| Revision as of 11:34, 31 October 2011 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'ChEBI', 'CAS_number').← Previous edit | Latest revision as of 01:35, 9 September 2024 edit undo98.191.202.231 (talk) Cat. | ||
| (137 intermediate revisions by 82 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Chemical compound}} | |||
| {{Drugbox | |||
| {{cs1 config|name-list-style=vanc|display-authors=6}} | |||
| ⚫ | | verifiedrevid = |
||
| {{Infobox drug | |||
| | IUPAC_name = 2-trimethylazaniumylethyl phosphate | |||
| | Verifiedfields = changed | |||
| | image = Choline alfoscerate.svg | |||
| | Watchedfields = changed | |||
| ⚫ | | verifiedrevid = 458279808 | ||
| | IUPAC_name = 2-trimethylazaniumylethyl phosphate <!-- see www.fda.gov/ucm/groups/fdagov-public/@fdagov-foods-gen/documents/document/ucm299330.pdf#page=12 --> | |||
| | image = L-alpha-GPC Structural Formula V.1.svg | |||
| <!--Clinical data--> | <!--Clinical data--> | ||
| | tradename = |
| tradename = | ||
| | pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | | pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | ||
| | pregnancy_US = <!-- A / B / C / D / X --> | | pregnancy_US = <!-- A / B / C / D / X --> | ||
| Line 15: | Line 19: | ||
| <!--Identifiers--> | <!--Identifiers--> | ||
| | CAS_number_Ref = {{cascite| |
| CAS_number_Ref = {{cascite|changed|??}} | ||
| | CAS_number = |
| CAS_number = 28319-77-9 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | UNII = 60M22SGW66 | |||
| | ATC_prefix = N07 | | ATC_prefix = N07 | ||
| | ATC_suffix = AX02 | | ATC_suffix = AX02 | ||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | ChEBI = 55397 | | ChEBI = 55397 | ||
| | PubChem = |
| PubChem = 657272 | ||
| | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 571409 | | ChemSpiderID = 571409 | ||
| | ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| | ChEMBL = 1567463 | |||
| | smiles = P(=O)(OC(O)CO)OCC(C)(C)C | | smiles = P(=O)(OC(O)CO)OCC(C)(C)C | ||
| | InChI = 1/C8H20NO6P/c1-9(2,3)4-5-14-16(12,13)15-7-8(11)6-10/h8,10-11H,4-7H2,1-3H3/t8-/m1/s1 | |||
| | InChIKey = SUHOQUVVVLNYQR-MRVPVSSYBR | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/C8H20NO6P/c1-9(2,3)4-5-14-16(12,13)15-7-8(11)6-10/h8,10-11H,4-7H2,1-3H3/t8-/m1/s1 | | StdInChI = 1S/C8H20NO6P/c1-9(2,3)4-5-14-16(12,13)15-7-8(11)6-10/h8,10-11H,4-7H2,1-3H3/t8-/m1/s1 | ||
| Line 33: | Line 40: | ||
| <!--Chemical data--> | <!--Chemical data--> | ||
| | C=8 | H=20 | N=1 | O=6 | P=1 |
| C=8 | H=20 | N=1 | O=6 | P=1 | ||
| | molecular_weight = 257.221 g/mol | |||
| }} | }} | ||
| '''L- |
'''<small>L</small>-α-Glycerophosphorylcholine''' ('''alpha-GPC''', '''choline alfoscerate''', '''''sn''-glycero-3-phosphocholine''') is a natural ] compound found in the brain. It is also a ] ] precursor<ref name="pmid12637119">{{cite journal | vauthors = De Jesus Moreno Moreno M | title = Cognitive improvement in mild to moderate Alzheimer's dementia after treatment with the acetylcholine precursor choline alfoscerate: a multicenter, double-blind, randomized, placebo-controlled trial | journal = Clinical Therapeutics | volume = 25 | issue = 1 | pages = 178–93 | date = January 2003 | pmid = 12637119 | doi = 10.1016/S0149-2918(03)90023-3 }}</ref> which has been investigated for its potential for the treatment of ]<ref name="Parnetti">{{cite journal | vauthors = Parnetti L, Mignini F, Tomassoni D, Traini E, Amenta F | title = Cholinergic precursors in the treatment of cognitive impairment of vascular origin: ineffective approaches or need for re-evaluation? | journal = Journal of the Neurological Sciences | volume = 257 | issue = 1–2 | pages = 264–9 | date = June 2007 | pmid = 17331541 | doi = 10.1016/j.jns.2007.01.043 | s2cid = 34661218 }} | ||
| </ref> and other ]s.<ref name="Doggrell">{{cite journal | vauthors = Doggrell SA, Evans S | title = Treatment of dementia with neurotransmission modulation | journal = Expert Opinion on Investigational Drugs | volume = 12 | issue = 10 | pages = 1633–54 | date = October 2003 | pmid = 14519085 | doi = 10.1517/13543784.12.10.1633 | s2cid = 46175609 }}</ref> | |||
| Alpha-GPC rapidly delivers ] to the brain across the ] and is a biosynthetic precursor of ].<ref name="Parnetti" /> It is a non-prescription drug in most countries. The ] determined that intake of no more than 196.2 mg/person/day is considered ] (GRAS).<ref>{{cite web |url= https://www.accessdata.fda.gov/scripts/fcn/gras_notices/GRN000419.pdf | archive-url = https://web.archive.org/web/20131224102629/https://www.accessdata.fda.gov/scripts/fcn/gras_notices/GRN000419.pdf | archive-date = 24 December 2013 |title= Generally Recognized as Safe (GRAS) Determination for the Use of AlphaSize® Alpha-Glycerylphosphoryl Choline | date = 25 January 2012 | publisher = United States Food and Drug Administration }}</ref> | |||
| Alpha GPC rapidly delivers ] to the brain across the blood-brain barrier and is a biosynthetic precursor of the acetylcholine neurotransmitter.<ref>{{cite journal | |||
| | last =Parnetti | |||
| | first = Lucilla | |||
| | coauthors = et al. | |||
| | year = 2007 | |||
| | title = Cholinergic precursors in the treatment of cognitive impairment of vascular origin: Ineffective approaches or need for re-evaluation? | |||
| | journal = Journal of the Neurological Sciences | |||
| | volume = 257 | |||
| | pages = 264–9 | |||
| | pmid = 17331541 | |||
| | issue =1–2 | |||
| | doi =10.1016/j.jns.2007.01.043 | |||
| }} | |||
| </ref> Alpha GPC is derived from highly purified soy ]. | |||
| == Production == | |||
| Studies have investigated its efficacy for cognitive disorders including stroke and ]. An Italian multicentre clinical trial on 2,044 patients suffering from recent stroke were supplied alpha-GPC in doses of 1,000 mg/day for 28 days and 400 mg three times per day for the five ensuing months. The trial confirmed the therapeutic role of alpha-GPC on the cognitive recovery of patients based on four measurement scales, three of which reached statistical significance.<ref>Barbagallo Sangiorgi G, et al. "Alpha-Glycerophosphocholine in the mental recovery of cerebral ischemic attacks." An Italian multicenter clinical trial. Ann NY Acad Sci 1994; 717:253-69.</ref> | |||
| Industrially, alpha-GPC is produced by the chemical or enzymatic deacylation of ] enriched soya ] followed by ]. Alpha-GPC may also be derived in small amounts from highly purified soy ] as well as from purified sunflower lecithin.<ref name="Traini_2013">{{cite journal | vauthors = Traini E, Bramanti V, Amenta F | title = Choline alphoscerate (alpha-glyceryl-phosphoryl-choline) an old choline- containing phospholipid with a still interesting profile as cognition enhancing agent | journal = Current Alzheimer Research | volume = 10 | issue = 10 | pages = 1070–9 | date = December 2013 | pmid = 24156263 | doi = 10.2174/15672050113106660173 }}</ref><ref name="Scapicchio_2013">{{cite journal | vauthors = Scapicchio PL | title = Revisiting choline alphoscerate profile: a new, perspective, role in dementia? | journal = The International Journal of Neuroscience | volume = 123 | issue = 7 | pages = 444–9 | date = July 2013 | pmid = 23387341 | doi = 10.3109/00207454.2013.765870 | url = }}</ref> | |||
| == Safety == | |||
| Commonly used doses are 300-1,200 mg daily. | |||
| Alpha-GPC metabolizes to ] in the gastrointestinal tract, which has implications for cardiovascular health. In one study, risk of stroke over a ten-year period was increased by about 40% in users of alpha-GPC.<ref>{{cite journal | vauthors = Lee G, Choi S, Chang J, Choi D, Son JS, Kim K, Kim SM, Jeong S, Park SM | title = Association of L-α Glycerylphosphorylcholine With Subsequent Stroke Risk After 10 Years | journal = JAMA Network Open | volume = 4 | issue = 11 | pages = e2136008 | date = November 2021 | pmid = 34817582 | pmc = 8613599 | doi = 10.1001/jamanetworkopen.2021.36008 }}</ref> | |||
| == References == | == References == | ||
| {{Reflist|2}} | {{Reflist|2}} | ||
| == External links == | |||
| * | |||
| {{Dietary_supplements}} | {{Dietary_supplements}} | ||
| {{Nootropics}} | |||
| {{Acetylcholine receptor modulators}} | |||
| {{Cholinergics}} | |||
| {{DEFAULTSORT:Alpha-Gpc}} | {{DEFAULTSORT:Alpha-Gpc}} | ||
| ⚫ | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ⚫ | ] | ||
| ] | |||
| ] | |||
Latest revision as of 01:35, 9 September 2024
Chemical compoundPharmaceutical compound
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.044.496 |
| Chemical and physical data | |
| Formula | C8H20NO6P |
| Molar mass | 257.223 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (what is this?) (verify) | |
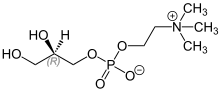
L-α-Glycerophosphorylcholine (alpha-GPC, choline alfoscerate, sn-glycero-3-phosphocholine) is a natural choline compound found in the brain. It is also a parasympathomimetic acetylcholine precursor which has been investigated for its potential for the treatment of Alzheimer's disease and other dementias.
Alpha-GPC rapidly delivers choline to the brain across the blood–brain barrier and is a biosynthetic precursor of acetylcholine. It is a non-prescription drug in most countries. The FDA determined that intake of no more than 196.2 mg/person/day is considered generally recognized as safe (GRAS).
Production
Industrially, alpha-GPC is produced by the chemical or enzymatic deacylation of phosphatidylcholine enriched soya phospholipids followed by chromatographic purification. Alpha-GPC may also be derived in small amounts from highly purified soy lecithin as well as from purified sunflower lecithin.
Safety
Alpha-GPC metabolizes to trimethylamine n-oxide in the gastrointestinal tract, which has implications for cardiovascular health. In one study, risk of stroke over a ten-year period was increased by about 40% in users of alpha-GPC.
References
- De Jesus Moreno Moreno M (January 2003). "Cognitive improvement in mild to moderate Alzheimer's dementia after treatment with the acetylcholine precursor choline alfoscerate: a multicenter, double-blind, randomized, placebo-controlled trial". Clinical Therapeutics. 25 (1): 178–93. doi:10.1016/S0149-2918(03)90023-3. PMID 12637119.
- ^ Parnetti L, Mignini F, Tomassoni D, Traini E, Amenta F (June 2007). "Cholinergic precursors in the treatment of cognitive impairment of vascular origin: ineffective approaches or need for re-evaluation?". Journal of the Neurological Sciences. 257 (1–2): 264–9. doi:10.1016/j.jns.2007.01.043. PMID 17331541. S2CID 34661218.
- Doggrell SA, Evans S (October 2003). "Treatment of dementia with neurotransmission modulation". Expert Opinion on Investigational Drugs. 12 (10): 1633–54. doi:10.1517/13543784.12.10.1633. PMID 14519085. S2CID 46175609.
- "Generally Recognized as Safe (GRAS) Determination for the Use of AlphaSize® Alpha-Glycerylphosphoryl Choline" (PDF). United States Food and Drug Administration. 25 January 2012. Archived from the original (PDF) on 24 December 2013.
- Traini E, Bramanti V, Amenta F (December 2013). "Choline alphoscerate (alpha-glyceryl-phosphoryl-choline) an old choline- containing phospholipid with a still interesting profile as cognition enhancing agent". Current Alzheimer Research. 10 (10): 1070–9. doi:10.2174/15672050113106660173. PMID 24156263.
- Scapicchio PL (July 2013). "Revisiting choline alphoscerate profile: a new, perspective, role in dementia?". The International Journal of Neuroscience. 123 (7): 444–9. doi:10.3109/00207454.2013.765870. PMID 23387341.
- Lee G, Choi S, Chang J, Choi D, Son JS, Kim K, et al. (November 2021). "Association of L-α Glycerylphosphorylcholine With Subsequent Stroke Risk After 10 Years". JAMA Network Open. 4 (11): e2136008. doi:10.1001/jamanetworkopen.2021.36008. PMC 8613599. PMID 34817582.