| Revision as of 10:46, 1 November 2011 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'DrugBank').← Previous edit | Latest revision as of 20:08, 30 August 2024 edit undo174.66.87.253 (talk) Sort cats. | ||
| (72 intermediate revisions by 54 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Chemical compound}} | |||
| {{Drugbox | {{Drugbox | ||
| | Watchedfields = changed | |||
| | verifiedrevid = |
| verifiedrevid = 458440771 | ||
| | IUPAC_name = |
| IUPAC_name = bis(propan-2-yl) fluorophosphonate | ||
| | image = Diisopropylfluorophosphate.svg | | image = Diisopropylfluorophosphate.svg | ||
| | image2 = Diisopropyl-fluorophosphate-3D-balls.png | | image2 = Diisopropyl-fluorophosphate-3D-balls.png | ||
| <!--Clinical data--> | <!--Clinical data--> | ||
| | tradename = |
| tradename = | ||
| <!--Identifiers--> | <!--Identifiers--> | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | CAS_number_Ref = {{cascite|correct|??}} | | CAS_number_Ref = {{cascite|correct|??}} | ||
| | CAS_number = 55-91-4 | | CAS_number = 55-91-4 | ||
| Line 27: | Line 26: | ||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | | ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| | ChEMBL = 1025 | | ChEMBL = 1025 | ||
| |synonyms= Isofluorophate, Isofluorphate, DFP, DIFP, DIPF, Diisopropyl phosphorofluoridate, EA-1152, PF-3, T-1703, TL-466 | |||
| <!--Chemical data--> | <!--Chemical data--> | ||
| | C=6 | H=14 | F=1 | O=3 | P=1 |
| C=6 | H=14 | F=1 | O=3 | P=1 | ||
| | molecular_weight = 184.146 g/mol | |||
| | smiles = FP(=O)(OC(C)C)OC(C)C | | smiles = FP(=O)(OC(C)C)OC(C)C | ||
| | InChI = 1/C6H14FO3P/c1-5(2)9-11(7,8)10-6(3)4/h5-6H,1-4H3 | |||
| | InChIKey = MUCZHBLJLSDCSD-UHFFFAOYAT | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChI = 1S/C6H14FO3P/c1-5(2)9-11(7,8)10-6(3)4/h5-6H,1-4H3 | | StdInChI = 1S/C6H14FO3P/c1-5(2)9-11(7,8)10-6(3)4/h5-6H,1-4H3 | ||
| Line 43: | Line 39: | ||
| }} | }} | ||
| '''Diisopropyl fluorophosphate''' ('''DFP''' |
'''Diisopropyl fluorophosphate''' ('''DFP''') or '''Isoflurophate''' is an oily, colorless liquid with the chemical formula C<sub>6</sub>H<sub>14</sub>FO<sub>3</sub>P. It is used in medicine<ref name=drugsIsofluorphate>{{cite web|title=Isofluorphate definition|url=https://www.drugs.com/dict/isofluorphate.html|publisher=Drugs.com|access-date=6 September 2017|archive-url=https://web.archive.org/web/20170906134840/https://www.drugs.com/dict/isofluorphate.html|archive-date=6 September 2017|url-status=dead}}</ref> and as an ] ].<ref>{{Cite web |title=Isoflurophate |url=https://ncithesaurus.nci.nih.gov/ncitbrowser/ConceptReport.jsp?dictionary=NCI_Thesaurus&ns=ncit&code=C65979 |access-date=2022-10-30 |website=National Cancer Institute}}</ref> It is stable, but undergoes hydrolysis when subjected to moisture. | ||
| It is known also under names '''Difluorophate''', '''Diflupyl''', '''Diflurphate''', '''Dyflos''', '''Dyphlos''', '''Fluropryl''', '''Fluostigmine''', '''isofluorophate''', '''isofluorphate''', '''Neoglaucit''', '''PF-3''', '''PF3''', '''T-1703''', '''TL 466''', and others. | |||
| == |
==Uses in medicine== | ||
| ⚫ | Diisopropyl fluorophosphate is a ] ] and has been used in ] as a ] agent in treatment of chronic ], as a miotic in veterinary medicine, and as an experimental agent in neuroscience because of its ] ] properties and ability to induce ].<ref name=drugsIsofluorphate/> | ||
| ⚫ | ==Uses as toxin== | ||
| ⚫ | Diisopropyl fluorophosphate is a parasympathomimetic drug |
||
| ⚫ | ] | ||
| ⚫ | The marked toxicity of esters of ] was discovered in 1932, when ] and his PhD student Gerda von Krueger prepared the methyl, ethyl, n-propyl, and n-butyl esters and incidentally experienced their toxic effects. Another homologue of this series of esters, Diisopropyl fluorophosphate, was developed by British scientist ]. On his search for compounds to be used as ] agents, Saunders was inspired by the report by Lange and Krueger and decided to prepare the new homologue which he labeled PF-3. It was much less effective as a chemical weapon than the G series agents. It was often mixed with ], forming a more effective mixture with significantly lower melting point, resulting in an agent suitable for use in cold weather. .]] In military research, due to its physical and chemical similarities and comparatively low toxicity, it is used as a simulant of ]s (], ], ], and ]). Diisopropyl fluorophosphate is used in civilian laboratories to mimic lethal nerve gas exposure or organophosphate toxicities.<ref>{{cite journal | vauthors = Deshpande LS, Carter DS, Blair RE, DeLorenzo RJ | title = Development of a prolonged calcium plateau in hippocampal neurons in rats surviving status epilepticus induced by the organophosphate diisopropylfluorophosphate | journal = Toxicological Sciences | volume = 116 | issue = 2 | pages = 623–31 | date = August 2010 | pmid = 20498005 | pmc = 2905411 | doi = 10.1093/toxsci/kfq157 }}</ref><ref>{{cite journal | vauthors = Pessah IN, Rogawski MA, Tancredi DJ, Wulff H, Zolkowska D, Bruun DA, Hammock BD, Lein PJ | display-authors = 6 | title = Models to identify treatments for the acute and persistent effects of seizure-inducing chemical threat agents | journal = Annals of the New York Academy of Sciences | volume = 1378 | issue = 1 | pages = 124–136 | date = August 2016 | pmid = 27467073 | pmc = 5063690 | doi = 10.1111/nyas.13137 }}</ref><ref>{{cite journal | vauthors = Kadriu B, Guidotti A, Costa E, Davis JM, Auta J | title = Acute imidazenil treatment after the onset of DFP-induced seizure is more effective and longer lasting than midazolam at preventing seizure activity and brain neuropathology | journal = Toxicological Sciences | volume = 120 | issue = 1 | pages = 136–45 | date = March 2011 | pmid = 21097996 | doi = 10.1093/toxsci/kfq356 | doi-access = free }}</ref> It has also been used to develop a rodent model of ].<ref>{{cite journal | vauthors = Phillips KF, Deshpande LS | title = Repeated low-dose organophosphate DFP exposure leads to the development of depression and cognitive impairment in a rat model of Gulf War Illness | journal = Neurotoxicology | volume = 52 | pages = 127–33 | date = January 2016 | pmid = 26619911 | doi = 10.1016/j.neuro.2015.11.014 | url = https://zenodo.org/record/894926 | doi-access = free }}</ref> | ||
| ⚫ | == |
||
| ⚫ | Diisopropyl fluorophosphate is a very potent ]. Its {{LD50}} in rats is 6 mg/kg (oral). It combines with the ] ] at the active site of the enzyme ],<ref>{{cite journal | vauthors = Millard CB, Kryger G, Ordentlich A, Greenblatt HM, Harel M, Raves ML, Segall Y, Barak D, Shafferman A, Silman I, Sussman JL | display-authors = 6 | title = Crystal structures of aged phosphonylated acetylcholinesterase: nerve agent reaction products at the atomic level | journal = Biochemistry | volume = 38 | issue = 22 | pages = 7032–9 | date = June 1999 | pmid = 10353814 | doi = 10.1021/bi982678l }}</ref> an enzyme that deactivates the ] ]. Neurotransmitters are needed to continue the passage of ]s from one ] to another across the ]. Once the impulse has been transmitted, acetylcholinesterase functions to deactivate the acetylcholine almost immediately by breaking it down. If the enzyme is inhibited, acetylcholine accumulates and nerve impulses cannot be stopped, causing prolonged muscle contraction. ] occurs and ] may result since the ] are affected. | ||
| ⚫ | ] | ||
| ⚫ | DFP also inhibits some ]s. It is a useful additive for protein or cell isolation procedure. | ||
| ⚫ | The marked toxicity of esters of monofluorophosphoric acid was discovered in 1932, when ] and his PhD student Gerda von Krueger prepared the methyl, ethyl, n-propyl, and n-butyl esters and incidentally experienced their toxic effects. Another homologue of this series of esters, Diisopropyl fluorophosphate, was developed by British scientist ]. On his search for compounds to be used as ] agents, Saunders was inspired by the report by Lange |
||
| Diisopropyl fluorophosphate (DFP) was a nerve gas developed by the German during the ]. DFP irreversibly binds with the enzymes containing serine at the active site, e g. Serine proteases, acetylcholine esterase. | |||
| ⚫ | Diisopropyl fluorophosphate is a very potent ]. Its {{LD50}} in rats is |
||
| ==Production== | |||
| ⚫ | DFP also inhibits some |
||
| ⚫ | Isoflurophate, the diisopropyl ester of fluorophosphoric acid, is made by reacting ] with ], forming diisopropylphosphite, which is chlorinated and further reacted with ] to replace the chlorine atom with fluorine, thus giving diisopropyl fluorophosphate.<ref>{{cite patent | inventor1-first=Edgar E|inventor1-last=Hardy|inventor2-first=Gennady M|inventor2-last= Kosoloapoff| country = US | assign = ] | gdate = 1946-10-08 | number = 2409039 | title = Halogenated compounds and process for making same | status = patent}}</ref> | ||
| ⚫ | :] | ||
| ==Biochemistry== | |||
| DFP is a diagnostic test for the presence of the active site Ser in serine proteases, as well as a serine protease inhibitor. ] and ] are alternative, less toxic, but presumably also less reactive, reagents for these same applications. DFP and other analogous organophosphate neurotoxins are inactivated by the enzyme ], which is present in widely varying levels in humans. | |||
| ==Society and culture== | |||
| It is marketed under many brand names including Difluorophate, Diflupyl, Diflurphate, Dyflos, Dyphlos, Fluropryl, Fluostigmine, Neoglaucit.{{citation needed|date=September 2017}} | |||
| ==Chemistry== | |||
| ⚫ | Isoflurophate, the |
||
| ⚫ | ] | ||
| *E.E. Hardy, G.M. Kosoloapoff, {{US Patent|2409039}} (1946). | |||
| == See also == | == See also == | ||
| * |
*] (methoxy arachidonoylfluorophosphonate), a mechanistically related inhibitor | ||
| *], a cyclic analogue | |||
| *] (isopropyl methylphosphonofluoridate), a related phosphofluoridate | |||
| *] (4-(2-aminoethyl)benzenesulfonyl fluoride), an analogous sulfonyl fluoride sulfonating reagent | |||
| *] (phenylmethylsulfonyl fluoride), an analogous sulfonyl fluoride sulfonating reagent | |||
| == References == | == References == | ||
| {{ |
{{reflist|32em}} | ||
| ⚫ | * Brenner |
||
| ⚫ | * Meiers |
||
| == Further reading == | |||
| ⚫ | ==External links== | ||
| {{refbegin}} | |||
| ⚫ | * |
||
| ⚫ | * {{cite book| vauthors = Brenner GM |year=2000|title= Pharmacology|location= Philadelphia, PA|publisher= W.B. Saunders Company. |isbn =0-7216-7757-6}} | ||
| ⚫ | * {{cite web| vauthors = Meiers P |year=2006|url=http://www.fluoride-history.de/p-mfp.htm|title= History of the fluorophosphates}} | ||
| {{refend}} | |||
| ⚫ | == External links == | ||
| ⚫ | *The ] online database for peptidases and their inhibitors: | ||
| {{Chemical agents}} | |||
| {{Opthalmologicals}} | {{Opthalmologicals}} | ||
| {{Acetylcholine metabolism and transport modulators}} | |||
| {{Cholinergics}} | |||
| {{DEFAULTSORT:Diisopropyl Fluorophosphate}} | {{DEFAULTSORT:Diisopropyl Fluorophosphate}} | ||
| ⚫ | ] | ||
| ] | ] | ||
| ] | ] | ||
| ⚫ | ] | ||
| ] | ] | ||
| ⚫ | ] | ||
| ⚫ | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 20:08, 30 August 2024
Chemical compound Pharmaceutical compound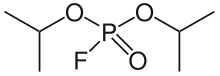 | |
 | |
| Clinical data | |
|---|---|
| Other names | Isofluorophate, Isofluorphate, DFP, DIFP, DIPF, Diisopropyl phosphorofluoridate, EA-1152, PF-3, T-1703, TL-466 |
| ATC code | |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.225 |
| Chemical and physical data | |
| Formula | C6H14FO3P |
| Molar mass | 184.147 g·mol |
| 3D model (JSmol) | |
| Melting point | −82 °C (−116 °F) |
| Boiling point | 183 °C (361 °F) 1013 mbar |
SMILES
| |
InChI
| |
| (verify) | |
Diisopropyl fluorophosphate (DFP) or Isoflurophate is an oily, colorless liquid with the chemical formula C6H14FO3P. It is used in medicine and as an organophosphorus insecticide. It is stable, but undergoes hydrolysis when subjected to moisture.
Uses in medicine
Diisopropyl fluorophosphate is a parasympathomimetic drug irreversible anti-cholinesterase and has been used in ophthalmology as a miotic agent in treatment of chronic glaucoma, as a miotic in veterinary medicine, and as an experimental agent in neuroscience because of its acetylcholinesterase inhibitory properties and ability to induce delayed peripheral neuropathy.
Uses as toxin

The marked toxicity of esters of monofluorophosphoric acid was discovered in 1932, when Willy Lange and his PhD student Gerda von Krueger prepared the methyl, ethyl, n-propyl, and n-butyl esters and incidentally experienced their toxic effects. Another homologue of this series of esters, Diisopropyl fluorophosphate, was developed by British scientist Bernard Charles Saunders. On his search for compounds to be used as chemical warfare agents, Saunders was inspired by the report by Lange and Krueger and decided to prepare the new homologue which he labeled PF-3. It was much less effective as a chemical weapon than the G series agents. It was often mixed with mustard gas, forming a more effective mixture with significantly lower melting point, resulting in an agent suitable for use in cold weather.

In military research, due to its physical and chemical similarities and comparatively low toxicity, it is used as a simulant of G-agents (GA, GB, GD, and GF). Diisopropyl fluorophosphate is used in civilian laboratories to mimic lethal nerve gas exposure or organophosphate toxicities. It has also been used to develop a rodent model of Gulf War Syndrome.
Diisopropyl fluorophosphate is a very potent neurotoxin. Its LD50 in rats is 6 mg/kg (oral). It combines with the amino acid serine at the active site of the enzyme acetylcholinesterase, an enzyme that deactivates the neurotransmitter acetylcholine. Neurotransmitters are needed to continue the passage of nerve impulses from one neuron to another across the synapse. Once the impulse has been transmitted, acetylcholinesterase functions to deactivate the acetylcholine almost immediately by breaking it down. If the enzyme is inhibited, acetylcholine accumulates and nerve impulses cannot be stopped, causing prolonged muscle contraction. Paralysis occurs and death may result since the respiratory muscles are affected.
DFP also inhibits some proteases. It is a useful additive for protein or cell isolation procedure.
Diisopropyl fluorophosphate (DFP) was a nerve gas developed by the German during the Second World War. DFP irreversibly binds with the enzymes containing serine at the active site, e g. Serine proteases, acetylcholine esterase.
Production
Isoflurophate, the diisopropyl ester of fluorophosphoric acid, is made by reacting isopropyl alcohol with phosphorus trichloride, forming diisopropylphosphite, which is chlorinated and further reacted with sodium fluoride to replace the chlorine atom with fluorine, thus giving diisopropyl fluorophosphate.
Biochemistry
DFP is a diagnostic test for the presence of the active site Ser in serine proteases, as well as a serine protease inhibitor. PMSF and AEBSF are alternative, less toxic, but presumably also less reactive, reagents for these same applications. DFP and other analogous organophosphate neurotoxins are inactivated by the enzyme paraoxonase, which is present in widely varying levels in humans.
Society and culture
It is marketed under many brand names including Difluorophate, Diflupyl, Diflurphate, Dyflos, Dyphlos, Fluropryl, Fluostigmine, Neoglaucit.
See also
- MAFP (methoxy arachidonoylfluorophosphonate), a mechanistically related inhibitor
- Neopentylene fluorophosphate, a cyclic analogue
- Sarin (isopropyl methylphosphonofluoridate), a related phosphofluoridate
- PMSF (4-(2-aminoethyl)benzenesulfonyl fluoride), an analogous sulfonyl fluoride sulfonating reagent
- AEBSF (phenylmethylsulfonyl fluoride), an analogous sulfonyl fluoride sulfonating reagent
References
- ^ "Isofluorphate definition". Drugs.com. Archived from the original on 6 September 2017. Retrieved 6 September 2017.
- "Isoflurophate". National Cancer Institute. Retrieved 2022-10-30.
- Deshpande LS, Carter DS, Blair RE, DeLorenzo RJ (August 2010). "Development of a prolonged calcium plateau in hippocampal neurons in rats surviving status epilepticus induced by the organophosphate diisopropylfluorophosphate". Toxicological Sciences. 116 (2): 623–31. doi:10.1093/toxsci/kfq157. PMC 2905411. PMID 20498005.
- Pessah IN, Rogawski MA, Tancredi DJ, Wulff H, Zolkowska D, Bruun DA, et al. (August 2016). "Models to identify treatments for the acute and persistent effects of seizure-inducing chemical threat agents". Annals of the New York Academy of Sciences. 1378 (1): 124–136. doi:10.1111/nyas.13137. PMC 5063690. PMID 27467073.
- Kadriu B, Guidotti A, Costa E, Davis JM, Auta J (March 2011). "Acute imidazenil treatment after the onset of DFP-induced seizure is more effective and longer lasting than midazolam at preventing seizure activity and brain neuropathology". Toxicological Sciences. 120 (1): 136–45. doi:10.1093/toxsci/kfq356. PMID 21097996.
- Phillips KF, Deshpande LS (January 2016). "Repeated low-dose organophosphate DFP exposure leads to the development of depression and cognitive impairment in a rat model of Gulf War Illness". Neurotoxicology. 52: 127–33. doi:10.1016/j.neuro.2015.11.014. PMID 26619911.
- Millard CB, Kryger G, Ordentlich A, Greenblatt HM, Harel M, Raves ML, et al. (June 1999). "Crystal structures of aged phosphonylated acetylcholinesterase: nerve agent reaction products at the atomic level". Biochemistry. 38 (22): 7032–9. doi:10.1021/bi982678l. PMID 10353814.
- US patent 2409039, Hardy, Edgar E & Kosoloapoff, Gennady M, "Halogenated compounds and process for making same", issued 1946-10-08, assigned to Monsanto Chemical Company
Further reading
- Brenner GM (2000). Pharmacology. Philadelphia, PA: W.B. Saunders Company. ISBN 0-7216-7757-6.
- Meiers P (2006). "History of the fluorophosphates".
External links
| Drugs used for glaucoma preparations and miosis (S01E) | |||||||
|---|---|---|---|---|---|---|---|
| Sympathomimetics | |||||||
| Parasympathomimetics |
| ||||||
| Carbonic anhydrase inhibitors/ (sulfonamides) | |||||||
| Beta blocking agents | |||||||
| Prostaglandin analogues (F2α) | |||||||
| Other agents | |||||||
| Acetylcholine metabolism and transport modulators | |||||||
|---|---|---|---|---|---|---|---|
| Enzyme (modulators) |
| ||||||
| Transporter (modulators) |
| ||||||
| Release (modulators) |
| ||||||