| Revision as of 10:13, 11 November 2011 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'ChEMBL', 'CASNo').← Previous edit |
Latest revision as of 14:42, 30 April 2023 edit undoLegionMammal978 (talk | contribs)Extended confirmed users7,894 edits add semisystematic name |
| (31 intermediate revisions by 21 users not shown) |
| Line 1: |
Line 1: |
|
{{chembox |
|
{{chembox |
|
|
| Verifiedfields = changed |
|
| verifiedrevid = 399731015 |
|
| verifiedrevid = 460107369 |
|
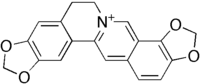
|ImageFile=coptisine.png |
|
| ImageFile=coptisine.png |
|
|ImageSize=200px |
|
| ImageSize=200px |
|
|IUPACName=6,7-Dihydro-bis(1,3)benzodioxolo (5,6-a:4',5'-g)quinolizinium |
|
|
|
| IUPACName=7,8,13,13a-Tetradehydro-2′''H'',2′′''H''-bis(dioxolo)berbin-7-ium |
| ⚫ |
|OtherNames= |
|
|
|
| SystematicName=6,7-Dihydro-2''H'',10''H''-5λ<sup>5</sup>-dioxolodioxoloisoquinolinoisoquinolin-5-ylium |
| ⚫ |
|Section1= {{Chembox Identifiers |
|
|
⚫ |
| OtherNames= |
| ⚫ |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|
|
⚫ |
|Section1={{Chembox Identifiers |
|
⚫ |
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} |
|
| ChemSpiderID = 65268 |
|
| ChemSpiderID = 65268 |
|
| InChI = 1/C19H14NO4/c1-2-16-19(24-10-21-16)14-8-20-4-3-12-6-17-18(23-9-22-17)7-13(12)15(20)5-11(1)14/h1-2,5-8H,3-4,9-10H2/q+1 |
|
| InChI = 1/C19H14NO4/c1-2-16-19(24-10-21-16)14-8-20-4-3-12-6-17-18(23-9-22-17)7-13(12)15(20)5-11(1)14/h1-2,5-8H,3-4,9-10H2/q+1 |
| Line 14: |
Line 16: |
|
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} |
|
| StdInChIKey = XYHOBCMEDLZUMP-UHFFFAOYSA-N |
|
| StdInChIKey = XYHOBCMEDLZUMP-UHFFFAOYSA-N |
|
|
| CASNo_Ref = {{cascite|correct|CAS}} |
|
| CASNo = <!-- blanked - oldvalue: 3486-66-6 --> |
|
|
|
| CASNo=3486-66-6 |
| ⚫ |
| ChEMBL = 362071 |
|
|
|
| UNII_Ref = {{fdacite|correct|FDA}} |
|
|
| UNII = 0GCL71VN14 |
|
|
| ChEMBL_Ref = {{ebicite|correct|EBI}} |
|
⚫ |
| ChEMBL = 362071 |
|
| PubChem=72322 |
|
| PubChem=72322 |
|
| SMILES = O1c3c(OC1)c2c6c(cc2cc3)c5cc4OCOc4cc5CC6 |
|
| SMILES = O1c3c(OC1)c2c6c(cc2cc3)c5cc4OCOc4cc5CC6 |
|
}} |
|
}} |
|
|Section2= {{Chembox Properties |
|
|Section2={{Chembox Properties |
|
| Formula=C<sub>19</sub>H<sub>14</sub>NO<sub>4</sub>+ |
|
| Formula=C<sub>19</sub>H<sub>14</sub>NO<sub>4</sub>+ |
|
| MolarMass=320.319 |
|
| MolarMass=320.319 |
|
| Appearance= |
|
| Appearance= |
|
| Density= |
|
| Density= |
|
| MeltingPt= |
|
| MeltingPt= |
|
| BoilingPt= |
|
| BoilingPt= |
|
| Solubility= |
|
| Solubility= |
|
}} |
|
}} |
|
|Section3= {{Chembox Hazards |
|
|Section3={{Chembox Hazards |
|
| MainHazards= |
|
| MainHazards= |
|
| FlashPt= |
|
| FlashPt= |
|
|
| AutoignitionPt = |
|
| Autoignition= |
|
|
}} |
|
}} |
|
}} |
|
}} |
|
|
|
|
|
|
'''Coptisine''' is an ] found in Chinese goldthread ('']''),<ref name="pmid18395058">{{cite journal | vauthors = Chen J, Wang F, Liu J, Lee FS, Wang X, Yang H | title = Analysis of alkaloids in Coptis chinensis Franch by accelerated solvent extraction combined with ultra performance liquid chromatographic analysis with photodiode array and tandem mass spectrometry detections | journal = Analytica Chimica Acta | volume = 613 | issue = 2 | pages = 184–95 | date = April 2008 | pmid = 18395058 | doi = 10.1016/j.aca.2008.02.060 }}</ref> ], and opium.<ref>{{cite journal |pages=198–201 |doi=10.1038/189198a0 |title=Distribution of Certain Poppy-Fumaria Alkaloids and a Possible Link with the Incidence of Glaucoma |year=1961 |last1=Hakim |first1=Sohrab A. E. |last2=Mijović |first2=Valerie |last3=Walker |first3=James |journal=Nature |volume=189 |issue=4760 |pmid=13710637}}</ref> Famous for the bitter taste that it produces, it is used in ] along with the related compound ] for digestive disorders caused by bacterial infections.<ref name="pmid19686830">{{cite journal | vauthors = Tang J, Feng Y, Tsao S, Wang N, Curtain R, Wang Y | title = Berberine and Coptidis rhizoma as novel antineoplastic agents: a review of traditional use and biomedical investigations | journal = Journal of Ethnopharmacology | volume = 126 | issue = 1 | pages = 5–17 | date = October 2009 | pmid = 19686830 | doi = 10.1016/j.jep.2009.08.009 | hdl = 10722/127599 | hdl-access = free }}</ref> |
|
'''Coptisine''' is an ] found in Chinese goldthread ('']'').<ref></ref> Famous for the bitter taste that it produces, it is used in ] along with the related compound ] for treating digestive disorders caused by bacterial infections. |
|
|
|
|
|
|
|
== References== |
|
Also found in ] and has also been detected in ].<ref>Hakim et al., 1961</ref> {{Fact|date=September 2008}} |
|
|
|
|
|
Coptisine has been found to reversibly inhibit ] in mice, pointing to a potential role as a natural antidepressant<ref></ref>. However, this may also imply a hazard for those taking other medications or with a natural functional disorder in Monoamine oxidase A. |
|
|
|
|
|
Coptisine was found to be toxic to larval ] and a variety of human cell lines, potentially implying a therapeutic effect on cancer or alternatively a generally toxic character. The same authors illustrate a four-step process to produce Coptisine from ]<ref></ref>. |
|
|
|
|
|
==Footnotes== |
|
|
{{reflist}} |
|
{{reflist}} |
|
|
|
|
|
] |
|
] |
|
|
] |
|
|
|
|
|
] |
|
|
|
|
|
{{organic-compound-stub}} |
|
{{alkaloid-stub}} |