| Revision as of 16:52, 10 November 2011 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'ChEBI').← Previous edit | Latest revision as of 20:23, 25 November 2024 edit undoLaura240406 (talk | contribs)Extended confirmed users887 edits →History: added linkTag: Visual edit: Switched | ||
| (74 intermediate revisions by 49 users not shown) | |||
| Line 1: | Line 1: | ||
| {{ |
{{Chembox | ||
| | Verifiedfields = changed | |||
| ⚫ | | verifiedrevid = |
||
| | Watchedfields = changed | |||
| ⚫ | | |
||
| ⚫ | | verifiedrevid = 459990955 | ||
| | ImageSizeL1 = 120px | |||
| | |
| ImageFile = Cacodylic acid.svg | ||
| | |
| ImageSize = 160px | ||
| | ImageAlt = Structural formula | |||
| ⚫ | | |
||
| ⚫ | | ImageFile1 = cacodylic-acid-3D-balls.png | ||
| | ImageSize1 = 160px | |||
| | ImageAlt1 = Ball-and-stick model | |||
| | ImageCaption1 = ]<ref>{{ Cite journal | url = https://dx.doi.org/10.5517/ccx73mv | title = CSD Entry: CADYLA01 : Dimethylarsinic acid | website = ]: Access Structures | year = 2011 | publisher = ] | doi = 10.5517/ccx73mv | access-date = 2021-12-21 | last1 = Betz | first1 = R. | last2 = McCleland | first2 = C. | last3 = Marchand | first3 = H. }}</ref><ref name="Betz">{{ cite journal | title = The monoclinic polymorph of dimethylarsinic acid | first1 = R. | last1 = Betz | first2 = C. | last2 = McCleland | first3 = H. | last3 = Marchand | journal = ] | year = 2011 | volume = 67 | issue = 8 | pages = m1013 | doi = 10.1107/S1600536811025505 | pmid = 22090811 | pmc = 3212109 }}</ref> | |||
| ⚫ | | PIN = Dimethylarsinic acid | ||
| | OtherNames = Dimethylarsenic acid, Cacodylic acid, Hydroxydimethylarsine oxide, Arsecodile, Ansar, Silvisar, Phytar 560, DMAA, UN 1572 | | OtherNames = Dimethylarsenic acid, Cacodylic acid, Hydroxydimethylarsine oxide, Arsecodile, Ansar, Silvisar, Phytar 560, DMAA, UN 1572 | ||
| | |
|Section1={{Chembox Identifiers | ||
| | |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 2418 | | ChemSpiderID = 2418 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | | UNII_Ref = {{fdacite|correct|FDA}} | ||
| Line 21: | Line 26: | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | CASNo = 75-60-5 | | CASNo = 75-60-5 | ||
| | |
| PubChem = 2513 | ||
| | |
| EINECS = 200-883-4 | ||
| | |
| ChEBI_Ref = {{ebicite|changed|EBI}} | ||
| | ChEBI = |
| ChEBI = 29839 | ||
| | |
| ChEMBL = 1231644 | ||
| | RTECS = CH7525000 | |||
| | |
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| | DrugBank = DB02994 | | DrugBank = DB02994 | ||
| | UNNumber = 1572 | |||
| | Gmelin = 130562 | |||
| | Beilstein = 1736965 | |||
| | SMILES = O=(O)(C)C | | SMILES = O=(O)(C)C | ||
| | |
| InChI = 1/C2H7AsO2/c1-3(2,4)5/h1-2H3,(H,4,5) | ||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| | |
| Formula = C<sub>2</sub>H<sub>7</sub>AsO<sub>2</sub> | ||
| | |
| MolarMass = 137.9977 g/mol | ||
| | |
| Appearance = White crystals or powder | ||
| | Density = > 1.1 g/cm<sup>3</sup> | | Odor = odorless | ||
| | Density = > 1.1 g/cm<sup>3</sup> | |||
| | |
| MeltingPtC = 192 to 198 | ||
| | MeltingPt_notes = | |||
| | |
| BoilingPt= > | ||
| ⚫ | | |
||
| | |
| BoilingPtC = 200 | ||
| ⚫ | | Solubility = 66.7 g/100 ml | ||
| | SolubleOther = soluble in ], ] <br /> insoluble in ] | |||
| | pKa = 6 | |||
| | MagSus = -79.9·10<sup>−6</sup> cm<sup>3</sup>/mol | |||
| }} | }} | ||
| | |
|Section3={{Chembox Structure | ||
| | CrystalStruct = triclinic,<ref>{{ cite journal | first1 = J. | last1 = Trotter | first2 = T. | last2 = Zobel | journal = ] | year = 1965 | pages=4466–4471 | title = 826. Stereochemistry of arsenic. Part XVI. Cacodylic acid | doi = 10.1039/JR9650004466 }}</ref> monoclinic<ref name="Betz" /> | |||
| | EUClass = {{Hazchem T+}} | |||
| }} | |||
| ⚫ | | |
||
| |Section7={{Chembox Hazards | |||
| ⚫ | | |
||
| | GHSPictograms = {{GHS06}}{{GHS09}} | |||
| | Autoignition = | |||
| | GHSSignalWord = Danger | |||
| | RPhrases = {{R26/27/28}}, {{R40}} | |||
| | HPhrases = {{H-phrases|301|331|410}} | |||
| | PPhrases = {{P-phrases|261|264|270|271|273|301+310|304+340|311|321|330|391|403+233|405|501}} | |||
| | NFPA-H = 4 | |||
| | NFPA-F = 0 | |||
| | NFPA-R = 0 | |||
| ⚫ | | ExternalSDS = | ||
| ⚫ | | FlashPt = | ||
| | AutoignitionPt = | |||
| | LD50 = 23-100 mg/kg (rat and mouse, oral) | |||
| }} | }} | ||
| }} | }} | ||
| '''Cacodylic acid''' is |
'''Cacodylic acid''' is an ] with the ] (CH<sub>3</sub>)<sub>2</sub>]O<sub>2</sub>H. With the formula R<sub>2</sub>As(O)OH, it is the simplest of the ]. It is a colorless solid that is soluble in water. | ||
| Neutralization of cacodylic acid with base gives '''cacodylate''' salts, e.g. '''sodium cacodylate'''. They are potent ]s. Cacodylic acid/sodium cacodylate is a ] in the preparation and fixation of biological samples for ] and in ]. | |||
| == History == | == History == | ||
| ⚫ | In the 18th century it was found that combining ] ({{chem2|As2O3}}) and four equivalents of ] ({{chem2|CH3CO2K}}) gives a product called "]" which contains ], {{chem2|((CH3)2As)2O}} and ], {{chem2|((CH3)2As)2}}. | ||
| ⚫ | |||
| Early research into "]s" was reported by ] at the ]. Bunsen said of the compounds, | |||
| ⚫ | |||
| ⚫ | <blockquote>"The smell of this body produces instantaneous tingling of the hands and feet, and even giddiness and insensibility... It is remarkable that when one is exposed to the smell of these compounds the tongue becomes covered with a black coating, even when no further evil effects are noticeable".</blockquote> | ||
| == Synthesis and reactions== | |||
| ⚫ | In the 18th century it was |
||
| His work in this field led to an increased understanding of the ]. | |||
| ⚫ | Cacodylic acid can be reduced to dimethylarsine |
||
| :(CH<sub>3</sub>)<sub>2</sub>AsO<sub>2</sub>H + 2 Zn + 4 HCl → (CH<sub>3</sub>)<sub>2</sub>AsH + 2 ZnCl<sub>2</sub> + 2 H<sub>2</sub>O | |||
| :(CH<sub>3</sub>)<sub>2</sub>AsO<sub>2</sub>H + SO<sub>2</sub> + HI → (CH<sub>3</sub>)<sub>2</sub>AsI + SO<sub>3</sub> + H<sub>2</sub>O | |||
| ⚫ | Cacodyl oxide, {{chem2|((CH3)2As)2O}}, is often considered the first ] to be prepared synthetically. | ||
| ⚫ | == Health effects == | ||
| Cacodylic acid and its salts were incorporated into ]s by a large variety of manufacturers under numerous brand names. APC Holdings Corp. sold cacodylic acid and its salts under the ] brand name.<ref name="Greene2005">{{cite book|author=Stanley A. Greene|title=Sittig's Handbook of Pesticides and Agricultural Chemicals|url=https://books.google.com/books?id=hAoKEHpyu6wC&pg=PA132|year=2005|publisher=William Andrew|isbn=978-0-8155-1903-4|page=132}}</ref> The variety Phytar 560G, a mixture of cacodylic acid and sodium cacodylate, was used during the ] as a ] under the name "]".<ref name="HerbicidesMedicine1994">{{cite book |author1=Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides |url=https://books.google.com/books?id=RjCHcoUE3B8C&pg=PA89 |title=Veterans and Agent Orange: Health Effects of Herbicides Used in Vietnam |author2=Institute of Medicine |publisher=National Academies Press |year=1994 |isbn=978-0-309-55619-4 |pages=89–90}}</ref> | |||
| Cacodylic acid is highly toxic by ingestion, inhalation, or skin contact. Once thought to be a byproduct of inorganic arsenic detoxification, it is now believed to have serious health consequences of its own. It has been shown to be ] in rodents, most often causing cleft palate but also fetal fatality at high doses. It has been shown to be genotoxic in human cells, causing ] and also decreased DNA production and shorter DNA strands. While not itself a strong carcinogen, cacodylic acid does promote tumors in the presence of carcinogens in organs such as the kidneys and liver. | |||
| ==Reactions== | |||
| Cacodylic acid is a ] with a pK<sub>a</sub> of around 6.25.<ref>{{cite journal |vauthors=((Shin, T.-W.)), ((Kim, K.)), ((Lee, I.-J.)) |date=April 1997 |title=Spectrophotometric determination of the acid dissociation constants for cacodylic acid and p-Nitrophenol at elevated temperatures |journal=Journal of Solution Chemistry |volume=26 |issue=4 |pages=379–390 |doi=10.1007/BF02767677 |issn=0095-9782}}</ref> | |||
| ⚫ | Cacodylic acid can be reduced to dimethylarsine , which is a versatile intermediate for the synthesis of other organoarsenic compounds:<ref>{{cite journal|author1=Feltham, R. D. |author2=Kasenally, A. |author3=Nyholm, R. S. |title=A New Synthesis of Di- and Tri-Tertiary Arsines|journal=Journal of Organometallic Chemistry|year=1967|volume=7|issue=2|pages=285–288|doi=10.1016/S0022-328X(00)91079-9}}</ref><ref>Burrows, G. J. and Turner, E. E., "A New Type of Compound containing Arsenic", Journal of the Chemical Society Transactions, 1920, 1374-1383</ref> | ||
| :{{chem2|(CH3)2AsO2H + 2 Zn + 4 HCl → (CH3)2AsH + 2 ZnCl2 + 2 H2O}} | |||
| :{{chem2|(CH3)2AsO2H + SO2 + HI → (CH3)2AsI + SO3 + H2O}} | |||
| ⚫ | == Health effects == | ||
| Cacodylic acid is highly toxic by ], ], or skin contact. The U.S. EPA states that all forms of arsenic are a serious risk to human health and the United States ] ranked arsenic as number 1 in its 2001 Priority List of Hazardous Substances at Superfund sites.<ref name="EPA1">{{cite web |url=https://cfpub.epa.gov/ncer_abstracts/index.cfm/fuseaction/display.highlight/abstract/6015 |title=Biogeochemistry of Arsenic in Contaminated Soils of Superfund Sites |last1=Dibyendu |first1=Sarkar |last2=Datta |first2=Rupali |date=2007 |website=EPA |publisher=United States Environmental Protection Agency |access-date=25 February 2018 |archive-url=https://web.archive.org/web/20200317143949/https://cfpub.epa.gov/ncer_abstracts/index.cfm/fuseaction/display.highlight/abstract/6015 |archive-date=17 March 2020 |url-status=dead}}</ref> Arsenic is classified as a Group-A ].<ref name="EPA1"/> | |||
| == See also == | == See also == | ||
| Line 72: | Line 106: | ||
| * ] | * ] | ||
| * ] | * ] | ||
| * ] | |||
| == References == | == References == | ||
| {{reflist}} | |||
| <references/> | |||
| ⚫ | * {{cite journal | |
||
| ==Further reading== | |||
| ⚫ | * {{cite journal |author1=Kenyon, E. M. |author2=Hughes, M. F. | title = A Concise Review of the Toxicity and Carcinogenicity of Dimethylarsenic Acid | journal = ] | volume = 160 | year = 2001 | issue = 1–3 | pages = 227–236 | doi = 10.1016/S0300-483X(00)00458-3|pmid=11246143 |url=https://zenodo.org/record/1259985 }} | ||
| * Elschenbroich, C; Salzer, A. (1992) Organometallics, 2nd Edition | * Elschenbroich, C; Salzer, A. (1992) Organometallics, 2nd Edition | ||
| ⚫ | * | ||
| == External links == | == External links == | ||
| * | * | ||
| ⚫ | * | ||
| {{DEFAULTSORT:Cacodylic Acid}} | {{DEFAULTSORT:Cacodylic Acid}} | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 20:23, 25 November 2024

| |
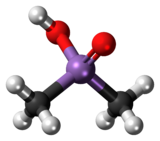 Ball-and-stick model | |
| Names | |
|---|---|
| Preferred IUPAC name Dimethylarsinic acid | |
| Other names Dimethylarsenic acid, Cacodylic acid, Hydroxydimethylarsine oxide, Arsecodile, Ansar, Silvisar, Phytar 560, DMAA, UN 1572 | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Beilstein Reference | 1736965 |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.000.804 |
| EC Number |
|
| Gmelin Reference | 130562 |
| KEGG | |
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 1572 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C2H7AsO2 |
| Molar mass | 137.9977 g/mol |
| Appearance | White crystals or powder |
| Odor | odorless |
| Density | > 1.1 g/cm |
| Melting point | 192 to 198 °C (378 to 388 °F; 465 to 471 K) |
| Boiling point | > 200 °C (392 °F; 473 K) |
| Solubility in water | 66.7 g/100 ml |
| Solubility | soluble in ethanol, acetic acid insoluble in diethyl ether |
| Acidity (pKa) | 6 |
| Magnetic susceptibility (χ) | -79.9·10 cm/mol |
| Structure | |
| Crystal structure | triclinic, monoclinic |
| Hazards | |
| GHS labelling: | |
| Pictograms |  
|
| Signal word | Danger |
| Hazard statements | H301, H331, H410 |
| Precautionary statements | P261, P264, P270, P271, P273, P301+P310, P304+P340, P311, P321, P330, P391, P403+P233, P405, P501 |
| NFPA 704 (fire diamond) |
 |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 23-100 mg/kg (rat and mouse, oral) |
| Safety data sheet (SDS) | External MSDS |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Cacodylic acid is an organoarsenic compound with the formula (CH3)2AsO2H. With the formula R2As(O)OH, it is the simplest of the arsinic acids. It is a colorless solid that is soluble in water.
Neutralization of cacodylic acid with base gives cacodylate salts, e.g. sodium cacodylate. They are potent herbicides. Cacodylic acid/sodium cacodylate is a buffering agent in the preparation and fixation of biological samples for electron microscopy and in protein crystallography.
History
In the 18th century it was found that combining arsenic trioxide (As2O3) and four equivalents of potassium acetate (CH3CO2K) gives a product called "Cadet's fuming liquid" which contains cacodyl oxide, ((CH3)2As)2O and cacodyl, ((CH3)2As)2.
Early research into "cacodyls" was reported by Robert Bunsen at the University of Marburg. Bunsen said of the compounds,
"The smell of this body produces instantaneous tingling of the hands and feet, and even giddiness and insensibility... It is remarkable that when one is exposed to the smell of these compounds the tongue becomes covered with a black coating, even when no further evil effects are noticeable".
His work in this field led to an increased understanding of the methyl group.
Cacodyl oxide, ((CH3)2As)2O, is often considered the first organometallic compound to be prepared synthetically.
Cacodylic acid and its salts were incorporated into herbicides by a large variety of manufacturers under numerous brand names. APC Holdings Corp. sold cacodylic acid and its salts under the Phytar brand name. The variety Phytar 560G, a mixture of cacodylic acid and sodium cacodylate, was used during the Vietnam War as a defoliant under the name "Agent Blue".
Reactions
Cacodylic acid is a weak acid with a pKa of around 6.25.
Cacodylic acid can be reduced to dimethylarsine , which is a versatile intermediate for the synthesis of other organoarsenic compounds:
- (CH3)2AsO2H + 2 Zn + 4 HCl → (CH3)2AsH + 2 ZnCl2 + 2 H2O
- (CH3)2AsO2H + SO2 + HI → (CH3)2AsI + SO3 + H2O
Health effects
Cacodylic acid is highly toxic by ingestion, inhalation, or skin contact. The U.S. EPA states that all forms of arsenic are a serious risk to human health and the United States Agency for Toxic Substances and Disease Registry ranked arsenic as number 1 in its 2001 Priority List of Hazardous Substances at Superfund sites. Arsenic is classified as a Group-A carcinogen.
See also
References
- Betz, R.; McCleland, C.; Marchand, H. (2011). "CSD Entry: CADYLA01 : Dimethylarsinic acid". Cambridge Structural Database: Access Structures. Cambridge Crystallographic Data Centre. doi:10.5517/ccx73mv. Retrieved 2021-12-21.
- ^ Betz, R.; McCleland, C.; Marchand, H. (2011). "The monoclinic polymorph of dimethylarsinic acid". Acta Crystallogr. E. 67 (8): m1013. doi:10.1107/S1600536811025505. PMC 3212109. PMID 22090811.
- Trotter, J.; Zobel, T. (1965). "826. Stereochemistry of arsenic. Part XVI. Cacodylic acid". J. Chem. Soc.: 4466–4471. doi:10.1039/JR9650004466.
- Stanley A. Greene (2005). Sittig's Handbook of Pesticides and Agricultural Chemicals. William Andrew. p. 132. ISBN 978-0-8155-1903-4.
- Committee to Review the Health Effects in Vietnam Veterans of Exposure to Herbicides; Institute of Medicine (1994). Veterans and Agent Orange: Health Effects of Herbicides Used in Vietnam. National Academies Press. pp. 89–90. ISBN 978-0-309-55619-4.
- Shin, T.-W., Kim, K., Lee, I.-J. (April 1997). "Spectrophotometric determination of the acid dissociation constants for cacodylic acid and p-Nitrophenol at elevated temperatures". Journal of Solution Chemistry. 26 (4): 379–390. doi:10.1007/BF02767677. ISSN 0095-9782.
- Feltham, R. D.; Kasenally, A.; Nyholm, R. S. (1967). "A New Synthesis of Di- and Tri-Tertiary Arsines". Journal of Organometallic Chemistry. 7 (2): 285–288. doi:10.1016/S0022-328X(00)91079-9.
- Burrows, G. J. and Turner, E. E., "A New Type of Compound containing Arsenic", Journal of the Chemical Society Transactions, 1920, 1374-1383
- ^ Dibyendu, Sarkar; Datta, Rupali (2007). "Biogeochemistry of Arsenic in Contaminated Soils of Superfund Sites". EPA. United States Environmental Protection Agency. Archived from the original on 17 March 2020. Retrieved 25 February 2018.
Further reading
- Kenyon, E. M.; Hughes, M. F. (2001). "A Concise Review of the Toxicity and Carcinogenicity of Dimethylarsenic Acid". Toxicology. 160 (1–3): 227–236. doi:10.1016/S0300-483X(00)00458-3. PMID 11246143.
- Elschenbroich, C; Salzer, A. (1992) Organometallics, 2nd Edition