb.com/press-release/press-release-major-league-baseball-pioneering-cbd-brand-charlotte-s-web-strike- |publisher=Major League Baseball}}</ref><ref>{{cite press release |date=March 18, 2021 |title=UFC Names Love Hemp Official Global CBD Partner |url=https://www.ufc.com/news/ufc-names-love-hemp-official-global-cbd-partner |publisher=Ultimate Fighting Championship}}</ref> Numerous professional athletes use CBD, primarily for treating pain.<ref name=spencer/><ref name="loudin">{{cite news |vauthors=Loudin A |title=As more pro athletes use cannabis for aches and pain, the more they run afoul of rules |url=https://www.washingtonpost.com/health/as-more-pro-athletes-use-cannabis-for-aches-and-pain-the-more-they-run-afoul-of-rules/2019/12/06/7dbd82fc-00c7-11ea-8bab-0fc209e065a8_story.html |access-date=February 17, 2020 |newspaper=The Washington Post |date=December 7, 2019 |archive-date=November 24, 2020 |archive-url=https://web.archive.org/web/20201124094701/https://www.washingtonpost.com/health/as-more-pro-athletes-use-cannabis-for-aches-and-pain-the-more-they-run-afoul-of-rules/2019/12/06/7dbd82fc-00c7-11ea-8bab-0fc209e065a8_story.html |url-status=live }}</ref><ref name="harrison">{{cite magazine |vauthors=Harrison S |title=Lots of athletes say CBD Is a better painkiller. Is It? |url=https://www.wired.com/story/lots-of-athletes-say-cbd-is-a-better-painkiller-is-it/ |access-date=February 17, 2020 |magazine=Wired |date=September 28, 2019 |archive-date=July 12, 2021 |archive-url=https://web.archive.org/web/20210712160248/https://www.wired.com/story/lots-of-athletes-say-cbd-is-a-better-painkiller-is-it/ |url-status=live }}</ref> | |||
| ===Legal status=== | |||
| ====Australia==== | |||
| Prescription medicine (Schedule 4) for therapeutic use containing two percent (2.0%) or less of other cannabinoids commonly found in cannabis (such as ∆{{sup|9}}-THC). A Schedule 4 drug under the ] is a Prescription Only Medicine, or Prescription Animal Remedy – Substances, the use or supply of which should be by or on the order of persons permitted by state or territory legislation to prescribe and should be available from a pharmacist on prescription.<ref>{{cite web |url=https://www.legislation.gov.au/Details/F2017L00605 |title=Poisons Standard June 2017 |publisher=Legislation.gov.au |access-date=December 4, 2016 |archive-date=December 13, 2020 |archive-url=https://web.archive.org/web/20201213060305/https://www.legislation.gov.au/Details/F2017L00605/ |url-status=live }}</ref> | |||
| In June 2020, the Australian Therapeutic Goods Administration (TGA) published a consultation on a proposal to pave the way to make "low dose" CBD available to consumer/patients via pharmacists only through moving products from Schedule 4 to 3.<ref>{{Cite web|author=Australian Government Department of Health Therapeutic Goods Administration|date=April 24, 2020|title=Consultation: Proposed amendments to the Poisons Standard – Joint ACMS/ACCS meetings, June 2020|url=https://www.tga.gov.au/consultation-invitation/consultation-proposed-amendments-poisons-standard-joint-acmsaccs-meetings-june-2020|access-date=November 25, 2020|website=Therapeutic Goods Administration (TGA)|language=en|archive-date=May 11, 2021|archive-url=https://web.archive.org/web/20210511053302/https://www.tga.gov.au/consultation-invitation/consultation-proposed-amendments-poisons-standard-joint-acmsaccs-meetings-june-2020|url-status=live}}</ref> Any products sold would need to have their safety, quality and efficacy pre-assessed by the TGA and be formally approved for sale (details to be outlined by TGA). They would be made available to over 18s only, with the maximum daily dose of 60{{nbsp}}mg/day, up to 2% THC finished product allowed, 30-day maximum supply, plant-derived or synthetic. This proposal is based on an initial literature review on the safety of low dose CBD published by the TGA in April 2020.<ref>{{cite web | title = Safety of low dose cannabidiol | date = April 2020 | agency = Therapeutic Goods Administration | publisher = Government of Australia | url = https://www.tga.gov.au/sites/default/files/review-safety-low-dose-cannabidiol.pdf | access-date = November 25, 2020 | archive-date = September 7, 2021 | archive-url = https://web.archive.org/web/20210907175547/https://www.tga.gov.au/sites/default/files/review-safety-low-dose-cannabidiol.pdf | url-status = live }}</ref> Epidyolex was approved for the adjunctive therapy of seizures associated with ] or with ] in September 2020.<ref name="Epidyolex TGA" /> | |||
| ====Bulgaria==== | |||
| In 2020, Bulgaria became the first country in the European Union to allow retail sales of food products and supplements containing CBD, despite the ongoing discussion within the EU about the classification of CBD as a ].<ref>{{Cite web|url=https://www.forbes.com/sites/javierhasse/2019/05/23/this-eu-country-has-apparently-become-the-first-to-allow-free-sale-of-cbd/|title=This EU country has become the first to allow free sale Of CBD|vauthors=Hasse J|work=Forbes|access-date=February 21, 2020|archive-date=October 6, 2021|archive-url=https://web.archive.org/web/20211006020147/https://www.forbes.com/sites/javierhasse/2019/05/23/this-eu-country-has-apparently-become-the-first-to-allow-free-sale-of-cbd/|url-status=live}}</ref> However, there exists a legal gap because of the lack of a legally-permissible minimum amount of THC in the products containing cannabinoids.<ref>{{Cite web|vauthors=Todorova SE|title=Growing cannabis in Bulgaria: Legal but still stigmatised|url=https://www.lexology.com/library/detail.aspx?g=b18a928d-6437-4544-b834-12cd169bd9b2|access-date=September 3, 2020|website=Lexology|date=July 29, 2019|archive-date=April 11, 2021|archive-url=https://web.archive.org/web/20210411021658/https://www.lexology.com/library/detail.aspx?g=b18a928d-6437-4544-b834-12cd169bd9b2|url-status=live}}</ref> | |||
| ====Canada==== | |||
| In October 2018, cannabidiol became legal for recreational and medical use by the federal '']''.<ref name="cbdact">{{cite web |title=Cannabidiol (CBD) |url=https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/about/cannabidiol.html#a4 |publisher=Government of Canada |access-date=October 11, 2019 |date=August 13, 2019 |archive-date=September 18, 2019 |archive-url=https://web.archive.org/web/20190918124123/https://www.canada.ca/en/health-canada/services/drugs-medication/cannabis/about/cannabidiol.html#a4 |url-status=live }}</ref><ref>{{cite web |title=Health products containing cannabis or for use with cannabis: Guidance for the Cannabis Act, the Food and Drugs Act, and related regulations |url=https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/guidance-documents/guidance-cannabis-act-food-and-drugs-act-related-regulations/document.html |publisher=Government of Canada |access-date=October 19, 2018 |date=July 11, 2018 |archive-date=October 19, 2018 |archive-url=https://web.archive.org/web/20181019121912/https://www.canada.ca/en/health-canada/services/drugs-health-products/drug-products/applications-submissions/guidance-documents/guidance-cannabis-act-food-and-drugs-act-related-regulations/document.html |url-status=live }}</ref><ref>{{cite web|url=https://www.justice.gc.ca/eng/cj-jp/cannabis/|title=Cannabis Legalization and Regulation|publisher=Government of Canada|work=Department of Justice, Electronic Communications|date=June 20, 2018|access-date=November 24, 2018|archive-date=August 24, 2021|archive-url=https://web.archive.org/web/20210824170353/https://www.justice.gc.ca/eng/cj-jp/cannabis/|url-status=live}}</ref> {{as of|2019|August}}, CBD products in Canada could only be sold by authorized retailers or federally licensed medical companies, limiting their access to the general public.<ref name="cbc19">{{cite web |title='It should be available': Natural health food stores hope to cash in on CBD craze |url=https://www.cbc.ca/news/canada/ottawa/natural-health-food-retailers-cannabis-cbd-market-1.5237911 |work=CBC News |access-date=October 11, 2019 |date=August 6, 2019 |archive-date=October 12, 2019 |archive-url=https://web.archive.org/web/20191012060444/https://www.cbc.ca/news/canada/ottawa/natural-health-food-retailers-cannabis-cbd-market-1.5237911 |url-status=live }}</ref> Nonetheless, with online delivery services and over 2,600 authorized cannabis retail stores {{as of|October 2021|lc=y}}, accessibility has steadily increased over time.<ref>{{cite web |url=https://www.statista.com/statistics/1035996/number-of-cannabis-stores-by-region-canada/ |title=Number of cannabis stores in Canada as of June 2021, by region |vauthors=Conway J |publisher=Statista |date=September 17, 2021 |access-date=May 2, 2022 |archive-date=May 2, 2022 |archive-url=https://web.archive.org/web/20220502201541/https://www.statista.com/statistics/1035996/number-of-cannabis-stores-by-region-canada/ |url-status=live }}</ref><ref>{{cite news |vauthors=Armstrong M |url=https://theconversation.com/amp/cannabis-store-openings-in-canada-only-slightly-affected-the-number-of-users-169055 |title=Cannabis store openings in Canada only slightly affected the number of users |work=The Conversation |date=October 13, 2021 |access-date=May 2, 2022 |archive-date=May 2, 2022 |archive-url=https://web.archive.org/web/20220502201541/https://theconversation.com/amp/cannabis-store-openings-in-canada-only-slightly-affected-the-number-of-users-169055 |url-status=live }}</ref> The Canadian government states that CBD products "are subject to all of the rules and requirements that apply to cannabis under the Cannabis Act and its regulations."<ref name=cbdact/> It requires "a processing licence to manufacture products containing CBD for sale, no matter what the source of the CBD is, and that CBD and products containing CBD, such as cannabis oil, may only be sold by an authorized retailer or licensed seller of medical CBD."<ref name=cbdact/> Edible CBD products were scheduled to be permitted for sale in Canada on October 17, 2019, for human consumption.<ref name=cbdact/> | |||
| {{As of|August 2020}}, it was still illegal to carry cannabis and cannabis-derived products (including products containing CBD) across the Canadian border. If one carries any amount of cannabis for any purpose (including medical), it needs to be declared to the ]. Not declaring it is a serious criminal offence.<ref>{{Cite web |author=Canada Health |date=June 20, 2018 |title=Cannabis and Canadian borders |url=https://www.canada.ca/en/services/health/campaigns/cannabis/border.html |access-date=September 3, 2020 |website=aem |archive-date=August 14, 2021 |archive-url=https://web.archive.org/web/20210814083741/https://www.canada.ca/en/services/health/campaigns/cannabis/border.html |url-status=live }}</ref> | |||
| ====Czech Republic==== | |||
| As of May 2023, the ] of the Czech Republic is putting together broad regulations regarding a ban on CBD products.<ref>{{Cite web |title=Ministerstvo zemědělství informuje o chystaném zákazu uvádění na trh produktů obsahujících kanabidiol (CBD) a jiné kanabinoidy (eAGRI) |url=https://eagri.cz/public/web/mze/tiskovy-servis/tiskove-zpravy/x2023_ministerstvo-zemedelstvi-informuje-o.html |access-date=May 8, 2023 |website=eagri.cz |language=cs}}</ref> They will make it illegal to sell products containing cannabidiol and other cannabinoids derived from hemp, as a result of ]. In case of Czech Republic, ] has submitted an official request to the Czech Republic to recognize natural hemp extracts with cannabinoids as traditional food.<ref>{{Cite web | vauthors = Milosz F |date=May 6, 2023 |title=EIHA reacts about CBD and hemp extracts |url=https://cannabizeu.com/eiha-reacts-about-cbd-and-hemp-extracts/ | archive-url = https://web.archive.org/web/20230506175817/https://cannabizeu.com/eiha-reacts-about-cbd-and-hemp-extracts/ | archive-date = May 6, 2023 |access-date=May 8, 2023 |website=CannabizEU |language=en-US}}</ref> | |||
| ====European Union==== | |||
| ] | |||
| In 2019, the ] announced that CBD and other cannabinoids would be classified as "]s",<ref name="chu">{{cite web | title=Updated EC ruling for CBD classes supplement ingredient as Novel Food | publisher=NutraIngredients.com, William Reed Business Media Ltd. | vauthors=Chu W | date=January 31, 2019 | url=https://www.nutraingredients.com/Article/2019/01/31/Updated-EFSA-ruling-for-CBD-classes-supplement-ingredient-as-Novel-Food | access-date=January 1, 2019 | archive-date=November 1, 2021 | archive-url=https://web.archive.org/web/20211101213517/https://www.nutraingredients.com/Article/2019/01/31/Updated-EC-ruling-for-CBD-classes-supplement-ingredient-as-Novel-Food | url-status=live }}</ref> meaning that CBD products would require authorization under the ] stating that because "this product was not used as a food or food ingredient before May 15, 1997, before it may be placed on the market in the EU as a food or food ingredient, a safety assessment under the Novel Food Regulation is required."<ref name="ec2019">{{cite web | title=Cannabinoids, searched in the EU Novel food catalogue (v.1.1) | publisher=European Commission | url=http://ec.europa.eu/food/safety/novel_food/catalogue/search/public/index.cfm | access-date=February 1, 2019 | date=January 1, 2019 | archive-date=April 3, 2019 | archive-url=https://web.archive.org/web/20190403221542/http://ec.europa.eu/food/safety/novel_food/catalogue/search/public/index.cfm | url-status=live }}</ref> The recommendation – applying to CBD extracts, synthesized CBD, and all CBD products, including CBD oil – was scheduled for a final ruling by the European Commission in March 2019.<ref name=chu/> If approved, manufacturers of CBD products would be required to conduct safety tests and prove safe consumption, indicating that CBD products would not be eligible for legal commerce until at least 2021.<ref name=chu/> In December 2020, the European Commission concluded that CBD should not be considered as drug and can be qualified as food.<ref>{{Cite web|date=December 2, 2020|title=European Commission reverses course, says CBD should not be regulated as a narcotic|url=https://hempindustrydaily.com/breaking-european-commission-reverses-course-says-cbd-should-not-be-regulated-as-a-narcotic/|access-date=December 4, 2020|website=Hemp Industry Daily|language=en-US|archive-date=October 21, 2021|archive-url=https://web.archive.org/web/20211021163656/https://hempindustrydaily.com/breaking-european-commission-reverses-course-says-cbd-should-not-be-regulated-as-a-narcotic/|url-status=live}}</ref> | |||
| Cannabidiol is listed in the EU Cosmetics Ingredient Database (CosIng).<ref name="cos">{{cite web|url=http://ec.europa.eu/growth/tools-databases/cosing/index.cfm?fuseaction=search.details_v2&id=93486|title=CosIng – Cosmetics – Cannabidiol|publisher=European Commission|access-date=December 4, 2016|archive-date=January 13, 2019|archive-url=https://web.archive.org/web/20190113182333/http://ec.europa.eu/growth/tools-databases/cosing/index.cfm?fuseaction=search.details_v2&id=93486|url-status=live}}</ref> However, the listing of an ingredient, assigned with an ] name, in CosIng does not mean it is to be used in cosmetic products or is approved for such use.<ref name=cos/> | |||
| Several industrial hemp varieties can be legally cultivated in ]. A variety such as "Fedora 17" has a cannabinoid profile consistently around 1%, with ] less than 0.3%.<ref>{{cite journal | vauthors = Fournier G, Beherec O, Bertucelli S | title = Intérêt du rapport Δ-9-THC / CBD dans le contrôle des cultures de chanvre industriel |trans-title=The advantage of the Δ-9-THC / CBD ratio in the control of industrial hemp crops |language=fr | journal = Annales de Toxicologie Analytique | volume = 15 | issue = 4 | pages = 250–259 | year = 2003 | doi = 10.1051/ata/2003003 | doi-access = free }}</ref> | |||
| ====Hong Kong==== | |||
| In 2022, the ] proposed a ban on any use of cannabidiol (including for academic research and by medical professionals) within the Hong Kong territory, making ] the first jurisdiction in the world to have complete prohibition of cannabidiol, starting from February 1, 2023,<ref>{{cite web |title=Orders to amend Dangerous Drugs Ordinance and Control of Chemicals Ordinance to be gazetted on October 21 and cannabidiol to become dangerous drug |url=https://www.info.gov.hk/gia/general/202210/20/P2022102000492.htm?fontSize=1 |publisher=Hong Kong SAR Government |access-date=October 20, 2022 |archive-date=October 20, 2022 |archive-url=https://web.archive.org/web/20221020134136/https://www.info.gov.hk/gia/general/202210/20/P2022102000492.htm?fontSize=1 |url-status=live }}</ref> in part due to the possible presence of THC which is illegal in Hong Kong, according to a research subsidized by the Hong Kong SAR Government.<ref>{{Cite web |date=June 11, 2022 |title=What you need to know about a proposed ban on CBD products in Hong Kong |url=https://www.scmp.com/news/hong-kong/health-environment/article/3181288/hong-kong-has-proposed-banning-cbd-products |access-date=September 25, 2022 |website=South China Morning Post |language=en |archive-date=September 25, 2022 |archive-url=https://web.archive.org/web/20220925034831/https://www.scmp.com/news/hong-kong/health-environment/article/3181288/hong-kong-has-proposed-banning-cbd-products |url-status=live }}</ref><ref>{{Cite web |vauthors=Leung H |date=August 21, 2022 |title=Hong Kong's zero-tolerance approach to drugs leaves budding CBD industry high and dry |url=https://hongkongfp.com/2022/08/21/hong-kongs-zero-tolerance-approach-to-drugs-leaves-budding-cbd-industry-high-and-dry/ |access-date=September 25, 2022 |website=Hong Kong Free Press HKFP |language=en-GB |archive-date=April 20, 2023 |archive-url=https://web.archive.org/web/20230420184936/https://hongkongfp.com/2022/08/21/hong-kongs-zero-tolerance-approach-to-drugs-leaves-budding-cbd-industry-high-and-dry/ |url-status=live }}</ref><ref>{{Cite web | author = Legislative Council Panel on Security | date = June 7, 2022 | publisher = Government of Hong Kong | title = Proposed Control of Cannabidiol through Legislation | url = https://www.legco.gov.hk/yr2022/english/panels/se/papers/se20220607cb2-380-3-e.pdf | access-date = October 24, 2022 | archive-date = September 28, 2022 | archive-url = https://web.archive.org/web/20220928210959/https://www.legco.gov.hk/yr2022/english/panels/se/papers/se20220607cb2-380-3-e.pdf | url-status = live }}</ref> | |||
| ====New Zealand==== | |||
| In 2017, the New Zealand government made changes to the regulations so that restrictions would be removed, which meant a doctor was able to prescribe cannabidiol to patients.<ref>{{cite web|title=Doctors now able to prescribe cannabidiol|date=June 2, 2017|url=https://www.radionz.co.nz/news/political/332137/doctors-now-able-to-prescribe-cannabidiol|access-date=June 2, 2017|publisher=radionz.co.nz|archive-date=June 1, 2017|archive-url=https://web.archive.org/web/20170601230111/http://www.radionz.co.nz/news/political/332137/doctors-now-able-to-prescribe-cannabidiol|url-status=live}}</ref> | |||
| The passing of the ] in December 2018 means cannabidiol is no longer a controlled drug in New Zealand, but is a prescription medicine under the Medicines Act, with the restriction that "the tetrahydrocannabinols (THCs) and specified substances within the product must not exceed 2 percent of the total CBD, tetrahydrocannabinol (THC) and other specified substances."<ref>{{cite web|title=CBD products|url=https://www.health.govt.nz/our-work/regulation-health-and-disability-system/medicines-control/medicinal-cannabis/cbd-products|access-date=March 10, 2019|publisher=www.health.govt.nz|archive-date=February 4, 2019|archive-url=https://web.archive.org/web/20190204024704/https://www.health.govt.nz/our-work/regulation-health-and-disability-system/medicines-control/medicinal-cannabis/cbd-products|url-status=live}}</ref> | |||
| ====Russian Federation==== | |||
| According to a document received in response to an appeal to the Ministry of Internal Affairs of the Russian Federation, measures of state control in the Russian Federation regarding CBD have not been established. However, there is also a response from the Ministry of Health of the Russian Federation indicating that CBD can be considered as an isomer of restricted ]. The "]" argument is nonetheless vague, as ], which is freely sold in pharmacies, is also an isomer of THC, all three being {{chem|C|21|H|30|O|2}}.<ref>{{Cite web|url=https://pravoved.ru/question/2412784/|title=Является ли вещество CBD (каннабидиол) разрешенным к использованию на территории России? – Правовед.RU|access-date=February 19, 2021|archive-date=November 1, 2021|archive-url=https://web.archive.org/web/20211101213628/https://pravoved.ru/question/2412784/|url-status=live}}</ref> On February 17, 2020, the deputy of the Moscow City Duma ] sent an official request to the Prime Minister of the Russian Federation ] with a request to eliminate that legal ambiguity by publishing official explanations and, if necessary, making required changes in the corresponding government decree.<ref>{{Cite web|url=https://besedina.moscow/messaging/242|title=Журнал переписки депутата Бесединой|access-date=February 19, 2021|archive-date=February 19, 2021|archive-url=https://web.archive.org/web/20210219064618/https://besedina.moscow/messaging/242|url-status=live}}</ref> | |||
| ====Singapore==== | |||
| Singapore allows medical cannabis on a case-by-case basis, usually as a last resort drug. Each case is evaluated by the government, and largely comes in the form of Cannabidiol. However, the country is flexible to what is required for patient treatment, despite having some of the strictest drug laws in the world. | |||
| ====Sweden==== | |||
| Cannabidiol is classified as a medical product in Sweden.<ref>{{cite web|url=https://lakemedelsverket.se/Alla-nyheter/NYHETER---2018/CBD-produkter-ska-folja-lakemedelslagen/|title=CBD products should follow the drug laws|publisher=Swedish Medical Products Agency|date=April 4, 2018|access-date=July 31, 2018|archive-date=July 24, 2018|archive-url=https://web.archive.org/web/20180724032251/https://lakemedelsverket.se/Alla-nyheter/NYHETER---2018/CBD-produkter-ska-folja-lakemedelslagen/|url-status=live}}</ref> However, in July 2019, ] ruled that CBD oil with any concentration of THC falls under the narcotic control laws.<ref>{{Cite web|vauthors=Arnold M|date=July 30, 2019|title=Sweden Joins Italy In Path To Defining CBD Oil Regulations|url=https://cannabisindustryjournal.com/news_article/sweden-joins-italy-in-path-to-defining-cbd-oil-regulations/|access-date=September 3, 2020|website=Cannabis Industry Journal|archive-date=November 8, 2020|archive-url=https://web.archive.org/web/20201108021557/https://cannabisindustryjournal.com/news_article/sweden-joins-italy-in-path-to-defining-cbd-oil-regulations/|url-status=live}}</ref> | |||
| ====Switzerland==== | |||
| While THC remains illegal, cannabidiol is not subject to the Swiss Narcotic Acts because it does not produce a comparable psychoactive effect.<ref>{{Cite web|title=Cannabis: What is allowed, what is not allowed in Switzerland? – www.ch.ch|url=https://www.ch.ch/en/safety-and-justice/police/cannabis/|access-date=September 3, 2020|website=www.ch.ch|archive-date=January 21, 2021|archive-url=https://web.archive.org/web/20210121154628/https://www.ch.ch/en/safety-and-justice/police/cannabis/|url-status=live}}</ref> Cannabis products containing less than 1% THC can be sold and purchased legally.<ref>{{Cite web|collaboration=Vischer AG|vauthors=Kohler S, Benz AS, Pruschy D|title=Trends in the Swiss Cannabis Regulation {{!}} Lexology|url=https://www.lexology.com/library/detail.aspx?g=f50473b1-32a3-43a1-a05a-acafc5c937f6|access-date=September 3, 2020|website=www.lexology.com|date=April 11, 2019|archive-date=January 17, 2021|archive-url=https://web.archive.org/web/20210117113611/https://www.lexology.com/library/detail.aspx?g=f50473b1-32a3-43a1-a05a-acafc5c937f6|url-status=live}}</ref><ref>{{cite web|title=Cannabis à faible teneur en THC et CBD|url=https://www.bag.admin.ch/bag/fr/home/themen/mensch-gesundheit/sucht/cannabis/thc-armer-cannabis-cbd.html|url-status=dead|archive-url=https://web.archive.org/web/20170326123332/https://www.bag.admin.ch/bag/fr/home/themen/mensch-gesundheit/sucht/cannabis/thc-armer-cannabis-cbd.html|archive-date=March 26, 2017|access-date=May 20, 2017|publisher=BAG.Admin.ch|language=fr}}</ref> | |||
| ====Ukraine==== | |||
| On April 7, 2021, the Ukrainian government legalised use of isolated cannabidiol. Additionally, it approved ], a cannabidiol-containing drug, for medical use.<ref>{{cite news |url=https://life.pravda.com.ua/health/2021/04/9/244505/ |vauthors=Cross D |work=УП.Життя (UP.Life) |date=April 9, 2021 |language=Ukrainian |title=В Україні легалізували використання медичного канабісу, але не всього |trans-title=Ukraine has legalized the use of medical cannabis, but not all of it |access-date=April 10, 2021 |archive-date=April 9, 2021 |archive-url=https://web.archive.org/web/20210409202156/https://life.pravda.com.ua/health/2021/04/9/244505/ |url-status=live }}</ref> | |||
| ====United Kingdom==== | |||
| Cannabidiol, in an oral-mucosal spray formulation combined with ], is a product available by prescription for the relief of severe spasticity due to multiple sclerosis (where other anti-]s have not been effective) in the United Kingdom.<ref name="Sativex SmPC">{{cite web | title=Sativex Oromucosal Spray – Summary of Product Characteristics (SmPC) | website=(emc) | date=August 25, 2020 | url=https://www.medicines.org.uk/emc/product/602/smpc | access-date=September 11, 2020 | archive-date=February 4, 2021 | archive-url=https://web.archive.org/web/20210204162637/https://www.medicines.org.uk/emc/product/602/smpc | url-status=live }}</ref> | |||
| Until 2017, products containing cannabidiol marketed for medical purposes were classed as medicines by the UK ], the ] (MHRA), and could not be marketed without regulatory approval for the medical claims.<ref>{{cite news|title=MHRA statement on products containing Cannabidiol (CBD)|url=https://www.gov.uk/government/news/mhra-statement-on-products-containing-cannabidiol-cbd|publisher=Gov.uk|date=December 14, 2016|access-date=December 14, 2016|archive-date=May 10, 2019|archive-url=https://web.archive.org/web/20190510061128/https://www.gov.uk/government/news/mhra-statement-on-products-containing-cannabidiol-cbd|url-status=live}}</ref><ref>{{Cite web|title=UK Classifies CBD Oil as a Medicinal Ingredient|url=https://www.buycbd.net/blog/uk-classifies-cbd-oil-as-a-medicinal-ingredient/|access-date=September 3, 2020|website=BuyCBD.net|archive-date=November 1, 2021|archive-url=https://web.archive.org/web/20211101213519/https://www.buycbd.net/blog/uk-classifies-cbd-oil-as-a-medicinal-ingredient/|url-status=live}}</ref> {{as of|2018}}, cannabis oil is legal to possess, buy, and sell in the UK, providing the product does not contain more than 1 milligram of THC and is not advertised as providing a medicinal benefit.<ref name="Home Office">{{cite news|vauthors=Stallworth J|title=Drug licensing factsheet: cannabis, CBD and other cannabinoids|newspaper=The Home Office|url=https://www.gov.uk/government/publications/cannabis-cbd-and-other-cannabinoids-drug-licensing-factsheet/drug-licensing-factsheet-cannabis-cbd-and-other-cannabinoids#the-exempted-product-definition---regulation-2-of-the-mdr-2001|access-date=December 10, 2020|archive-date=January 17, 2021|archive-url=https://web.archive.org/web/20210117213102/https://www.gov.uk/government/publications/cannabis-cbd-and-other-cannabinoids-drug-licensing-factsheet/drug-licensing-factsheet-cannabis-cbd-and-other-cannabinoids#the-exempted-product-definition---regulation-2-of-the-mdr-2001|url-status=live}}</ref> Individual police officers and others who are ill-informed of the exact legislature pertaining to cannabidiol, however, may erroneously consider it of dubious legality, reflecting lack of awareness.<ref>Ukaegbu O, Smith J, Hall D, Frain T, Abbasian C. Staff awareness of the use of cannabidiol (CBD): a trust-wide survey study in the UK. Journal of Cannabis Research. 2021 Dec;3(1):1-0.</ref> | |||
| In January 2019, the UK ] indicated it would regard CBD products, including CBD oil, as a '']'' having no history of use before May 1997, and stated that such products must have authorisation and proven safety before being marketed.<ref name="chu" /><ref name="gunn">{{cite web | vauthors=Gunn L, Haigh L | title=British watchdog deems CBD a novel food, seeks to curtail sale on UK market | publisher=Nutrition Insight, CNS Media BV | date=January 29, 2019 | url=https://www.nutritioninsight.com/news/british-watchdog-deems-cbd-a-novel-food-seeks-to-ban-sale-on-uk-market.html | access-date=January 1, 2019 | archive-url=https://web.archive.org/web/20190202042806/https://www.nutritioninsight.com/news/british-watchdog-deems-cbd-a-novel-food-seeks-to-ban-sale-on-uk-market.html | archive-date=February 2, 2019 }}</ref> The deadline for companies with existing products to submit a full and validated novel foods application with the FSA was March 31, 2021; failure to do so before this date would exclude those companies from selling CBD.<ref name="fsa">{{cite web|date=September 24, 2020|title=Cannabidiol (CBD) guidance – Business guidance on cannabidiol (CBD) as a novel food|url=https://www.food.gov.uk/business-guidance/cannabidiol-cbd|access-date=December 8, 2020|publisher=UK Food Standards Agency|archive-date=October 30, 2021|archive-url=https://web.archive.org/web/20211030090033/https://www.food.gov.uk/business-guidance/cannabidiol-cbd|url-status=live}}</ref> New products containing CBD after this deadline would require a fully approved application.<ref>{{Cite web|vauthors=Marsat N|date=July 7, 2020|title=CBD and Novel Foods regulation in the UK: regulation then and now|url=https://www.healtheuropa.eu/cbd-and-novel-foods-regulation-in-the-uk-regulation-then-and-now/101187/|access-date=December 10, 2020|website=Health Europa|language=en-GB|archive-date=October 6, 2020|archive-url=https://web.archive.org/web/20201006014147/https://www.healtheuropa.eu/cbd-and-novel-foods-regulation-in-the-uk-regulation-then-and-now/101187/|url-status=live}}</ref> | |||
| In February 2020, the UK FSA advised vulnerable people, such as pregnant women, breastfeeding mothers, and those already taking medication for other medical concerns not to take CBD. The FSA further recommended that healthy adults should not consume more than 70{{nbsp}}mg CBD per day.<ref name="fsa" /> | |||
| ====United Nations==== | |||
| Cannabidiol is scheduled under the ] as cannabis. International Narcotics Control Board reminds Member States that, at the reconvened sixty-third session of the Commission on Narcotic Drugs, in December 2020, the States members of the Commission rejected the recommendation of WHO that a footnote be added to the entry for cannabis and cannabis resin in Schedule I of the 1961 Convention as amended to exempt from international control preparations containing predominantly CBD and not more than 0.2 per cent of delta-9-THC.<ref>{{Cite web |url=https://www.incb.org/documents/Publications/AnnualReports/AR2021/Annual_Report/E_INCB_2021_1_eng.pdf |title=Reports published by the International Narcotics Control Board for 2021 |access-date=December 1, 2022 |archive-date=November 30, 2022 |archive-url=https://web.archive.org/web/20221130103840/https://www.incb.org/documents/Publications/AnnualReports/AR2021/Annual_Report/E_INCB_2021_1_eng.pdf |url-status=live }}</ref> | |||
| ====United States==== | |||
| {{As of|2023}}, cannabidiol extracted from marijuana remains a ],<ref name="fdareg" /><ref name="Mead">{{cite journal | vauthors = Mead A | title = The legal status of cannabis (marijuana) and cannabidiol (CBD) under US law | journal = Epilepsy & Behavior | volume = 70 | issue = Pt B | pages = 288–291 | date = May 2017 | pmid = 28169144 | doi = 10.1016/j.yebeh.2016.11.021 | doi-access = free }}</ref><ref>{{Cite web|url=https://www.congress.gov/bill/115th-congress/house-bill/2/text|title=Text – H.R.2 – 115th Congress (2017-2018): Agriculture Improvement Act of 2018|vauthors=Conaway KM|date=December 20, 2018|website=Congress.gov|access-date=May 1, 2019|archive-date=October 6, 2021|archive-url=https://web.archive.org/web/20211006230238/https://www.congress.gov/bill/115th-congress/house-bill/2/text|url-status=live}}</ref> and is not approved as a ] or ] or allowed for interstate commerce in the United States.<ref name="fda11-26-19">{{cite web |title=What You Need to Know (And What We're Working to Find Out) About Products Containing Cannabis or Cannabis-derived Compounds, Including CBD |url=https://www.fda.gov/consumers/consumer-updates/what-you-need-know-and-what-were-working-find-out-about-products-containing-cannabis-or-cannabis |publisher=US Food and Drug Administration |access-date=June 23, 2022 |date=March 5, 2020 |archive-date=October 22, 2021 |archive-url=https://web.archive.org/web/20211022185821/https://www.fda.gov/consumers/consumer-updates/what-you-need-know-and-what-were-working-find-out-about-products-containing-cannabis-or-cannabis |url-status=live }}</ref> CBD derived from hemp (with 0.3% THC or lower) is legal to sell as a cosmetics ingredient or for other purposes not regulated by the FDA, but cannot be sold under federal law as an ingredient in food, dietary supplement, or animal feed.<ref name="fdareg" /><ref name="bell">{{cite web |publisher=] |url=https://sciencebasedmedicine.org/fda-no-cbd-in-dietary-supplements-or-foods-for-now-but-lets-talk/ |vauthors=Bellamy J |date=May 9, 2019 |title=FDA: No CBD in dietary supplements or foods for now, but let's talk |access-date=June 15, 2019 |archive-date=August 17, 2020 |archive-url=https://web.archive.org/web/20200817142121/https://sciencebasedmedicine.org/fda-no-cbd-in-dietary-supplements-or-foods-for-now-but-lets-talk/ |url-status=live }}</ref> It is a common misconception that the legal ability to sell hemp (which may contain CBD), and hemp extracts and derivatives (including CBD), makes CBD legal for sale as a supplement or medicine.<ref name="bell" /><ref name="fda-hemp">{{cite web |vauthors=Abernethy A, Schiller L |title=FDA is Committed to Sound, Science-based Policy on CBD |url=https://www.fda.gov/news-events/fda-voices-perspectives-fda-leadership-and-experts/fda-committed-sound-science-based-policy-cbd |publisher=US ] (FDA) |access-date=October 17, 2019 |date=July 17, 2019 |archive-date=November 14, 2019 |archive-url=https://web.archive.org/web/20191114232749/https://www.fda.gov/news-events/fda-voices-perspectives-fda-leadership-and-experts/fda-committed-sound-science-based-policy-cbd |url-status=live }}</ref> | |||
| In September 2018, the ] drug Epidiolex was placed in Schedule V of the ] by the ] (DEA),<ref name="CNBC">{{Cite news | url = https://www.cnbc.com/2018/09/27/dea-schedules-epidiolex-allowing-gw-pharma-to-start-selling-the-drug.html | title = DEA reschedules Epidiolex, marijuana-derived drug, paving the way for it to hit the market | publisher = CNBC | date = September 27, 2018 | access-date = October 3, 2018 | archive-date = April 26, 2021 | archive-url = https://web.archive.org/web/20210426202908/https://www.cnbc.com/2018/09/27/dea-schedules-epidiolex-allowing-gw-pharma-to-start-selling-the-drug.html | url-status = live }}</ref> following its approval by the FDA for rare types of childhood epilepsy.<ref name="Epidiolex FDA label"/> It was then removed from the Controlled Substances Act by the DEA in April 2020.<ref>{{cite news | vauthors = Wood S |title=DEA relaxes rules for the only federally approved drug derived from marijuana |url=https://www.inquirer.com/business/weed/epidiolex-cbd-cannabidiol-gw-pharma-roark-dea-fda-20200406.html |access-date=April 23, 2023 |work=The Philadelphia Inquirer |date=April 6, 2020 |archive-url=https://web.archive.org/web/20200609035500/https://www.inquirer.com/business/weed/epidiolex-cbd-cannabidiol-gw-pharma-roark-dea-fda-20200406.html |archive-date=June 9, 2020}}</ref> Epidiolex is available for prescription use in all 50 states.<ref>{{cite news | vauthors = Tinker B |title=First FDA-approved cannabis-based drug now available in the US |url=https://www.cnn.com/2018/11/01/health/marijuana-drug-epidiolex-prescription/index.html |access-date=April 23, 2023 |work=CNN |date=November 2, 2018}}</ref> | |||
| In 2013, a ] program that featured ] brought increased attention to the use of CBD for the treatment of seizure disorders in children.<ref name="MaaFigi2014">{{cite journal | vauthors = Maa E, Figi P | title = The case for medical marijuana in epilepsy | journal = Epilepsia | volume = 55 | issue = 6 | pages = 783–786 | date = June 2014 | pmid = 24854149 | doi = 10.1111/epi.12610 | s2cid = 4849160 | doi-access = free }}</ref><ref name="CNN charlotte">{{cite web | vauthors = Saundra Y | title = Marijuana stops child's severe seizures | url = http://www.cnn.com/2013/08/07/health/charlotte-child-medical-marijuana/index.html | website = CNN | date = August 7, 2013 | access-date = May 14, 2018 | archive-date = May 14, 2018 | archive-url = https://web.archive.org/web/20180514144200/https://www.cnn.com/2013/08/07/health/charlotte-child-medical-marijuana/index.html | url-status = live }}</ref> A number of states passed laws over the next few years to allow the use of low-THC, high-CBD cannabis oil in such situations.<ref name="ncsl states">{{cite web|title=State Medical Marijuana Laws|url=http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx|website=National Conference of State Legislatures|date=April 27, 2018|access-date=May 14, 2018|archive-date=December 11, 2018|archive-url=https://web.archive.org/web/20181211125951/http://www.ncsl.org/research/health/state-medical-marijuana-laws.aspx|url-status=live}}</ref> These states were in addition to the states that had already legalized cannabis for ] or ] use.<ref name="ncsl states" /> Many states further relaxed their laws regarding CBD following the passage of the 2018 Farm Bill.<ref>{{cite news | vauthors = Schuman B, Fisher J, Radke B, Faldetta G |title=A Survey of State CBD & Hemp Regulation Since The 2018 Farm Bill |url=https://cannabisindustryjournal.com/feature_article/a-survey-of-state-cbd-hemp-regulation-since-the-2018-farm-bill/ |access-date=April 24, 2023 |work=Cannabis Industry Journal |date=October 22, 2020}}</ref><ref>{{cite news | vauthors = Gebhart F |title=The Evolution of the CBD Regulatory Landscape |url=https://www.drugtopics.com/view/the-evolution-of-the-cbd-regulatory-landscape |access-date=April 24, 2023 |work=Drug Topics Journal |date=September 13, 2022}}</ref> | |||
| The ]<ref>{{Cite web|url=http://www.thefarmbill.com/the-bill|title=The 2014 Farm Bill|website=thefarmbill.com|access-date=November 27, 2018|archive-date=October 30, 2021|archive-url=https://web.archive.org/web/20211030111724/https://www.thefarmbill.com/the-bill|url-status=live}}</ref> legalized the sale of "non-viable hemp material" grown within states participating in the Hemp Pilot Program which defined ''hemp'' as cannabis containing less than 0.3% of THC.<ref>{{Cite news|url=https://www.forbes.com/sites/monazhang/2018/04/05/no-cbd-is-not-legal-in-all-50-states/|title=No, CBD Is Not 'Legal in All 50 States'|vauthors=Zhang M|work=Forbes|access-date=November 27, 2018|archive-date=December 5, 2020|archive-url=https://web.archive.org/web/20201205235942/https://www.forbes.com/sites/monazhang/2018/04/05/no-cbd-is-not-legal-in-all-50-states/|url-status=live}}</ref> The ] removed the hemp plant and all "derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a delta-9 tetrahydrocannabinol concentration of not more than 0.3 percent on a dry weight basis," including CBD, from the ], making them legal to manufacture in the United States.<ref name="Mead 2019"/><ref>{{USC|7|1639o}}</ref><ref>{{USC|21|802}}</ref> The FDA retains regulatory authority over hemp-derived CBD,<ref name="fda-hemp" /> while the ] is not involved in the regulation of legally-compliant hemp and hemp products.<ref name="dea">{{cite web |title=DEA announces steps necessary to improve access to marijuana research |url=https://www.dea.gov/press-releases/2019/08/26/dea-announces-steps-necessary-improve-access-marijuana-research |publisher=United States Drug Enforcement Administration |access-date=October 18, 2019 |date=August 26, 2019 |archive-date=October 18, 2019 |archive-url=https://web.archive.org/web/20191018222642/https://www.dea.gov/press-releases/2019/08/26/dea-announces-steps-necessary-improve-access-marijuana-research |url-status=live }}</ref> The 2018 Farm Bill requires that research and development of CBD for a therapeutic purpose would have to be conducted under notification and reporting to the FDA.<ref name="fda-hemp" /><ref name="hahn" /> | |||
| ==Veterinary use== | |||
| ===Research=== | |||
| The number of research projects and scientific publications on cannabidiol and other cannabinoids in pets surged in the late 2010s; nonetheless, {{As of|December 2020|lc=y}}, there were no hemp-derived, cannabinoid-rich registered veterinary medicinal products in any of the major regions (see ]). | |||
| In the US and other territories there are, however, numerous veterinary nutraceutical products available over the counter (OTC). The lack of clarity in the regulations governing veterinary hemp ]s allows for products of questionable quality to flood the market,<ref name="fda7-19">{{cite web |title=FDA warns company marketing unapproved cannabidiol products with unsubstantiated claims to treat cancer, Alzheimer's disease, opioid withdrawal, pain and pet anxiety |url=https://www.fda.gov/news-events/press-announcements/fda-warns-company-marketing-unapproved-cannabidiol-products-unsubstantiated-claims-treat-cancer |publisher=US Food and Drug Administration (FDA) |access-date=July 24, 2019 |date=July 23, 2019 |quote=Unlike drugs approved by the FDA, the manufacturing process of these products has not been subject to FDA review as part of the drug approval process, and there has been no FDA evaluation of whether these products are effective for their intended use, what the proper dosage is, how they could interact with FDA-approved drugs, or whether they have dangerous side effects or other safety concerns. |archive-date=August 14, 2019 |archive-url=https://web.archive.org/web/20190814014310/https://www.fda.gov/news-events/press-announcements/fda-warns-company-marketing-unapproved-cannabidiol-products-unsubstantiated-claims-treat-cancer |url-status=live }}</ref><ref name="Wakshlag_2020a">{{cite journal | vauthors = Wakshlag JJ, Cital S, Eaton SJ, Prussin R, Hudalla C | title = Cannabinoid, Terpene, and Heavy Metal Analysis of 29 Over-the-Counter Commercial Veterinary Hemp Supplements | journal = Veterinary Medicine: Research and Reports | volume = 11 | pages = 45–55 | date = April 15, 2020 | pmid = 32346530 | pmc = 7169471 | doi = 10.2147/vmrr.s248712 | doi-access = free }}</ref> which may pose a risk to the wellbeing of pets and owners. | |||
| To understand better the benefits of CBD and associated compounds for the quality of life of animals, companies specialized in CBD products for animals have been funding research projects.<ref name="McGrath_2019">{{cite journal | vauthors = McGrath S, Bartner LR, Rao S, Packer RA, Gustafson DL | title = Randomized blinded controlled clinical trial to assess the effect of oral cannabidiol administration in addition to conventional antiepileptic treatment on seizure frequency in dogs with intractable idiopathic epilepsy | journal = Journal of the American Veterinary Medical Association | volume = 254 | issue = 11 | pages = 1301–1308 | date = June 2019 | pmid = 31067185 | doi = 10.2460/javma.254.11.1301 | s2cid = 148569810 }}</ref><ref name="Verrico_2020">{{cite journal | vauthors = Verrico CD, Wesson S, Konduri V, Hofferek CJ, Vazquez-Perez J, Blair E, Dunner K, Salimpour P, Decker WK, Halpert MM | title = A randomized, double-blind, placebo-controlled study of daily cannabidiol for the treatment of canine osteoarthritis pain | journal = Pain | volume = 161 | issue = 9 | pages = 2191–2202 | date = September 2020 | pmid = 32345916 | pmc = 7584779 | doi = 10.1097/j.pain.0000000000001896 }}</ref><ref name="Mogi_2019">{{cite journal|vauthors=Mogi C, Fukuyama T|title=Cannabidiol as a potential anti-epileptic dietary supplement in dogs with suspected epilepsy: three case reports|url=http://www.uco.es/ucopress/ojs/index.php/pet/issue/view/960|journal=Pet Behaviour Science|year=2019|issue=7|pages=11–16|doi=10.21071/pbs.v0i7|issn=2445-2874|doi-access=free|access-date=December 17, 2020|archive-date=September 3, 2020|archive-url=https://web.archive.org/web/20200903190519/https://www.uco.es/ucopress/ojs/index.php/pet/issue/view/960|url-status=live}}</ref><ref name="Wakshlag_2020">{{cite journal | vauthors = Wakshlag JJ, Schwark WS, Deabold KA, Talsma BN, Cital S, Lyubimov A, Iqbal A, Zakharov A | title = Pharmacokinetics of Cannabidiol, Cannabidiolic Acid, Δ9-Tetrahydrocannabinol, Tetrahydrocannabinolic Acid and Related Metabolites in Canine Serum After Dosing With Three Oral Forms of Hemp Extract | journal = Frontiers in Veterinary Science | volume = 7 | pages = 505 | date = September 4, 2020 | pmid = 33102539 | pmc = 7498943 | doi = 10.3389/fvets.2020.00505 | doi-access = free }}</ref> | |||
| ===Canine osteoarthritis=== | |||
| CBD's ability to help regulate the endocannabinoid system<ref>{{cite journal | vauthors = Ibrahim MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP, Vanderah TW, Lai J, Porreca F, Makriyannis A, Malan TP | title = Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 100 | issue = 18 | pages = 10529–10533 | date = September 2003 | pmid = 12917492 | pmc = 193595 | doi = 10.1073/pnas.1834309100 | bibcode = 2003PNAS..10010529I | doi-access = free }}</ref><ref>{{cite journal | vauthors = Hohmann AG, Farthing JN, Zvonok AM, Makriyannis A | title = Selective activation of cannabinoid CB2 receptors suppresses hyperalgesia evoked by intradermal capsaicin | journal = The Journal of Pharmacology and Experimental Therapeutics | volume = 308 | issue = 2 | pages = 446–453 | date = February 2004 | pmid = 14610224 | doi = 10.1124/jpet.103.060079 | s2cid = 17663780 }}</ref><ref>{{cite book | vauthors = Jung KM, Piomelli D | chapter = Cannabinoids and Endocannabinoids|date=2015 | title =Neuroscience in the 21st Century|pages=1–31| veditors = Pfaff DW, Volkow ND |place=New York|publisher=Springer New York|language=en|doi=10.1007/978-1-4614-6434-1_136-1|isbn=978-1461464341 }}</ref> and reduce the release of excitatory neurotransmitters could result in a retrograde inhibitory signal that lessens chronic pain responses. Studies in dogs with chronic pain associated with osteoarthritis showed an increase in level of activity in animals receiving CBD-rich ]s.<ref name="Gamble_2018">{{cite journal |vauthors=Gamble LJ, Boesch JM, Frye CW, Schwark WS, Mann S, Wolfe L, Brown H, Berthelsen ES, Wakshlag JJ |date=July 23, 2018 |title=Pharmacokinetics, Safety, and Clinical Efficacy of Cannabidiol Treatment in Osteoarthritic Dogs |journal=Frontiers in Veterinary Science |volume=5 |pages=165 |doi=10.3389/fvets.2018.00165 |pmc=6065210 |pmid=30083539 |doi-access=free}}</ref><ref name="Brioschi_2020">{{cite journal |vauthors=Brioschi FA, Di Cesare F, Gioeni D, Rabbogliatti V, Ferrari F, D'Urso ES, Amari M, Ravasio G |date=August 2020 |title=Oral Transmucosal Cannabidiol Oil Formulation as Part of a Multimodal Analgesic Regimen: Effects on Pain Relief and Quality of Life Improvement in Dogs Affected by Spontaneous Osteoarthritis |journal=Animals |volume=10 |issue=9 |pages=1505 |doi=10.3390/ani10091505 |pmc=7552307 |pmid=32858828 |doi-access=free}}</ref><ref name="Kogan_2020">{{Cite journal |vauthors=Kogan L, Hellyer P, Downing R |year=2020 |title=Hemp Oil Extract to Treat Canine Osteoarthritis-Related Pain: A Pilot Study |url=https://www.researchgate.net/publication/339698157 |url-status=live |journal=Journal of the American Holistic Veterinary Medical Association |volume=58 |pages=35–45 |archive-url=https://web.archive.org/web/20211101213526/https://www.researchgate.net/publication/339698157_The_Use_of_Cannabidiol-Rich_Hemp_Oil_Extract_to_Treat_Canine_Osteoarthritis-Related_Pain_A_Pilot_Study |archive-date=November 1, 2021 |access-date=December 17, 2020}}</ref><ref name="Verrico_2020" /> | |||
| ===Epilepsy=== | |||
| From the results seen in humans with drugs such as Epidiolex and Sativex in scientific studies and reviews,<ref>{{Cite book| vauthors = Russo E |title=Handbook of psychotropic herbs: a scientific analysis of herbal remedies for psychiatric conditions|date=2001|publisher=Haworth Herbal Press|isbn=0789007185|location=New York|oclc=43810871}}</ref> it could be expected that CBD-based products would be helpful to manage seizures in dogs. However, despite the numerous case reports presented by veterinary neurologists supporting the benefits of CBD as adjunctive therapy, {{as of|December 2020|lc=y}}, published controlled studies have not shown a statistically significant decrease in the number of seizures across the groups receiving CBD.<ref name="McGrath_2019" /><ref name="Mogi_2019" /> | |||
| ===Pharmacokinetics=== | |||
| The oral bioavailability of CBD varies greatly across species and it is linked to the presentation and the time of administration.<ref name="Deabold_2019">{{cite journal |vauthors=Deabold KA, Schwark WS, Wolf L, Wakshlag JJ |date=October 2019 |title=Single-Dose Pharmacokinetics and Preliminary Safety Assessment with Use of CBD-Rich Hemp Nutraceutical in Healthy Dogs and Cats |journal=Animals |volume=9 |issue=10 |pages=832 |doi=10.3390/ani9100832 |pmc=6826847 |pmid=31635105 |doi-access=free}}</ref><ref name="Wakshlag_2020" /><ref name="Vaughn_2020">{{cite journal | vauthors = Vaughn D, Kulpa J, Paulionis L | title = Preliminary Investigation of the Safety of Escalating Cannabinoid Doses in Healthy Dogs | journal = Frontiers in Veterinary Science | volume = 7 | pages = 51 | date = February 11, 2020 | pmid = 32118071 | pmc = 7029731 | doi = 10.3389/fvets.2020.00051 | doi-access = free }}</ref> A 24-hour kinetic examination in dogs showed that the absorption of the cannabidiolic acid (CBDA) does occur, and that this molecule is absorbed least twice as well as CBD post oral ingestion.<ref name="Deabold_2019" /><ref name="Wakshlag_2020" /><ref>{{cite journal | vauthors = Della Rocca G, Di Salvo A | title = Hemp in Veterinary Medicine: From Feed to Drug | journal = Frontiers in Veterinary Science | volume = 7 | pages = 387 | date = July 28, 2020 | pmid = 32850997 | pmc = 7399642 | doi = 10.3389/fvets.2020.00387 | doi-access = free }}</ref> | |||
| It was found that the major metabolites of CBD in humans (] and 7-COOH-CBD) are not prevalent in dogs, while 6-OH-CBD was found to be the primary metabolite in dogs receiving a CBD-enriched cannabis-derived herbal extract,<ref>{{cite journal | vauthors = Chicoine A, Illing K, Vuong S, Pinto KR, Alcorn J, Cosford K | title = Pharmacokinetic and Safety Evaluation of Various Oral Doses of a Novel 1:20 THC:CBD ''Cannabis'' Herbal Extract in Dogs | journal = Frontiers in Veterinary Science | volume = 7 | pages = 583404 | date = September 29, 2020 | pmid = 33134364 | pmc = 7550466 | doi = 10.3389/fvets.2020.583404 | doi-access = free }}</ref> suggesting that canine and human CBD metabolic route might be somewhat different.<ref name="Vaughn_2020" /> | |||
| ==Research== | |||
| {{As of|2022}}, little evidence exists for cannabidiol reducing ]s in humans.<ref name=kirkland/><ref name="black" /><ref name="mayo" /><ref name="prud" /><ref name="Hoch">{{cite journal | vauthors = Hoch E, Niemann D, von Keller R, Schneider M, Friemel CM, Preuss UW, Hasan A, Pogarell O | title = How effective and safe is medical cannabis as a treatment of mental disorders? A systematic review | journal = European Archives of Psychiatry and Clinical Neuroscience | volume = 269 | issue = 1 | pages = 87–105 | date = February 2019 | pmid = 30706168 | pmc = 6595000 | doi = 10.1007/s00406-019-00984-4 }}</ref> | |||
| In the United States, federal illegality complicates conducting historic research on CBD.<ref>{{cite news | vauthors = Sanders L |title=The CBD boom is way ahead of the science |url=https://www.sciencenews.org/article/cbd-product-boom-science-research-hemp-marijuana |access-date=April 24, 2023 |work=Science News |date=March 27, 2019}}</ref> | |||
| ==References== | |||
| {{Reflist}} | |||
| ==Further reading== | |||
| {{Refbegin}} | |||
| * | |||
| * {{Cite news |title=Why Is CBD Everywhere? |vauthors=Williams A |work=] |date=October 27, 2018 |url=https://www.nytimes.com/2018/10/27/style/cbd-benefits.html |issn=0362-4331 |access-date=November 17, 2018 |archive-date=October 22, 2021 |archive-url=https://web.archive.org/web/20211022054134/https://www.nytimes.com/2018/10/27/style/cbd-benefits.html |url-status=live }} | |||
| * {{Skeptoid | id= 4780| number= 780| title=CBD for Everything: Cannabidiol is sold as an additive to just about every kind of product you can imagine. Why?| date=May 18, 2021| access-date=May 21, 2022}} | |||
| {{Refend}} | |||
| {{Navboxes | |||
| |title = Articles related to Cannabidiol | |||
| |list = | |||
| {{Cannabis}} | |||
| {{Cannabinoids}} | {{Cannabinoids}} | ||
| {{Navboxes | |||
| | title = ] | |||
| | titlestyle = background:#ccccff | |||
| | list1 = | |||
| {{Analgesics}} | |||
| {{Anticonvulsants}} | |||
| }} | |||
| {{Navboxes | |||
| | title = ] | |||
| | titlestyle = background:#ccccff | |||
| | list1 = | |||
| {{Cannabinoid receptor modulators}} | |||
| {{Opioid receptor modulators}} | |||
| {{Serotonin receptor modulators}} | |||
| {{Transient receptor potential channel modulators}} | |||
| }} | |||
| }} | |||
| {{Portal bar | Medicine}} | |||
| {{Authority control}} | |||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 22:34, 4 January 2025
Phytocannabinoid discovered in 1940 Not to be confused with cannabinol or cannabinodiol.Pharmaceutical compound
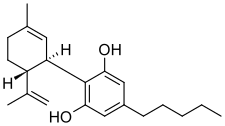 | |
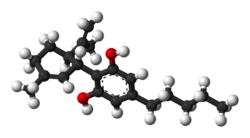 | |
| Clinical data | |
|---|---|
| Pronunciation | /kæ.nə.bə.ˈdaɪ.əl/ |
| Trade names | Epidiolex, Epidyolex |
| Other names | CBD, cannabidiolum, (−)-cannabidiol |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a618051 |
| License data |
|
| Pregnancy category |
|
| Addiction liability | None |
| Routes of administration | By mouth, sublingual, buccal,inhalation |
| Drug class | Cannabinoid |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability |
|
| Elimination half-life | 18–32 hours |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| PDB ligand | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.215.986 |
| Chemical and physical data | |
| Formula | C21H30O2 |
| Molar mass | 314.469 g·mol |
| 3D model (JSmol) | |
| Melting point | 66 °C (151 °F) |
| Boiling point | 160–180 °C (320–356 °F) |
| Solubility in water | Insoluble |
SMILES
| |
InChI
| |
| (verify) | |
| Part of a series on |
| Cannabis |
|---|
 |
|
Chemistry
|
Pharmacology
|
| Consumption |
| Economics |
| Effects |
| Forms |
Law
|
Variants
|
| Related |
Cannabidiol (CBD) is a phytocannabinoid, one of 113 identified cannabinoids in cannabis plants, along with tetrahydrocannabinol (THC), and accounts for up to 40% of the plant's extract. Medically, it is an anticonvulsant used to treat multiple forms of epilepsy. It was discovered in 1940 and, as of 2024 clinical research on CBD included studies related to the treatment of anxiety, addiction, psychosis, movement disorders, and pain, but there is insufficient high-quality evidence that CBD is effective for these conditions. CBD is sold as an herbal dietary supplement and promoted with yet unproven claims of particular therapeutic effects.
Cannabidiol can be taken internally in multiple ways, including by inhaling cannabis smoke or vapor, swallowing it by mouth, and through use of an aerosol spray into the cheek. It may be supplied as CBD oil containing only CBD as the active ingredient (excluding THC or terpenes), CBD-dominant hemp extract oil, capsules, dried cannabis, or prescription liquid solution. CBD does not have the same psychoactivity as THC, and can modulate the psychoactive effects of THC on the body if both are present. Conversion of CBD to THC can occur when CBD is heated to temperatures between 250–300 °C, potentially leading to its partial transformation into THC.
In the United States, the cannabidiol drug Epidiolex was approved by the Food and Drug Administration (FDA) in 2018 for the treatment of two seizure disorders. While the 2018 United States Farm Bill removed hemp and hemp extracts (including CBD) from the Controlled Substances Act, the marketing and sale of CBD formulations for medical use or as an ingredient in dietary supplements or manufactured foods remains illegal under FDA regulation, as of 2024.
Medical uses
Epilepsy
See also: Charlotte's Web (cannabis)In the United States, the FDA has indicated only one brand of prescription cannabidiol called Epidiolex for the treatment of seizures associated with Dravet syndrome, Lennox–Gastaut syndrome, or tuberous sclerosis complex in people one year of age and older. While Epidiolex treatment is generally well tolerated, it is associated with minor adverse effects, such as gastrointestinal upset, decreased appetite, lethargy, sleepiness, and poor sleep quality.
In the European Union, Epidyolex is indicated for use as adjunctive therapy of seizures associated with Lennox–Gastaut syndrome or Dravet syndrome, in conjunction with clobazam, for people two years of age and older. In 2020, the label for Epidiolex in the US was expanded to include seizures associated with tuberous sclerosis complex. Epidiolex/Epidyolex is the first prescription formulation of plant-derived cannabidiol approved by regulatory bodies in the US and Europe.
Other uses
Research on other uses for cannabidiol includes several neurological disorders, but the findings have not been confirmed to establish such uses in clinical practice. In October 2019, the FDA issued an advisory warning that the effects of CBD during pregnancy or breastfeeding are unknown, indicating that the safety, doses, interactions with other drugs or foods, and side effects of CBD are not clinically defined, and may pose a risk to the mother and infant.
Many claims are made for the therapeutic benefit of cannabidiol that are not backed by sound evidence. Some claims, such as treatment of cancer, are pseudoscience.
In 2020, the label for Epidiolex in the US was expanded to include treatment of seizures associated with tuberous sclerosis.
Acclaimed for relieving chronic pain, some researchers conclude that the evidence is insufficient to determine the effectiveness of CBD in pain relief, primarily due to the challenging access to pure CBD.
CBD oil is used for massage therapy as a substitute for body oil and for its health benefits.
Non-intoxicating effects
Cannabidiol does not appear to have any intoxicating effects such as those caused by ∆-THC in cannabis, but it is under preliminary research for its possible anxiolytic and antipsychotic effects. As the legal landscape and understanding about the differences in medical cannabinoids unfolds, experts are working to distinguish "medical cannabis" (with varying degrees of psychotropic effects and deficits in executive function) from "medical CBD therapies", which would commonly present as having a reduced or non-psychoactive side-effect profile.
Various strains of "medical cannabis" are found to have a significant variation in the ratios of CBD-to-THC and are known to contain other non-psychotropic cannabinoids. Any psychoactive cannabis, regardless of its CBD content, is derived from the flower (or bud) of the genus Cannabis. As defined by US federal law, non-psychoactive hemp (also commonly termed "industrial hemp"), regardless of its CBD content, is any part of the cannabis plant, whether growing or not, containing a ∆-tetrahydrocannabinol concentration of no more than 0.3% on a dry-weight basis. In the United States, certain standards are required for legal growing, cultivating, and producing the hemp plant, but there are no federal standards for quality being enforced in the hemp industry. Certain state regulations are in place, but vary state to state. For instance, the Colorado Industrial Hemp Program registers growers of industrial hemp and samples crops to verify that the dry-weight THC concentration does not exceed 0.3%.
Available forms
CBD is present as an active constituent of cannabis, which is used both recreationally and medically.
Nabiximols (brand name Sativex), an oromucosal spray made of a complex botanical mixture containing cannabidiol (CBD), delta-9-tetrahydrocannabinol (THC), and additional cannabinoid and non-cannabinoid constituents from cannabis sativa plants, was approved by Health Canada in 2005, to treat central neuropathic pain in multiple sclerosis, and in 2007, for cancer-related pain. In New Zealand, Sativex is "approved for use as an add-on treatment for symptom improvement in people with moderate to severe spasticity due to multiple sclerosis who have not responded adequately to other anti-spasticity medication."
Epidiolex (Epidyolex in Europe) is an orally administered cannabidiol solution. It was approved in 2018 for treatment of two rare forms of childhood epilepsy, Lennox–Gastaut syndrome and Dravet syndrome, and seizures associated with tuberous sclerosis complex. In the US, it is approved in these indications for people one year of age and older.
Side effects
Research indicates that cannabidiol may reduce adverse effects of THC, particularly those causing intoxication and sedation, but only at high doses. Safety studies of cannabidiol showed it is well tolerated, but may cause fatigue, somnolence, sedation, diarrhea, or changes in appetite as common adverse effects, with the most common being somnolence and sedation. Side effects of CBD are dose related. Epidiolex documentation lists sleepiness, insomnia and poor quality sleep, decreased appetite, diarrhea, and fatigue.
In November 2019, the FDA issued concerns about the safety of cannabidiol, stating that CBD use has potential to cause hepatotoxicity, interfere with the mechanisms of prescription drugs, produce gastrointestinal disorders, or affect alertness and mood. Over 2020–23, the FDA updated its safety concerns about CBD, acknowledging the unknown effects of protracted use, how it affects the developing brain, fetus, or infants during breastfeeding, whether it interacts with dietary supplements or prescription drugs, whether male fertility is affected, and its possible side effects, such as drowsiness.
As of September 2019, 1,085 people contacted US poison control centers about CBD-induced illnesses, doubling the number of cases over the 2018 rate and increasing by 9 times the case numbers of 2017. Of cases reported in 2019, more than 33% received medical attention and 46 people were admitted to a hospital intensive care unit, possibly due to exposure to other products, or drug interactions with CBD.
In 2022, the FDA stated that "scientific studies show possible harm to the male reproductive system, including testicular atrophy, harm to the liver, and interactions with certain medications. The FDA has not found adequate information showing how much CBD can be consumed, and for how long, before causing harm. This is particularly true for vulnerable populations like children and those who are pregnant."
Interactions
Laboratory evidence indicated that cannabidiol may reduce THC clearance, increasing plasma concentrations which may raise THC availability to receptors and enhance its effect in a dose-dependent manner. In vitro, cannabidiol inhibited the activity of voltage-dependent sodium and potassium channels, which may affect neural activity. A recent study using X-ray crystallography showed that CBD binds inside the sodium channel pore at a novel site at the interface of the fenestrations and the central hydrophobic cavity of the channel. Binding at this site blocks the transmembrane-spanning sodium ion translocation pathway, providing a molecular mechanism for channel inhibition, which could contribute to a reduced excitability. A small clinical trial reported that CBD partially inhibited the CYP2C-catalyzed hydroxylation of THC to 11-OH-THC. Little is known about potential drug interactions, but CBD mediates a decrease in clobazam metabolism. Work with human liver microsomes shows that cannabidiol inhibits CYP3A5 and CYP3A4 to some degree.
Pharmacology
Pharmacodynamics
In vitro, cannabidiol has low affinity for, and acts as a negative allosteric modulator of the CB1 cannabinoid receptor
Cannabidiol may be an antagonist of GPR55, a G protein-coupled receptor and putative non-homologous CB3 cannabinoid receptor shown by in vitro studies to be widely distributed in the brain. Cannabidiol may interact with various neurotransmitters, such as serotonin, dopamine, and GABA.
As of 2024, the cellular effects and mechanisms of cannabidiol in vivo are unknown, as research to date has been inconclusive and based on laboratory studies. The anticonvulsant effects provided by cannabidiol (Epidiolex) in people with certain forms of epilepsy do not appear to involve cannabinoid receptors. A possible mechanism for the effects of cannabidiol on seizures is by affecting the neuronal movement of calcium in brain structures involved in the excessive electrical activity of seizures.
Pharmacokinetics
The oral bioavailability of cannabidiol is approximately 6% in fasting state and 36.5—57.3% in fed-state in humans, while its bioavailability via inhalation is 11 to 45% (mean 31%). The oral bioavailability of cannabidiol varies based on several factors such as formulation, dose, and food intake. The sublingual bioavailability of cannabidiol is approximately 12 to 35%. The elimination half-life of cannabidiol in blood is 56 to 61 hours after oral doses twice per day over 7 days. Based on the pharmacokinetic analysis of long-term dosing of cannabidiol in humans, the terminal elimination half-life is estimated to be >134 h. Cannabidiol is metabolized in the liver as well as in the intestines by cytochrome P450 enzymes.
Chemistry
See also: Conversion of CBD to THCAt room temperature, cannabidiol is a colorless crystalline solid. In strongly basic media and the presence of air, it is oxidized to cannabinodiol (CBND) and a quinone called HU-331. Under acidic conditions it cyclizes to THC, which also occurs during pyrolysis, and during smoking. The synthesis of cannabidiol has been accomplished by several research groups.

Overview
| Category | Compound | Description |
|---|---|---|
| Analog | 4'-Fluorocannabidiol | fluorinated cannabidiol derivative of CBD |
| Analog | Cannabidibutol (CBDB) | an analogue to tetrahydrocannabutol (THCB) |
| Analog | Cannabidiol diacetate | a semi-synthetic derivative of CBD |
| Analog | Cannabidiol dimethyl ether (CBDD) | an analog of CBD |
| Analog | H4-CBD | a structural analog of CBD |
| Analog | HU-320 | a structural analog of CBD |
| Analog | KLS-13019 | a structural analog of CBD |
| Homologue | 8,9-Dihydrocannabidiol (H2CBD) | synthetic derivative of CBD |
| Homologue | Cannabidiorcol (CBD-C1) | a homologue of CBD |
| Homologue | Cannabidiphorol (CBDP) | the heptyl-homologue of CBD |
| Homologue | Cannabidivarin (CBDV) | a homologue of CBD |
| Homologue | CBD-DMH | synthetic homologue of CBD |
| Homologue | Δ-6-Cannabidiol | a positional isomer of CBD |
| Isomer | Abnormal cannabidiol | a synthetic regioisomer of CBD |
| Metabolite | 7-Hydroxycannabidiol (7-OH-CBD) | an active metabolite of CBD |
| Precursor | Cannabidiolic acid (CBDA) | precursor to CBD |
Biosynthesis

Cannabis produces CBD through the same metabolic pathway as THC, until the next to last step, where CBDA synthase performs catalysis instead of THCA synthase.
Isomerism
See also: Tetrahydrocannabinol § Isomerism, and Abnormal cannabidiol
| Formal numbering | Terpenoid numbering | Number of stereoisomers | Natural occurrence | Convention on Psychotropic Substances Schedule | Structure | |||
|---|---|---|---|---|---|---|---|---|
| Short name | Chiral centers | Full name | Short name | Chiral centers | ||||
| Δ-Cannabidiol | 1 and 3 | 2-(6-isopropenyl-3-methyl-5-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ-Cannabidiol | 1 and 3 | 4 | No | Unscheduled | 
|
| Δ-Cannabidiol | 1, 3 and 6 | 2-(6-isopropenyl-3-methyl-4-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ-Cannabidiol | 1, 3 and 4 | 8 | No | Unscheduled | 
|
| Δ-Cannabidiol | 1 and 6 | 2-(6-isopropenyl-3-methyl-3-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ-Cannabidiol | 3 and 4 | 4 | Yes | Unscheduled | 
|
| Δ-Cannabidiol | 1 and 6 | 2-(6-isopropenyl-3-methylenecyclohex-1-yl)-5-pentyl-1,3-benzenediol | Δ-Cannabidiol | 3 and 4 | 4 | No | Unscheduled | 
|
| Δ-Cannabidiol | 1 and 6 | 2-(6-isopropenyl-3-methyl-2-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ-Cannabidiol | 3 and 4 | 4 | Yes | Unscheduled | 
|
| Δ-Cannabidiol | 3 and 6 | 2-(6-isopropenyl-3-methyl-1-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ-Cannabidiol | 1 and 4 | 4 | No | Unscheduled | 
|
| Δ-Cannabidiol | 3 | 2-(6-isopropenyl-3-methyl-6-cyclohexen-1-yl)-5-pentyl-1,3-benzenediol | Δ-Cannabidiol | 1 | 2 | No | Unscheduled | 
|
Pyrolysis
In the typical operating temperature range of e-cigarettes (250–400 °C (482–752 °F)), 25–52% of CBD is transformed into other chemical substances: Δ9-THC, Δ8-THC, cannabinol and cannabichromene as predominant pyrolysates. From a chemical point of view, CBD in e-cigarettes can be considered a precursor of THC.
Synthetic derivatives
Numerous synthetic derivatives of CBD are known, and have been researched for generally similar applications as CBD itself.
History
Efforts to isolate the active ingredients in cannabis were made in the 19th century. Cannabidiol was studied in 1940 from Minnesota wild hemp and Egyptian Cannabis indica resin. The chemical formula of CBD was proposed from a method for isolating it from wild hemp. Its structure and stereochemistry were determined in 1963.
Plant breeding
Selective breeding of cannabis plants has expanded and diversified as commercial and therapeutic markets develop. Some growers in the US succeeded in lowering the proportion of CBD-to-THC to accommodate customers who preferred varietals that were more mind-altering due to the higher THC and lower CBD content. In the US, hemp is classified by the federal government as cannabis containing no more than 0.3% THC by dry weight. This classification was established in the 2018 Farm Bill and was refined to include hemp-sourced extracts, cannabinoids, and derivatives in the definition of hemp.
Society and culture
Names
Cannabidiol is the generic name of the drug and its INNTooltip International Nonproprietary Name.
Foods and beverages

Food and beverage products containing cannabidiol were widely marketed in the United States as early as 2017. Hemp seed ingredients which do not naturally contain THC or CBD (but which may be contaminated with trace amounts on the outside during harvesting) were declared by the US Food and Drug Administration as generally recognized as safe (GRAS) in December 2018. CBD itself has not been declared GRAS, and under US federal law is illegal to sell as a food, dietary supplement, or animal feed. State laws vary considerably as non-medical cannabis and derived products have been legalized in various jurisdictions. Despite once having a promising market, the industry for CBD stalled out during the COVID-19 pandemic beginning in 2020, and, by 2024, it collapsed due to withdrawal of investors, the absence of a FDA ruling on efficacy and safety, inconsistent state-by-state legislation, and consumer ambivalence.
Similar to energy drinks and protein bars which may contain vitamin or herbal additives, food and beverage items can be infused with CBD as an alternative means of ingesting the substance. In the United States, numerous products are marketed as containing CBD, but in reality contain little or none. Some companies marketing CBD-infused food products with claims that are similar to the effects of prescription drugs have received warning letters from the FDA for making unsubstantiated health claims. In February 2019, the New York City Department of Health announced plans to fine restaurants that sell food or drinks containing CBD, beginning in October 2019.
Labeling and advertising
Studies conducted by the FDA from 2014 through 2019 have determined that a majority of CBD products are not accurately labeled with the amount of CBD they contain. For example, a 2017 analysis of cannabidiol content in oil, tincture, or liquid vape products purchased online in the United States showed that 69% were mislabeled, with 43% having higher and 26% having lower content than stated on product labels. In 2020, the FDA conducted a study of 147 CBD products and found that half contained THC.
From 2015 to November 2022, the FDA issued dozens of warning letters to American manufacturers of CBD products for false advertising and illegal interstate marketing of CBD as an unapproved drug to treat diseases, such as cancer, osteoarthritis, symptoms of opioid withdrawal, Alzheimer's disease, and pet disorders. Chemical analysis of CBD products found that many did not contain the levels of CBD claimed in advertising.
In December 2020, the Federal Trade Commission initiated a law enforcement crackdown on American companies marketing CBD products as unapproved drugs. The warning also applied to hemp CBD capsules and oil that were being marketed illegally while not adhering to the federal definition of a dietary supplement.
Sports
See also: Cannabis and sportsCannabidiol has been used by professional and amateur athletes across disciplines and countries, with the World Anti-Doping Agency removing CBD from its banned substances list. The United States Anti-Doping Agency and United Kingdom-Anti-Doping Agency do not have anti-CBD policies, with the latter stating that, "CBD is not currently listed on the World Anti-Doping Agency Prohibited List. As a result, it is permitted to use in sport, though the intended benefits are unclear and not backed by clinical evidence. All other cannabinoids (including but not limited to cannabis, hashish, marijuana, and THC) are prohibited in-competition. The intention of the regulations is to prohibit cannabinoids that activate the same receptors in the brain as activated by THC."
In 2019, the cannabis manufacturer Canopy Growth acquired majority ownership of BioSteel Sports Nutrition, which is developing CBD products under endorsement by numerous professional athletes. The National Hockey League Alumni Association began a project with Canopy Growth to determine if CBD or other cannabis products might improve neurological symptoms and quality of life in head-injured players. Some sports leagues have announced sponsorships with CBD companies, such as Major League Baseball (Charlotte's Web) and Ultimate Fighting Championship (Love Hemp). Numerous professional athletes use CBD, primarily for treating pain.
Legal status
Australia
Prescription medicine (Schedule 4) for therapeutic use containing two percent (2.0%) or less of other cannabinoids commonly found in cannabis (such as ∆-THC). A Schedule 4 drug under the SUSMP is a Prescription Only Medicine, or Prescription Animal Remedy – Substances, the use or supply of which should be by or on the order of persons permitted by state or territory legislation to prescribe and should be available from a pharmacist on prescription.
In June 2020, the Australian Therapeutic Goods Administration (TGA) published a consultation on a proposal to pave the way to make "low dose" CBD available to consumer/patients via pharmacists only through moving products from Schedule 4 to 3. Any products sold would need to have their safety, quality and efficacy pre-assessed by the TGA and be formally approved for sale (details to be outlined by TGA). They would be made available to over 18s only, with the maximum daily dose of 60 mg/day, up to 2% THC finished product allowed, 30-day maximum supply, plant-derived or synthetic. This proposal is based on an initial literature review on the safety of low dose CBD published by the TGA in April 2020. Epidyolex was approved for the adjunctive therapy of seizures associated with Lennox–Gastaut syndrome or with Dravet syndrome in September 2020.
Bulgaria
In 2020, Bulgaria became the first country in the European Union to allow retail sales of food products and supplements containing CBD, despite the ongoing discussion within the EU about the classification of CBD as a novel food. However, there exists a legal gap because of the lack of a legally-permissible minimum amount of THC in the products containing cannabinoids.
Canada
In October 2018, cannabidiol became legal for recreational and medical use by the federal Cannabis Act. As of August 2019, CBD products in Canada could only be sold by authorized retailers or federally licensed medical companies, limiting their access to the general public. Nonetheless, with online delivery services and over 2,600 authorized cannabis retail stores as of October 2021, accessibility has steadily increased over time. The Canadian government states that CBD products "are subject to all of the rules and requirements that apply to cannabis under the Cannabis Act and its regulations." It requires "a processing licence to manufacture products containing CBD for sale, no matter what the source of the CBD is, and that CBD and products containing CBD, such as cannabis oil, may only be sold by an authorized retailer or licensed seller of medical CBD." Edible CBD products were scheduled to be permitted for sale in Canada on October 17, 2019, for human consumption.
As of August 2020, it was still illegal to carry cannabis and cannabis-derived products (including products containing CBD) across the Canadian border. If one carries any amount of cannabis for any purpose (including medical), it needs to be declared to the Canada Border Services Agency. Not declaring it is a serious criminal offence.
Czech Republic
As of May 2023, the State Agricultural and Food Inspection of the Czech Republic is putting together broad regulations regarding a ban on CBD products. They will make it illegal to sell products containing cannabidiol and other cannabinoids derived from hemp, as a result of EU Novel Food Regulation. In case of Czech Republic, European Industrial Hemp Association has submitted an official request to the Czech Republic to recognize natural hemp extracts with cannabinoids as traditional food.
European Union

In 2019, the European Commission announced that CBD and other cannabinoids would be classified as "novel foods", meaning that CBD products would require authorization under the EU Novel Food Regulation stating that because "this product was not used as a food or food ingredient before May 15, 1997, before it may be placed on the market in the EU as a food or food ingredient, a safety assessment under the Novel Food Regulation is required." The recommendation – applying to CBD extracts, synthesized CBD, and all CBD products, including CBD oil – was scheduled for a final ruling by the European Commission in March 2019. If approved, manufacturers of CBD products would be required to conduct safety tests and prove safe consumption, indicating that CBD products would not be eligible for legal commerce until at least 2021. In December 2020, the European Commission concluded that CBD should not be considered as drug and can be qualified as food.
Cannabidiol is listed in the EU Cosmetics Ingredient Database (CosIng). However, the listing of an ingredient, assigned with an INCI name, in CosIng does not mean it is to be used in cosmetic products or is approved for such use.
Several industrial hemp varieties can be legally cultivated in Western Europe. A variety such as "Fedora 17" has a cannabinoid profile consistently around 1%, with THC less than 0.3%.
Hong Kong
In 2022, the HKSAR Government proposed a ban on any use of cannabidiol (including for academic research and by medical professionals) within the Hong Kong territory, making Hong Kong the first jurisdiction in the world to have complete prohibition of cannabidiol, starting from February 1, 2023, in part due to the possible presence of THC which is illegal in Hong Kong, according to a research subsidized by the Hong Kong SAR Government.
New Zealand
In 2017, the New Zealand government made changes to the regulations so that restrictions would be removed, which meant a doctor was able to prescribe cannabidiol to patients.
The passing of the Misuse of Drugs (Medicinal Cannabis) Amendment Act in December 2018 means cannabidiol is no longer a controlled drug in New Zealand, but is a prescription medicine under the Medicines Act, with the restriction that "the tetrahydrocannabinols (THCs) and specified substances within the product must not exceed 2 percent of the total CBD, tetrahydrocannabinol (THC) and other specified substances."
Russian Federation
According to a document received in response to an appeal to the Ministry of Internal Affairs of the Russian Federation, measures of state control in the Russian Federation regarding CBD have not been established. However, there is also a response from the Ministry of Health of the Russian Federation indicating that CBD can be considered as an isomer of restricted THC. The "isomer" argument is nonetheless vague, as progesterone, which is freely sold in pharmacies, is also an isomer of THC, all three being C
21H
30O
2. On February 17, 2020, the deputy of the Moscow City Duma Darya Besedina sent an official request to the Prime Minister of the Russian Federation Mikhail Mishustin with a request to eliminate that legal ambiguity by publishing official explanations and, if necessary, making required changes in the corresponding government decree.
Singapore
Singapore allows medical cannabis on a case-by-case basis, usually as a last resort drug. Each case is evaluated by the government, and largely comes in the form of Cannabidiol. However, the country is flexible to what is required for patient treatment, despite having some of the strictest drug laws in the world.
Sweden
Cannabidiol is classified as a medical product in Sweden. However, in July 2019, Supreme Court of Sweden ruled that CBD oil with any concentration of THC falls under the narcotic control laws.
Switzerland
While THC remains illegal, cannabidiol is not subject to the Swiss Narcotic Acts because it does not produce a comparable psychoactive effect. Cannabis products containing less than 1% THC can be sold and purchased legally.
Ukraine
On April 7, 2021, the Ukrainian government legalised use of isolated cannabidiol. Additionally, it approved Nabiximols, a cannabidiol-containing drug, for medical use.
United Kingdom
Cannabidiol, in an oral-mucosal spray formulation combined with delta-9-tetrahydrocannabinol, is a product available by prescription for the relief of severe spasticity due to multiple sclerosis (where other anti-spasmodics have not been effective) in the United Kingdom.
Until 2017, products containing cannabidiol marketed for medical purposes were classed as medicines by the UK regulatory body, the Medicines and Healthcare products Regulatory Agency (MHRA), and could not be marketed without regulatory approval for the medical claims. As of 2018, cannabis oil is legal to possess, buy, and sell in the UK, providing the product does not contain more than 1 milligram of THC and is not advertised as providing a medicinal benefit. Individual police officers and others who are ill-informed of the exact legislature pertaining to cannabidiol, however, may erroneously consider it of dubious legality, reflecting lack of awareness.
In January 2019, the UK Food Standards Agency indicated it would regard CBD products, including CBD oil, as a novel food having no history of use before May 1997, and stated that such products must have authorisation and proven safety before being marketed. The deadline for companies with existing products to submit a full and validated novel foods application with the FSA was March 31, 2021; failure to do so before this date would exclude those companies from selling CBD. New products containing CBD after this deadline would require a fully approved application.
In February 2020, the UK FSA advised vulnerable people, such as pregnant women, breastfeeding mothers, and those already taking medication for other medical concerns not to take CBD. The FSA further recommended that healthy adults should not consume more than 70 mg CBD per day.
United Nations
Cannabidiol is scheduled under the Single Convention on Narcotic Drugs as cannabis. International Narcotics Control Board reminds Member States that, at the reconvened sixty-third session of the Commission on Narcotic Drugs, in December 2020, the States members of the Commission rejected the recommendation of WHO that a footnote be added to the entry for cannabis and cannabis resin in Schedule I of the 1961 Convention as amended to exempt from international control preparations containing predominantly CBD and not more than 0.2 per cent of delta-9-THC.
United States
As of 2023, cannabidiol extracted from marijuana remains a Schedule I Controlled Substance, and is not approved as a prescription drug or dietary supplement or allowed for interstate commerce in the United States. CBD derived from hemp (with 0.3% THC or lower) is legal to sell as a cosmetics ingredient or for other purposes not regulated by the FDA, but cannot be sold under federal law as an ingredient in food, dietary supplement, or animal feed. It is a common misconception that the legal ability to sell hemp (which may contain CBD), and hemp extracts and derivatives (including CBD), makes CBD legal for sale as a supplement or medicine.
In September 2018, the GW Pharmaceuticals drug Epidiolex was placed in Schedule V of the Controlled Substances Act by the Drug Enforcement Administration (DEA), following its approval by the FDA for rare types of childhood epilepsy. It was then removed from the Controlled Substances Act by the DEA in April 2020. Epidiolex is available for prescription use in all 50 states.
In 2013, a CNN program that featured Charlotte's Web cannabis brought increased attention to the use of CBD for the treatment of seizure disorders in children. A number of states passed laws over the next few years to allow the use of low-THC, high-CBD cannabis oil in such situations. These states were in addition to the states that had already legalized cannabis for medical or recreational use. Many states further relaxed their laws regarding CBD following the passage of the 2018 Farm Bill.
The 2014 Farm Bill legalized the sale of "non-viable hemp material" grown within states participating in the Hemp Pilot Program which defined hemp as cannabis containing less than 0.3% of THC. The 2018 Farm Bill removed the hemp plant and all "derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a delta-9 tetrahydrocannabinol concentration of not more than 0.3 percent on a dry weight basis," including CBD, from the Controlled Substances Act, making them legal to manufacture in the United States. The FDA retains regulatory authority over hemp-derived CBD, while the DEA is not involved in the regulation of legally-compliant hemp and hemp products. The 2018 Farm Bill requires that research and development of CBD for a therapeutic purpose would have to be conducted under notification and reporting to the FDA.
Veterinary use
Research
The number of research projects and scientific publications on cannabidiol and other cannabinoids in pets surged in the late 2010s; nonetheless, as of December 2020, there were no hemp-derived, cannabinoid-rich registered veterinary medicinal products in any of the major regions (see #Legal status).
In the US and other territories there are, however, numerous veterinary nutraceutical products available over the counter (OTC). The lack of clarity in the regulations governing veterinary hemp food supplements allows for products of questionable quality to flood the market, which may pose a risk to the wellbeing of pets and owners.
To understand better the benefits of CBD and associated compounds for the quality of life of animals, companies specialized in CBD products for animals have been funding research projects.
Canine osteoarthritis
CBD's ability to help regulate the endocannabinoid system and reduce the release of excitatory neurotransmitters could result in a retrograde inhibitory signal that lessens chronic pain responses. Studies in dogs with chronic pain associated with osteoarthritis showed an increase in level of activity in animals receiving CBD-rich food supplements.
Epilepsy
From the results seen in humans with drugs such as Epidiolex and Sativex in scientific studies and reviews, it could be expected that CBD-based products would be helpful to manage seizures in dogs. However, despite the numerous case reports presented by veterinary neurologists supporting the benefits of CBD as adjunctive therapy, as of December 2020, published controlled studies have not shown a statistically significant decrease in the number of seizures across the groups receiving CBD.
Pharmacokinetics
The oral bioavailability of CBD varies greatly across species and it is linked to the presentation and the time of administration. A 24-hour kinetic examination in dogs showed that the absorption of the cannabidiolic acid (CBDA) does occur, and that this molecule is absorbed least twice as well as CBD post oral ingestion.
It was found that the major metabolites of CBD in humans (7-OH-CBD and 7-COOH-CBD) are not prevalent in dogs, while 6-OH-CBD was found to be the primary metabolite in dogs receiving a CBD-enriched cannabis-derived herbal extract, suggesting that canine and human CBD metabolic route might be somewhat different.
Research
As of 2022, little evidence exists for cannabidiol reducing psychiatric disorders in humans.
In the United States, federal illegality complicates conducting historic research on CBD.
References
- "cannabidiol (CHEBI:69478)". ebi.ac.uk. Archived from the original on May 12, 2021. Retrieved February 12, 2019.
- ^ "Epidyolex". Therapeutic Goods Administration (TGA). September 29, 2020. Archived from the original on October 30, 2021. Retrieved September 30, 2020.
- "Schedules of Controlled Substances: Placement in Schedule V of Certain FDA-Approved Drugs Containing Cannabidiol; Corresponding Change to Permit Requirements". Federal Register, US Federal Government. September 28, 2018. Retrieved March 10, 2024.
- ^ "Epidiolex – cannabidiol solution". DailyMed. August 26, 2020. Archived from the original on February 25, 2021. Retrieved September 11, 2020.
- "Sativex (Cannabidiol/Tetrahydrocannabinol) Bayer Label" (PDF). bayer.ca. Archived (PDF) from the original on January 16, 2021. Retrieved June 28, 2018.
- Anvisa (July 24, 2023). "RDC Nº 804 - Listas de Substâncias Entorpecentes, Psicotrópicas, Precursoras e Outras sob Controle Especial" [Collegiate Board Resolution No. 804 - Lists of Narcotic, Psychotropic, Precursor, and Other Substances under Special Control] (in Brazilian Portuguese). Diário Oficial da União (published July 25, 2023). Archived from the original on August 27, 2023. Retrieved August 27, 2023.
- ^ Stallworth J. "Drug licensing factsheet: cannabis, CBD and other cannabinoids". The Home Office. Archived from the original on January 17, 2021. Retrieved December 10, 2020.
- "DEA announces steps necessary to improve access to marijuana research". DEA. August 26, 2019. Retrieved September 7, 2024.
- "7 U.S. Code § 1639o". LII / Legal Information Institute. Retrieved September 7, 2024.
- Laurence E (May 31, 2022). "Your Guide To CBD Legalization By State". Forbes Health. Retrieved September 7, 2024.
- ^ "Epidyolex EPAR". European Medicines Agency (EMA). June 24, 2019. Archived from the original on August 9, 2021. Retrieved September 11, 2020. Text was copied from this source which is copyrighted by the European Medicines Agency. Reproduction is authorized provided the source is acknowledged.
- ^ Perucca E, Bialer M (June 5, 2020). "Critical Aspects Affecting Cannabidiol Oral Bioavailability and Metabolic Elimination, and Related Clinical Implications". CNS Drugs. 34 (8): 795–800. doi:10.1007/s40263-020-00741-5. PMID 32504461. S2CID 219313952.
- ^ Scuderi C, Filippis DD, Iuvone T, Blasio A, Steardo A, Esposito G (May 2009). "Cannabidiol in medicine: a review of its therapeutic potential in CNS disorders". Phytotherapy Research (Review). 23 (5): 597–602. doi:10.1002/ptr.2625. PMID 18844286. S2CID 21836765. Archived from the original on April 11, 2021. Retrieved May 22, 2020.
- Hossain K (September 25, 2023). "Current Challenges and Opportunities for Improved Cannabidiol Solubility". International Journal of Molecular Sciences. 24 (19): 14514. doi:10.3390/ijms241914514. PMC 10572536. PMID 37833962.
- ^ Devinsky O, Cilio MR, Cross H, Fernandez-Ruiz J, French J, Hill C, et al. (June 2014). "Cannabidiol: pharmacology and potential therapeutic role in epilepsy and other neuropsychiatric disorders". Epilepsia. 55 (6): 791–802. doi:10.1111/epi.12631. PMC 4707667. PMID 24854329.
- "Phytocannabinoid Boiling Points" (PDF). Project CBD. Archived (PDF) from the original on April 8, 2019. Retrieved August 20, 2021.
- ^ Campos AC, Moreira FA, Gomes FV, Del Bel EA, Guimarães FS (December 2012). "Multiple mechanisms involved in the large-spectrum therapeutic potential of cannabidiol in psychiatric disorders". Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences (Review). 367 (1607): 3364–3378. doi:10.1098/rstb.2011.0389. PMC 3481531. PMID 23108553.
- ^ Kirkland AE, Fadus MC, Gruber SA, Gray KM, Wilens TE, Squeglia LM (February 2022). "A scoping review of the use of cannabidiol in psychiatric disorders". Psychiatry Research. 308: 114347. doi:10.1016/j.psychres.2021.114347. PMC 8799523. PMID 34952255.
- ^ Black N, Stockings E, Campbell G, Tran LT, Zagic D, Hall WD, et al. (December 2019). "Cannabinoids for the treatment of mental disorders and symptoms of mental disorders: a systematic review and meta-analysis". The Lancet. Psychiatry. 6 (12): 995–1010. doi:10.1016/S2215-0366(19)30401-8. PMC 6949116. PMID 31672337.
- ^ VanDolah HJ, Bauer BA, Mauck KF (September 2019). "Clinicians' Guide to Cannabidiol and Hemp Oils". Mayo Clinic Proceedings. 94 (9): 1840–1851. doi:10.1016/j.mayocp.2019.01.003. PMID 31447137.
- Prud'homme M, Cata R, Jutras-Aswad D (January 2015). "Cannabidiol as an Intervention for Addictive Behaviors: A Systematic Review of the Evidence". Substance Abuse. 9: 33–38. doi:10.4137/SART.S25081. PMC 4444130. PMID 26056464.
- ^ Novella S (September 30, 2020). "Where Are We With CBD?". Science-Based Medicine. Archived from the original on August 12, 2021. Retrieved October 1, 2020.
- Itin C, Barasch D, Domb AJ, Hoffman A (May 2020). "Prolonged oral transmucosal delivery of highly lipophilic drug cannabidiol". International Journal of Pharmaceutics. 581: 119276. doi:10.1016/j.ijpharm.2020.119276. PMID 32243971. S2CID 214785913.
- Itin C, Domb AJ, Hoffman A (October 2019). "A meta-opinion: cannabinoids delivered to oral mucosa by a spray for systemic absorption are rather ingested into gastro-intestinal tract: the influences of fed / fasting states". Expert Opinion on Drug Delivery. 16 (10): 1031–1035. doi:10.1080/17425247.2019.1653852. PMID 31393180. S2CID 199505274.
- ^ Pisanti S, Malfitano AM, Ciaglia E, Lamberti A, Ranieri R, Cuomo G, et al. (July 2017). "Cannabidiol: State of the art and new challenges for therapeutic applications". Pharmacology & Therapeutics. 175: 133–150. doi:10.1016/j.pharmthera.2017.02.041. PMID 28232276.
- ^ Iseger TA, Bossong MG (March 2015). "A systematic review of the antipsychotic properties of cannabidiol in humans". Schizophrenia Research. 162 (1–3): 153–161. doi:10.1016/j.schres.2015.01.033. PMID 25667194. S2CID 3745655.
- Boggs DL, Nguyen JD, Morgenson D, Taffe MA, Ranganathan M (January 2018). "Clinical and Preclinical Evidence for Functional Interactions of Cannabidiol and Δ-Tetrahydrocannabinol". Neuropsychopharmacology. 43 (1): 142–154. doi:10.1038/npp.2017.209. PMC 5719112. PMID 28875990.
- Aizpurua-Olaizola O, Soydaner U, Öztürk E, Schibano D, Simsir Y, Navarro P, et al. (February 2016). "Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes". Journal of Natural Products. 79 (2): 324–331. doi:10.1021/acs.jnatprod.5b00949. hdl:1874/350973. PMID 26836472. Archived from the original on January 5, 2023. Retrieved November 27, 2022.
- ^ Czégény Z, Nagy G, Babinszki B, Bajtel Á, Sebestyén Z, Kiss T, et al. (April 2021). "CBD, a precursor of THC in e-cigarettes". Scientific Reports. 11 (1): 8951. Bibcode:2021NatSR..11.8951C. doi:10.1038/s41598-021-88389-z. PMC 8076212. PMID 33903673.
- ^ Mead A (June 14, 2019). "Legal and Regulatory Issues Governing Cannabis and Cannabis-Derived Products in the United States". Frontiers in Plant Science. 10: 697. doi:10.3389/fpls.2019.00697. PMC 6590107. PMID 31263468.
- "FDA Regulation of Cannabis and Cannabis-Derived Products, Including Cannabidiol (CBD). #2. How does the 2018 Farm Bill define hemp? What does it mean for FDA-regulated products?". US Food and Drug Administration (FDA). February 6, 2024. Retrieved February 6, 2024.
- ^ Office of the Commissioner (July 31, 2020). "FDA Approves New Indication for Drug Containing an Active Ingredient Derived from Cannabis to Treat Seizures in Rare Genetic Disease". FDA. Archived from the original on September 29, 2021. Retrieved November 25, 2020.
- ^ Stockings E, Zagic D, Campbell G, Weier M, Hall WD, Nielsen S, et al. (July 2018). "Evidence for cannabis and cannabinoids for epilepsy: a systematic review of controlled and observational evidence". Journal of Neurology, Neurosurgery, and Psychiatry. 89 (7): 741–753. doi:10.1136/jnnp-2017-317168. hdl:1959.4/unsworks_50076. PMID 29511052.
- ^ Elliott J, DeJean D, Clifford T, Coyle D, Potter BK, Skidmore B, et al. (February 2020). "Cannabis-based products for pediatric epilepsy: An updated systematic review". Seizure. 75: 18–22. doi:10.1016/j.seizure.2019.12.006. PMID 31865133. S2CID 208878465.
- "Cannabis derivative may reduce seizures in some severe drug-resistant epilepsies, but adverse events increase". NIHR Evidence (Plain English summary). June 26, 2018. doi:10.3310/signal-000606. S2CID 242083755. Archived from the original on July 24, 2021. Retrieved March 15, 2022.
- Office of the Commissioner (March 27, 2020). "FDA Approves First Drug Comprised of an Active Ingredient Derived from Marijuana to Treat Rare, Severe Forms of Epilepsy". FDA. Archived from the original on April 23, 2019. Retrieved May 28, 2021.
- ^ Prud'homme M, Cata R, Jutras-Aswad D (2015). "Cannabidiol as an Intervention for Addictive Behaviors: A Systematic Review of the Evidence". Substance Abuse. 9: 33–38. doi:10.4137/SART.S25081. PMC 4444130. PMID 26056464.
- Silva TB, Balbino CQ, Weiber AF (May 2015). "The relationship between cannabidiol and psychosis: A review". Annals of Clinical Psychiatry. 27 (2): 134–141. PMID 25954940.
- Blessing EM, Steenkamp MM, Manzanares J, Marmar CR (October 2015). "Cannabidiol as a Potential Treatment for Anxiety Disorders". Neurotherapeutics. 12 (4): 825–836. doi:10.1007/s13311-015-0387-1. PMC 4604171. PMID 26341731.
- "What You Should Know About Using Cannabis, Including CBD, When Pregnant or Breastfeeding". US Food and Drug Administration (FDA). October 16, 2019. Archived from the original on October 17, 2019. Retrieved October 17, 2019.
- Villanueva MR, Joshaghani N, Villa N, Badla O, Goit R, Saddik SE, et al. (July 2022). "Efficacy, Safety, and Regulation of Cannabidiol on Chronic Pain: A Systematic Review". Cureus. 14 (7): e26913. doi:10.7759/cureus.26913. PMC 9288157. PMID 35860716.
- "Experience best massage - CBD Oil - body health benefits". February 28, 2020. Retrieved October 22, 2024.
- Kicman A, Toczek M (September 2020). "The Effects of Cannabidiol, a Non-Intoxicating Compound of Cannabis, on the Cardiovascular System in Health and Disease". International Journal of Molecular Sciences. 21 (18): 6740. doi:10.3390/ijms21186740. PMC 7554803. PMID 32937917.
- Sachs J, McGlade E, Yurgelun-Todd D (October 2015). "Safety and Toxicology of Cannabinoids". Neurotherapeutics. 12 (4): 735–746. doi:10.1007/s13311-015-0380-8. PMC 4604177. PMID 26269228.
- Izzo AA, Borrelli F, Capasso R, Di Marzo V, Mechoulam R (October 2009). "Non-psychotropic plant cannabinoids: new therapeutic opportunities from an ancient herb". Trends in Pharmacological Sciences. 30 (10): 515–527. doi:10.1016/j.tips.2009.07.006. PMID 19729208.
- ^ "Industrial hemp". Department of Agriculture, State of Colorado. 2018. Archived from the original on August 26, 2018. Retrieved September 14, 2018.
- "Cannabinoid Clinical | Cannabinoids Research, Effects, and Uses". CannabinoidClinical.com. Archived from the original on June 26, 2021. Retrieved October 27, 2020.
- Russo EB (February 2008). "Cannabinoids in the management of difficult to treat pain". Therapeutics and Clinical Risk Management. 4 (1): 245–259. doi:10.2147/TCRM.S1928. PMC 2503660. PMID 18728714.
- "Sativex Oromucosal Spray". Medsafe, New Zealand Medicines and Medical Devices Safety Authority. December 19, 2018. Archived from the original on July 27, 2020. Retrieved April 3, 2019.
- Fischer B, Russell C, Sabioni P, van den Brink W, Le Foll B, Hall W, et al. (August 2017). "Lower-Risk Cannabis Use Guidelines: A Comprehensive Update of Evidence and Recommendations". American Journal of Public Health. 107 (8): e1 – e12. doi:10.2105/AJPH.2017.303818. PMC 5508136. PMID 28644037.
- Huestis MA, Solimini R, Pichini S, Pacifici R, Carlier J, Busardò FP (September 13, 2019). "Cannabidiol Adverse Effects and Toxicity". Current Neuropharmacology. 17 (10). Bentham Science Publishers Ltd.: 974–989. doi:10.2174/1570159x17666190603171901. PMC 7052834. PMID 31161980.
- "Cannabidiol (CBD): MedlinePlus Supplements". medlineplus.gov. Archived from the original on October 20, 2021. Retrieved January 15, 2021.
- ^ "What You Need to Know (And What We're Working to Find Out) About Products Containing Cannabis or Cannabis-derived Compounds, Including CBD". US Food and Drug Administration. March 5, 2020. Archived from the original on October 22, 2021. Retrieved June 23, 2022.
- ^ "FDA Regulation of Cannabis and Cannabis-Derived Products, Including Cannabidiol (CBD)". US Food and Drug Administration (FDA). September 28, 2023. Retrieved October 29, 2023.
- ^ Hahn SM (March 5, 2020). "FDA Advances Work Related to Cannabidiol Products with Focus on Protecting Public Health, Providing Market Clarity". US Food and Drug Administration. Archived from the original on September 29, 2021. Retrieved March 6, 2020.
- "Cannabidiol (CBD)". American Association of Poison Control Centers. September 30, 2019. Archived from the original on October 17, 2019. Retrieved October 17, 2019.
- ^ MacKeen D (October 16, 2019). "Scam or Not: What Are the Benefits of CBD?". The New York Times. Archived from the original on October 16, 2019. Retrieved October 17, 2019.
- ^ "FDA Warns Companies for Illegally Selling Food and Beverage Products that Contain CBD". US Food and Drug Administration. November 21, 2022. Archived from the original on November 23, 2022. Retrieved November 23, 2022.
These companies are selling CBD containing products that people may confuse for traditional foods or beverages which may result in unintentional consumption or overconsumption of CBD. CBD-containing products in forms that are appealing to children, such as gummies, hard candies and cookies, are especially concerning.
- Bornheim LM, Kim KY, Li J, Perotti BY, Benet LZ (August 1995). "Effect of cannabidiol pretreatment on the kinetics of tetrahydrocannabinol metabolites in mouse brain". Drug Metabolism and Disposition. 23 (8): 825–831. PMID 7493549.
- Klein C, Karanges E, Spiro A, Wong A, Spencer J, Huynh T, et al. (November 2011). "Cannabidiol potentiates Δ-tetrahydrocannabinol (THC) behavioural effects and alters THC pharmacokinetics during acute and chronic treatment in adolescent rats". Psychopharmacology. 218 (2): 443–457. doi:10.1007/s00213-011-2342-0. PMID 21667074. S2CID 6240926.
- Ghovanloo MR, Shuart NG, Mezeyova J, Dean RA, Ruben PC, Goodchild SJ (October 2018). "Inhibitory effects of cannabidiol on voltage-dependent sodium currents". The Journal of Biological Chemistry. 293 (43): 16546–16558. doi:10.1074/jbc.RA118.004929. PMC 6204917. PMID 30219789.
- Sait LG, Sula A, Ghovanloo MR, Hollingworth D, Ruben PC, Wallace BA (October 2020). "Cannabidiol interactions with voltage-gated sodium channels". eLife. 9. doi:10.7554/eLife.58593. PMC 7641581. PMID 33089780.
- Nadulski T, Pragst F, Weinberg G, Roser P, Schnelle M, Fronk EM, et al. (December 2005). "Randomized, double-blind, placebo-controlled study about the effects of cannabidiol (CBD) on the pharmacokinetics of Delta9-tetrahydrocannabinol (THC) after oral application of THC verses standardized cannabis extract". Therapeutic Drug Monitoring. 27 (6): 799–810. doi:10.1097/01.ftd.0000177223.19294.5c. PMID 16306858. S2CID 12979224.
- Lucas CJ, Galettis P, Schneider J (November 2018). "The pharmacokinetics and the pharmacodynamics of cannabinoids". British Journal of Clinical Pharmacology. 84 (11): 2477–2482. doi:10.1111/bcp.13710. PMC 6177698. PMID 30001569.
- Yamaori S, Ebisawa J, Okushima Y, Yamamoto I, Watanabe K (April 2011). "Potent inhibition of human cytochrome P450 3A isoforms by cannabidiol: role of phenolic hydroxyl groups in the resorcinol moiety". Life Sciences. 88 (15–16): 730–736. doi:10.1016/j.lfs.2011.02.017. PMID 21356216.
- Laprairie RB, Bagher AM, Kelly ME, Denovan-Wright EM (2015). "Cannabidiol is a negative allosteric modulator of the cannabinoid CB1 receptor". British Journal of Pharmacology. 172 (20): 4790–4805. doi:10.1111/bph.13250. PMC 4621983. PMID 26218440.
- Nguyen T, Li JX, Thomas BF, Wiley JL, Kenakin TP, Zhang Y (2016). "Allosteric Modulation: An Alternate Approach Targeting the Cannabinoid CB1 Receptor". Medicinal Research Reviews. 37 (3): 441–474. doi:10.1002/med.21418. PMC 5397374. PMID 27879006.
- ^ Schouten M, Dalle S, Mantini D, Koppo K (2023). "Cannabidiol and brain function: current knowledge and future perspectives". Frontiers in Pharmacology. 14: 1328885. doi:10.3389/fphar.2023.1328885. PMC 10823027. PMID 38288087.
- ^ "Cannabidiol". Drugs.com. July 14, 2023. Retrieved February 12, 2024.
- Gray RA, Whalley BJ (January 2020). "The proposed mechanisms of action of CBD in epilepsy". Epileptic Disorders. 22 (S1): 10–15. doi:10.1684/epd.2020.1135. PMID 32053110.
- Martínez-Aguirre C, et al. (December 2020). "Cannabidiol Acts at 5-HT1A Receptors in the Human Brain: Relevance for Treating Temporal Lobe Epilepsy". Front. Behav. Neurosci. 14: 611278. doi:10.3389/fnbeh.2020.611278. PMC 7770178. PMID 33384591.
- ^ Kolli AR, Hoeng J (April 2024). "Cannabidiol Bioavailability Is Nonmonotonic with a Long Terminal Elimination Half-Life: A Pharmacokinetic Modeling-Based Analysis". Cannabis and Cannabinoid Research. doi:10.1089/can.2023.0214. PMID 38624257.
- ^ O'Sullivan SE, Jensen SS, Kolli AR, Nikolajsen GN, Bruun HZ, Hoeng J (February 2024). "Strategies to Improve Cannabidiol Bioavailability and Drug Delivery". Pharmaceuticals. 17 (2). MDPI: 244. doi:10.3390/ph17020244. PMC 10892205. PMID 38399459.
- Hossain K (September 25, 2023). "Current Challenges and Opportunities for Improved Cannabidiol Solubility". International Journal of Molecular Sciences. 24 (19): 14514. doi:10.3390/ijms241914514. PMC 10572536. PMID 37833962.
- Jones PG, Falvello L, Kennard O, Sheldrick GM, Mechoulam R (1977). "Cannabidiol". Acta Crystallogr. B. 33 (10): 3211–3214. Bibcode:1977AcCrB..33.3211J. doi:10.1107/S0567740877010577.
- Mechoulam R, Ben-Zvi Z, Gaoni Y (August 1968). "Hashish – 13. On the nature of the Beam test". Tetrahedron. 24 (16): 5615–5624. doi:10.1016/0040-4020(68)88159-1. PMID 5732891.
- Gaoni Y, Mechoulam R (1966). "Hashish – VII The isomerization of cannabidiol to tetrahydrocannabinols". Tetrahedron. 22 (4): 1481–1488. doi:10.1016/S0040-4020(01)99446-3.
- Küppers FJ, Bercht CA, Salemink CA, Lousberg RC, Terlouw JK, Heerma W (1975). "Cannabis – XV: Pyrolysis of cannabidiol. Structure elucidation of four pyrolytic products". Tetrahedron. 31 (13–14): 1513–1516. doi:10.1016/0040-4020(75)87002-5.
- Quarles W, Ellman G, Jones R (1973). "Toxicology of marijuana: conditions for conversion of cannabidiol to THC upon smoking". Clinical Toxicology. 6 (2): 211–216. doi:10.3109/15563657308990520. PMID 4715204.
- Petrzilka T, Haefliger W, Sikemeier C, Ohloff G, Eschenmoser A (March 1967). "". Helvetica Chimica Acta. 50 (2): 719–723. doi:10.1002/hlca.19670500235. PMID 5587099.
- Gaoni Y, Mechoulam R (1985). "Boron trifluoride etherate on alumuna – a modified Lewis acid reagent. An improved synthesis of cannabidiol". Tetrahedron Letters. 26 (8): 1083–1086. doi:10.1016/S0040-4039(00)98518-6.
- Kobayashi Y, Takeuchi A, Wang YG (June 2006). "Synthesis of cannabidiols via alkenylation of cyclohexenyl monoacetate". Organic Letters. 8 (13): 2699–2702. doi:10.1021/ol060692h. PMID 16774235.
- Gaoni Y, Mechoulam R (January 1966). "Hashish – VII: The isomerization of cannabidiol to tetrahydrocannabinols". Tetrahedron. 22 (4): 1481–1488. doi:10.1016/S0040-4020(01)99446-3.
- Taura F, Sirikantaramas S, Shoyama Y, Yoshikai K, Shoyama Y, Morimoto S (June 2007). "Cannabidiolic-acid synthase, the chemotype-determining enzyme in the fiber-type Cannabis sativa". FEBS Letters. 581 (16): 2929–2934. Bibcode:2007FEBSL.581.2929T. doi:10.1016/j.febslet.2007.05.043. PMID 17544411. S2CID 20253070.
- Marks MD, Tian L, Wenger JP, Omburo SN, Soto-Fuentes W, He J, et al. (2009). "Identification of candidate genes affecting Delta9-tetrahydrocannabinol biosynthesis in Cannabis sativa". Journal of Experimental Botany. 60 (13): 3715–3726. doi:10.1093/jxb/erp210. PMC 2736886. PMID 19581347.
- Nelson KM, Bisson J, Singh G, Graham JG, Chen SN, Friesen JB, et al. (November 2020). "The Essential Medicinal Chemistry of Cannabidiol (CBD)". Journal of Medicinal Chemistry. 63 (21): 12137–12155. doi:10.1021/acs.jmedchem.0c00724. PMC 7666069. PMID 32804502.
- ^ Adams R, Hunt M, Clark JH (1940). "Structure of cannabidiol, a product isolated from the marihuana extract of Minnesota wild hemp". Journal of the American Chemical Society. 62 (1): 196–200. Bibcode:1940JAChS..62..196A. doi:10.1021/ja01858a058. ISSN 0002-7863.
- Jacob A, Todd AR (1940). "Cannabidiol and cannabol, constituents of Cannabis indica resin". Nature. 145 (3670): 350. Bibcode:1940Natur.145..350J. doi:10.1038/145350a0. ISSN 0028-0836. S2CID 4106662.
- Work TS, Bergel F, Todd AR (January 1939). "The active principles of Cannabis indica resin. I". The Biochemical Journal. 33 (1): 123–127. doi:10.1042/bj0330123. PMC 1264344. PMID 16746878.
- Mechoulam R, Shvo Y (December 1963). "Hashish. I. The structure of cannabidiol". Tetrahedron. 19 (12): 2073–2078. doi:10.1016/0040-4020(63)85022-x. PMID 5879214.
- Romney L (September 13, 2012). "On the frontier of medical pot to treat boy's epilepsy". Los Angeles Times. Archived from the original on October 21, 2018. Retrieved April 16, 2020.
- "7 U.S. Code § 5940 – Legitimacy of industrial hemp research". LII / Legal Information Institute. Archived from the original on December 22, 2020. Retrieved November 27, 2018.
- "International Nonproprietary Names for Pharmaceutical Substances (INN)" (PDF). WHO Drug Information. 30 (2): 241. 2016. Archived (PDF) from the original on February 5, 2018. Retrieved February 18, 2023.
- "Billboard featuring hemp leaf raises questions about new beverage for sale in Cincinnati | WLWT5". WLWT5. September 29, 2017. Archived from the original on December 26, 2020. Retrieved September 29, 2017.
- ^ Creswell, Julie (February 28, 2024). "Companies were big on CBD. Not anymore". The New York Times. Retrieved March 25, 2024.
- "Warning Letters and Test Results for Cannabidiol-Related Products". US Food and Drug Administration (FDA). November 2, 2017. Archived from the original on January 26, 2018. Retrieved January 2, 2018.
- Fox A, Ravitz JR, Leongini EM, Malkin BJ (November 20, 2017). "Companies Marketing CBD Products Be Warned: FDA Is Watching". Lexology. Archived from the original on August 3, 2020. Retrieved December 14, 2017.
- La Vito A, Franck T (February 15, 2019). "New York City plans to fine restaurants using CBD in food and drinks". CNBC. Archived from the original on November 7, 2020. Retrieved February 19, 2019.
- ^ "Sampling Study of the Current Cannabidiol Marketplace to Determine the Extent That Products are Mislabeled or Adulterated Report in Response to Further Consolidated Appropriations Act, 2020" (PDF). United States Food and Drug Administration. July 2020. Archived (PDF) from the original on June 23, 2021. Retrieved January 30, 2021.
- Bonn-Miller MO, Loflin MJ, Thomas BF, Marcu JP, Hyke T, Vandrey R (November 2017). "Labeling Accuracy of Cannabidiol Extracts Sold Online". JAMA. 318 (17): 1708–1709. doi:10.1001/jama.2017.11909. PMC 5818782. PMID 29114823.
- Evans DG (2020). "Medical Fraud, Mislabeling, Contamination: All Common in CBD Products". Missouri Medicine. 117 (5): 394–399. PMC 7723146. PMID 33311737.
- "FTC Announces Crackdown on Deceptively Marketed CBD Products". US Federal Trade Commission (FTC). December 17, 2020. Archived from the original on June 22, 2021. Retrieved January 12, 2021.
- ^ Ashley DD, Engle MK (October 22, 2019). "Warning letter: Rooted Apothecary LLC". Office of Compliance, Center for Drug Evaluation and Research, US Food and Drug Administration; US Federal Trade Commission. Archived from the original on December 12, 2019. Retrieved October 23, 2019.
- "Athlete Advisory Note: Cannabidiol (CBD)". United Kingdom Anti-Doping Agency. Archived from the original on October 9, 2019. Retrieved October 9, 2019.
- "Athletes: 6 things to know about cannabidiol". US Anti-Doping Agency. October 23, 2018. Archived from the original on October 11, 2021. Retrieved February 17, 2020.
- ^ Donna S (October 2, 2019). "Canopy cannabis company buys ex-NHL player's sports nutrition business". CTV Business. The Canadian Press. Archived from the original on November 26, 2020. Retrieved February 17, 2020.
BioSteel's brand ambassadors also include well-known athletes across major sports leagues in North America, which could be beneficial as the company's attempt to push regulated CBD nutrition products into the mainstream health and wellness segments
- "Major League Baseball, pioneering CBD brand Charlotte's Web strike groundbreaking deal" (Press release). Major League Baseball. October 12, 2022.
- "UFC Names Love Hemp Official Global CBD Partner" (Press release). Ultimate Fighting Championship. March 18, 2021.
- Loudin A (December 7, 2019). "As more pro athletes use cannabis for aches and pain, the more they run afoul of rules". The Washington Post. Archived from the original on November 24, 2020. Retrieved February 17, 2020.
- Harrison S (September 28, 2019). "Lots of athletes say CBD Is a better painkiller. Is It?". Wired. Archived from the original on July 12, 2021. Retrieved February 17, 2020.
- "Poisons Standard June 2017". Legislation.gov.au. Archived from the original on December 13, 2020. Retrieved December 4, 2016.
- Australian Government Department of Health Therapeutic Goods Administration (April 24, 2020). "Consultation: Proposed amendments to the Poisons Standard – Joint ACMS/ACCS meetings, June 2020". Therapeutic Goods Administration (TGA). Archived from the original on May 11, 2021. Retrieved November 25, 2020.
- "Safety of low dose cannabidiol" (PDF). Government of Australia. Therapeutic Goods Administration. April 2020. Archived (PDF) from the original on September 7, 2021. Retrieved November 25, 2020.
- Hasse J. "This EU country has become the first to allow free sale Of CBD". Forbes. Archived from the original on October 6, 2021. Retrieved February 21, 2020.
- Todorova SE (July 29, 2019). "Growing cannabis in Bulgaria: Legal but still stigmatised". Lexology. Archived from the original on April 11, 2021. Retrieved September 3, 2020.
- ^ "Cannabidiol (CBD)". Government of Canada. August 13, 2019. Archived from the original on September 18, 2019. Retrieved October 11, 2019.
- "Health products containing cannabis or for use with cannabis: Guidance for the Cannabis Act, the Food and Drugs Act, and related regulations". Government of Canada. July 11, 2018. Archived from the original on October 19, 2018. Retrieved October 19, 2018.
- "Cannabis Legalization and Regulation". Department of Justice, Electronic Communications. Government of Canada. June 20, 2018. Archived from the original on August 24, 2021. Retrieved November 24, 2018.
- "'It should be available': Natural health food stores hope to cash in on CBD craze". CBC News. August 6, 2019. Archived from the original on October 12, 2019. Retrieved October 11, 2019.
- Conway J (September 17, 2021). "Number of cannabis stores in Canada as of June 2021, by region". Statista. Archived from the original on May 2, 2022. Retrieved May 2, 2022.
- Armstrong M (October 13, 2021). "Cannabis store openings in Canada only slightly affected the number of users". The Conversation. Archived from the original on May 2, 2022. Retrieved May 2, 2022.
- Canada Health (June 20, 2018). "Cannabis and Canadian borders". aem. Archived from the original on August 14, 2021. Retrieved September 3, 2020.
- "Ministerstvo zemědělství informuje o chystaném zákazu uvádění na trh produktů obsahujících kanabidiol (CBD) a jiné kanabinoidy (eAGRI)". eagri.cz (in Czech). Retrieved May 8, 2023.
- Milosz F (May 6, 2023). "EIHA reacts about CBD and hemp extracts". CannabizEU. Archived from the original on May 6, 2023. Retrieved May 8, 2023.
- ^ Chu W (January 31, 2019). "Updated EC ruling for CBD classes supplement ingredient as Novel Food". NutraIngredients.com, William Reed Business Media Ltd. Archived from the original on November 1, 2021. Retrieved January 1, 2019.
- "Cannabinoids, searched in the EU Novel food catalogue (v.1.1)". European Commission. January 1, 2019. Archived from the original on April 3, 2019. Retrieved February 1, 2019.
- "European Commission reverses course, says CBD should not be regulated as a narcotic". Hemp Industry Daily. December 2, 2020. Archived from the original on October 21, 2021. Retrieved December 4, 2020.
- ^ "CosIng – Cosmetics – Cannabidiol". European Commission. Archived from the original on January 13, 2019. Retrieved December 4, 2016.
- Fournier G, Beherec O, Bertucelli S (2003). "Intérêt du rapport Δ-9-THC / CBD dans le contrôle des cultures de chanvre industriel" [The advantage of the Δ-9-THC / CBD ratio in the control of industrial hemp crops]. Annales de Toxicologie Analytique (in French). 15 (4): 250–259. doi:10.1051/ata/2003003.
- "Orders to amend Dangerous Drugs Ordinance and Control of Chemicals Ordinance to be gazetted on October 21 and cannabidiol to become dangerous drug". Hong Kong SAR Government. Archived from the original on October 20, 2022. Retrieved October 20, 2022.
- "What you need to know about a proposed ban on CBD products in Hong Kong". South China Morning Post. June 11, 2022. Archived from the original on September 25, 2022. Retrieved September 25, 2022.
- Leung H (August 21, 2022). "Hong Kong's zero-tolerance approach to drugs leaves budding CBD industry high and dry". Hong Kong Free Press HKFP. Archived from the original on April 20, 2023. Retrieved September 25, 2022.
- Legislative Council Panel on Security (June 7, 2022). "Proposed Control of Cannabidiol through Legislation" (PDF). Government of Hong Kong. Archived (PDF) from the original on September 28, 2022. Retrieved October 24, 2022.
- "Doctors now able to prescribe cannabidiol". radionz.co.nz. June 2, 2017. Archived from the original on June 1, 2017. Retrieved June 2, 2017.
- "CBD products". www.health.govt.nz. Archived from the original on February 4, 2019. Retrieved March 10, 2019.
- "Является ли вещество CBD (каннабидиол) разрешенным к использованию на территории России? – Правовед.RU". Archived from the original on November 1, 2021. Retrieved February 19, 2021.
- "Журнал переписки депутата Бесединой". Archived from the original on February 19, 2021. Retrieved February 19, 2021.
- "CBD products should follow the drug laws". Swedish Medical Products Agency. April 4, 2018. Archived from the original on July 24, 2018. Retrieved July 31, 2018.
- Arnold M (July 30, 2019). "Sweden Joins Italy In Path To Defining CBD Oil Regulations". Cannabis Industry Journal. Archived from the original on November 8, 2020. Retrieved September 3, 2020.
- "Cannabis: What is allowed, what is not allowed in Switzerland? – www.ch.ch". www.ch.ch. Archived from the original on January 21, 2021. Retrieved September 3, 2020.
- Kohler S, Benz AS, Pruschy D, et al. (Vischer AG) (April 11, 2019). "Trends in the Swiss Cannabis Regulation | Lexology". www.lexology.com. Archived from the original on January 17, 2021. Retrieved September 3, 2020.
- "Cannabis à faible teneur en THC et CBD" (in French). BAG.Admin.ch. Archived from the original on March 26, 2017. Retrieved May 20, 2017.
- Cross D (April 9, 2021). "В Україні легалізували використання медичного канабісу, але не всього" [Ukraine has legalized the use of medical cannabis, but not all of it]. УП.Життя (UP.Life) (in Ukrainian). Archived from the original on April 9, 2021. Retrieved April 10, 2021.
- "Sativex Oromucosal Spray – Summary of Product Characteristics (SmPC)". (emc). August 25, 2020. Archived from the original on February 4, 2021. Retrieved September 11, 2020.
- "MHRA statement on products containing Cannabidiol (CBD)". Gov.uk. December 14, 2016. Archived from the original on May 10, 2019. Retrieved December 14, 2016.
- "UK Classifies CBD Oil as a Medicinal Ingredient". BuyCBD.net. Archived from the original on November 1, 2021. Retrieved September 3, 2020.
- Ukaegbu O, Smith J, Hall D, Frain T, Abbasian C. Staff awareness of the use of cannabidiol (CBD): a trust-wide survey study in the UK. Journal of Cannabis Research. 2021 Dec;3(1):1-0.
- Gunn L, Haigh L (January 29, 2019). "British watchdog deems CBD a novel food, seeks to curtail sale on UK market". Nutrition Insight, CNS Media BV. Archived from the original on February 2, 2019. Retrieved January 1, 2019.
- ^ "Cannabidiol (CBD) guidance – Business guidance on cannabidiol (CBD) as a novel food". UK Food Standards Agency. September 24, 2020. Archived from the original on October 30, 2021. Retrieved December 8, 2020.
- Marsat N (July 7, 2020). "CBD and Novel Foods regulation in the UK: regulation then and now". Health Europa. Archived from the original on October 6, 2020. Retrieved December 10, 2020.
- "Reports published by the International Narcotics Control Board for 2021" (PDF). Archived (PDF) from the original on November 30, 2022. Retrieved December 1, 2022.
- Mead A (May 2017). "The legal status of cannabis (marijuana) and cannabidiol (CBD) under US law". Epilepsy & Behavior. 70 (Pt B): 288–291. doi:10.1016/j.yebeh.2016.11.021. PMID 28169144.
- Conaway KM (December 20, 2018). "Text – H.R.2 – 115th Congress (2017-2018): Agriculture Improvement Act of 2018". Congress.gov. Archived from the original on October 6, 2021. Retrieved May 1, 2019.
- ^ Bellamy J (May 9, 2019). "FDA: No CBD in dietary supplements or foods for now, but let's talk". Science-Based Medicine. Archived from the original on August 17, 2020. Retrieved June 15, 2019.
- ^ Abernethy A, Schiller L (July 17, 2019). "FDA is Committed to Sound, Science-based Policy on CBD". US Food and Drug Administration (FDA). Archived from the original on November 14, 2019. Retrieved October 17, 2019.
- "DEA reschedules Epidiolex, marijuana-derived drug, paving the way for it to hit the market". CNBC. September 27, 2018. Archived from the original on April 26, 2021. Retrieved October 3, 2018.
- Wood S (April 6, 2020). "DEA relaxes rules for the only federally approved drug derived from marijuana". The Philadelphia Inquirer. Archived from the original on June 9, 2020. Retrieved April 23, 2023.
- Tinker B (November 2, 2018). "First FDA-approved cannabis-based drug now available in the US". CNN. Retrieved April 23, 2023.
- Maa E, Figi P (June 2014). "The case for medical marijuana in epilepsy". Epilepsia. 55 (6): 783–786. doi:10.1111/epi.12610. PMID 24854149. S2CID 4849160.
- Saundra Y (August 7, 2013). "Marijuana stops child's severe seizures". CNN. Archived from the original on May 14, 2018. Retrieved May 14, 2018.
- ^ "State Medical Marijuana Laws". National Conference of State Legislatures. April 27, 2018. Archived from the original on December 11, 2018. Retrieved May 14, 2018.
- Schuman B, Fisher J, Radke B, Faldetta G (October 22, 2020). "A Survey of State CBD & Hemp Regulation Since The 2018 Farm Bill". Cannabis Industry Journal. Retrieved April 24, 2023.
- Gebhart F (September 13, 2022). "The Evolution of the CBD Regulatory Landscape". Drug Topics Journal. Retrieved April 24, 2023.
- "The 2014 Farm Bill". thefarmbill.com. Archived from the original on October 30, 2021. Retrieved November 27, 2018.
- Zhang M. "No, CBD Is Not 'Legal in All 50 States'". Forbes. Archived from the original on December 5, 2020. Retrieved November 27, 2018.
- 7 U.S.C. § 1639o
- 21 U.S.C. § 802
- "DEA announces steps necessary to improve access to marijuana research". United States Drug Enforcement Administration. August 26, 2019. Archived from the original on October 18, 2019. Retrieved October 18, 2019.
- "FDA warns company marketing unapproved cannabidiol products with unsubstantiated claims to treat cancer, Alzheimer's disease, opioid withdrawal, pain and pet anxiety". US Food and Drug Administration (FDA). July 23, 2019. Archived from the original on August 14, 2019. Retrieved July 24, 2019.
Unlike drugs approved by the FDA, the manufacturing process of these products has not been subject to FDA review as part of the drug approval process, and there has been no FDA evaluation of whether these products are effective for their intended use, what the proper dosage is, how they could interact with FDA-approved drugs, or whether they have dangerous side effects or other safety concerns.
- Wakshlag JJ, Cital S, Eaton SJ, Prussin R, Hudalla C (April 15, 2020). "Cannabinoid, Terpene, and Heavy Metal Analysis of 29 Over-the-Counter Commercial Veterinary Hemp Supplements". Veterinary Medicine: Research and Reports. 11: 45–55. doi:10.2147/vmrr.s248712. PMC 7169471. PMID 32346530.
- ^ McGrath S, Bartner LR, Rao S, Packer RA, Gustafson DL (June 2019). "Randomized blinded controlled clinical trial to assess the effect of oral cannabidiol administration in addition to conventional antiepileptic treatment on seizure frequency in dogs with intractable idiopathic epilepsy". Journal of the American Veterinary Medical Association. 254 (11): 1301–1308. doi:10.2460/javma.254.11.1301. PMID 31067185. S2CID 148569810.
- ^ Verrico CD, Wesson S, Konduri V, Hofferek CJ, Vazquez-Perez J, Blair E, et al. (September 2020). "A randomized, double-blind, placebo-controlled study of daily cannabidiol for the treatment of canine osteoarthritis pain". Pain. 161 (9): 2191–2202. doi:10.1097/j.pain.0000000000001896. PMC 7584779. PMID 32345916.
- ^ Mogi C, Fukuyama T (2019). "Cannabidiol as a potential anti-epileptic dietary supplement in dogs with suspected epilepsy: three case reports". Pet Behaviour Science (7): 11–16. doi:10.21071/pbs.v0i7. ISSN 2445-2874. Archived from the original on September 3, 2020. Retrieved December 17, 2020.
- ^ Wakshlag JJ, Schwark WS, Deabold KA, Talsma BN, Cital S, Lyubimov A, et al. (September 4, 2020). "Pharmacokinetics of Cannabidiol, Cannabidiolic Acid, Δ9-Tetrahydrocannabinol, Tetrahydrocannabinolic Acid and Related Metabolites in Canine Serum After Dosing With Three Oral Forms of Hemp Extract". Frontiers in Veterinary Science. 7: 505. doi:10.3389/fvets.2020.00505. PMC 7498943. PMID 33102539.
- Ibrahim MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP, et al. (September 2003). "Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: pain inhibition by receptors not present in the CNS". Proceedings of the National Academy of Sciences of the United States of America. 100 (18): 10529–10533. Bibcode:2003PNAS..10010529I. doi:10.1073/pnas.1834309100. PMC 193595. PMID 12917492.
- Hohmann AG, Farthing JN, Zvonok AM, Makriyannis A (February 2004). "Selective activation of cannabinoid CB2 receptors suppresses hyperalgesia evoked by intradermal capsaicin". The Journal of Pharmacology and Experimental Therapeutics. 308 (2): 446–453. doi:10.1124/jpet.103.060079. PMID 14610224. S2CID 17663780.
- Jung KM, Piomelli D (2015). "Cannabinoids and Endocannabinoids". In Pfaff DW, Volkow ND (eds.). Neuroscience in the 21st Century. New York: Springer New York. pp. 1–31. doi:10.1007/978-1-4614-6434-1_136-1. ISBN 978-1461464341.
- Gamble LJ, Boesch JM, Frye CW, Schwark WS, Mann S, Wolfe L, et al. (July 23, 2018). "Pharmacokinetics, Safety, and Clinical Efficacy of Cannabidiol Treatment in Osteoarthritic Dogs". Frontiers in Veterinary Science. 5: 165. doi:10.3389/fvets.2018.00165. PMC 6065210. PMID 30083539.
- Brioschi FA, Di Cesare F, Gioeni D, Rabbogliatti V, Ferrari F, D'Urso ES, et al. (August 2020). "Oral Transmucosal Cannabidiol Oil Formulation as Part of a Multimodal Analgesic Regimen: Effects on Pain Relief and Quality of Life Improvement in Dogs Affected by Spontaneous Osteoarthritis". Animals. 10 (9): 1505. doi:10.3390/ani10091505. PMC 7552307. PMID 32858828.
- Kogan L, Hellyer P, Downing R (2020). "Hemp Oil Extract to Treat Canine Osteoarthritis-Related Pain: A Pilot Study". Journal of the American Holistic Veterinary Medical Association. 58: 35–45. Archived from the original on November 1, 2021. Retrieved December 17, 2020.
- Russo E (2001). Handbook of psychotropic herbs: a scientific analysis of herbal remedies for psychiatric conditions. New York: Haworth Herbal Press. ISBN 0789007185. OCLC 43810871.
- ^ Deabold KA, Schwark WS, Wolf L, Wakshlag JJ (October 2019). "Single-Dose Pharmacokinetics and Preliminary Safety Assessment with Use of CBD-Rich Hemp Nutraceutical in Healthy Dogs and Cats". Animals. 9 (10): 832. doi:10.3390/ani9100832. PMC 6826847. PMID 31635105.
- ^ Vaughn D, Kulpa J, Paulionis L (February 11, 2020). "Preliminary Investigation of the Safety of Escalating Cannabinoid Doses in Healthy Dogs". Frontiers in Veterinary Science. 7: 51. doi:10.3389/fvets.2020.00051. PMC 7029731. PMID 32118071.
- Della Rocca G, Di Salvo A (July 28, 2020). "Hemp in Veterinary Medicine: From Feed to Drug". Frontiers in Veterinary Science. 7: 387. doi:10.3389/fvets.2020.00387. PMC 7399642. PMID 32850997.
- Chicoine A, Illing K, Vuong S, Pinto KR, Alcorn J, Cosford K (September 29, 2020). "Pharmacokinetic and Safety Evaluation of Various Oral Doses of a Novel 1:20 THC:CBD Cannabis Herbal Extract in Dogs". Frontiers in Veterinary Science. 7: 583404. doi:10.3389/fvets.2020.583404. PMC 7550466. PMID 33134364.
- Hoch E, Niemann D, von Keller R, Schneider M, Friemel CM, Preuss UW, et al. (February 2019). "How effective and safe is medical cannabis as a treatment of mental disorders? A systematic review". European Archives of Psychiatry and Clinical Neuroscience. 269 (1): 87–105. doi:10.1007/s00406-019-00984-4. PMC 6595000. PMID 30706168.
- Sanders L (March 27, 2019). "The CBD boom is way ahead of the science". Science News. Retrieved April 24, 2023.
Further reading
- CBD: What Does the Science Say?
- Williams A (October 27, 2018). "Why Is CBD Everywhere?". The New York Times. ISSN 0362-4331. Archived from the original on October 22, 2021. Retrieved November 17, 2018.
- Dunning B (May 18, 2021). "Skeptoid #780: CBD for Everything: Cannabidiol is sold as an additive to just about every kind of product you can imagine. Why?". Skeptoid. Retrieved May 21, 2022.
- 5-HT1A agonists
- Analgesics
- Anti-inflammatory agents
- Anticonvulsants
- Cyclohexenes
- CYP2D6 inhibitors
- CYP3A4 inhibitors
- Drugs with unknown mechanisms of action
- Endocannabinoid reuptake inhibitors
- Experimental anxiolytics
- Natural phenols
- Opioid receptor negative allosteric modulators
- Orphan drugs
- Phytocannabinoids
- Resorcinols
- Transient receptor potential channel modulators