| Revision as of 09:06, 24 November 2011 editLamro (talk | contribs)Autopatrolled, Extended confirmed users84,272 edits link← Previous edit | Latest revision as of 17:52, 25 November 2024 edit undo5.178.188.143 (talk)No edit summaryTag: Visual edit | ||
| (64 intermediate revisions by 39 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Chembox | {{Chembox | ||
| |Watchedfields = changed | |||
| | ImageFile = Triphenylmethylradical.png | |||
| |verifiedrevid = 462238281 | |||
| ⚫ | | |
||
| |ImageFile = Triphenylmethyl radical.svg | |||
| ⚫ | | |
||
| |ImageSize = 175 | |||
| ⚫ | | |
||
| ⚫ | |ImageFile_Ref = {{chemboximage|correct|??}} | ||
| ⚫ | | |
||
| ⚫ | |ImageName = Kekulé, skeletal formula of the triphenylmethyl radical | ||
| ⚫ | | |
||
| |ImageFile1 = Triphenylmethyl radical ball.png | |||
| ⚫ | | |
||
| |ImageAlt1 = Ball-and-stick model of the triphenylmethyl radical | |||
| ⚫ | | |
||
| |PIN = Triphenylmethyl | |||
| ⚫ | | |
||
| ⚫ | |Section1={{Chembox Identifiers | ||
| ⚫ | | |
||
| |CASNo = 2216-49-1 | |||
| ⚫ | | |
||
| |CASNo_Ref = {{Cascite|changed|CAS}} | |||
| ⚫ | | |
||
| ⚫ | |ChemSpiderID = 10627185 | ||
| ⚫ | |ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| |PubChem = 5374035 | |||
| ⚫ | |SMILES = c1ccc(cc1)(c1ccccc1)c1ccccc1 | ||
| ⚫ | |SMILES1 = C1=CC=C(C=C1)(C1=CC=CC=C1)C1=CC=CC=C1 | ||
| ⚫ | |StdInChI = 1S/C19H15/c1-4-10-16(11-5-1)19(17-12-6-2-7-13-17)18-14-8-3-9-15-18/h1-15H | ||
| ⚫ | |StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| ⚫ | |StdInChIKey = OHSJPLSEQNCRLW-UHFFFAOYSA-N | ||
| ⚫ | |StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| | |
|C=19 | H=15 | ||
| | H = 15 | |||
| | ExactMass = 243.117375480 g mol<sup>-1</sup> | |||
| }} | }} | ||
| }} | }} | ||
| The '''triphenylmethyl radical''' (often shortened to '''trityl radical''' after 1927 suggestion by ] et al.<ref>{{Cite journal |last=Helferich |first=B. |last2=Bredereck |first2=H. |last3=Schneidmüller |first3=A. |date=1927 |title=Acylwanderung an partiell acylierten Methyl‐glucosiden |url=https://chemistry-europe.onlinelibrary.wiley.com/doi/10.1002/jlac.19274580108 |journal=Justus Liebigs Annalen der Chemie |language=de |volume=458 |issue=1 |pages=111–116 |doi=10.1002/jlac.19274580108 |issn=0075-4617}}</ref>) is an ] with the formula (C<sub>6</sub>H<sub>5</sub>)<sub>3</sub>C. It is a ]. It was the first ] ever to be described in ]. Because of its accessibility, the trityl radical has been heavily exploited.<ref>{{cite book |doi=10.1002/9780470666975.ch1|chapter=Triarylmethyl and Related Radicals|title=Stable Radicals|year=2010|last1=Tidwell|first1=Thomas T.|pages=1–31|isbn=9780470666975}}</ref> | |||
| ⚫ | The |
||
| == Preparation and properties == | |||
| ⚫ | ] | ||
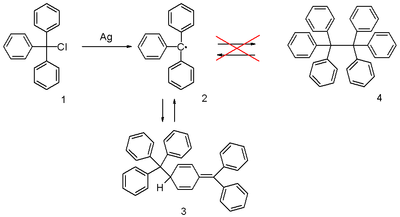
| ⚫ | The triphenylmethyl radical can be prepared by ] of ] '''1''' by a metal like ] or ] in ] or ]. The radical '''2''' forms a ] with the ]-type ] '''3''' (]). In benzene the concentration of the radical is 2%.<ref>{{March6th}} </ref> | ||
| ⚫ | ] | ||
| Solutions containing the radical are ] and when the temperature of the solution is increased the yellow color becomes more intense as the equilibrium is shifted in favor of the radical following ]. Conversely when the solution is cooled it becomes less yellow. | |||
| Solutions containing the radical are ]; when the temperature of the solution is raised, the yellow color becomes more intense as the equilibrium is shifted in favor of the radical rather than the colorless dimer, in accordance with ]. | |||
| The triphenylmethyl radical exhibits green ]. Further reaction of the quinoid dimer with another triphenylmethyl radical produces a quinoid radical that exhibits red photoluminescence.<ref name="Red photoluminescence">{{cite journal |doi=10.1070/MC2000v010n01ABEH001115 |title=Red photoluminescence in the synthesis of triphenylmethyl radicals by the Gomberg method |date=2000 |last1=Bulgakov |first1=Ramil G. |last2=Kuleshov |first2=Sergei P. |last3=Valiullina |first3=Zemfira S. |journal=Mendeleev Communications |volume=10 |pages=22–23 }}</ref> | |||
| ⚫ | ] | ||
| When exposed to air, the radical rapidly oxidizes to the ], and the color of the solution changes from yellow to colorless. Likewise, the radical reacts with ] to triphenylmethyl iodide. | |||
| The radical was discovered by ] in 1900.<ref>{{cite journal | title = An instance of trivalent carbon: triphenylmethyl | author = ] | journal = ] | year = 1900 | volume = 22 | issue = 11 | pages = 757–771 | doi = 10.1021/ja02049a006}}</ref><ref>{{cite journal | title = On trivalent carbon | author = ] | journal = ] | year = 1901 | volume = 23 | issue = 7 | pages = 496–502 | doi = 10.1021/ja02033a015}} (Note: radical is also called a ''cadicle'')</ref><ref>{{cite journal | title = On trivalent carbon | author = ] | journal = ] | year = 1902 | volume = 24 | issue = 7 | pages = 597–628 | doi = 10.1021/ja02021a001}}</ref> He tried to prepare hexaphenylethane from ] and ] in ] in a ] and found that the product, based on its behaviour towards iodine and oxygen, was far more reactive than anticipated. | |||
| ⚫ | ] | ||
| ⚫ | The correct quinoid structure for the dimer was suggested as early as 1904 but this structure was soon after abandoned by the scientific community in favor of |
||
| While the triphenyl radical itself forms a quinoid dimer, derivatives of the triphenyl radical with certain ] phenyl groups do form dimers with a ]-like structure. For example, the tris(3,5-di-''tert''-butylphenyl) radical dimerizes to give hexakis(3,5-di-''t''-butylphenyl)ethane, with a ] of 1.67 Å for the central carbon–carbon bond. Theoretical calculations on a very high level of theory indicate that ] between the ] groups create a potential minimum that is absent in the unsubstituted molecule.<ref>{{Citation|last=Lewars|first=Errol|title=Modeling Marvels|year=2008|chapter=8. Hexaphenylethane|publisher=Springer|bibcode=2008moma.book.....L}}</ref><ref>{{cite journal|last1=Grimme|first1=Stefan|last2=Schreiner|first2=Peter R.|year=2011|title=Steric crowding can stabilize a labile molecule: Solving the hexaphenylethane riddle|journal=Angewandte Chemie International Edition|volume=50|issue=52|pages=12639–12642|doi=10.1002/anie.201103615|pmid=22025456}}</ref> Other derivatives have been reported as the quinoid dimer <ref>{{cite journal|last1=Uchimura|first1=Y.|last2=Takeda|first2=T.|last3=Katoono|first3=R.|last4=Fujiwara|first4=K.|last5=Suzuki|first5=T.|date=2015|title=New Insights into the Hexaphenylethane Riddle: Formation of an α,''o''-Dimer.|journal=Angewandte Chemie International Edition|volume=54|issue=13|pages=4010–4013|doi=10.1002/anie.201500122|pmid=25704856}}</ref> | |||
| ==Miscellany== | |||
| Gomberg concluded his 1900 article with the sentence "This work will be continued and I wish to reserve the field for myself." He ended his 1901 article by writing, "It is my intention to extend this study to other oxygen compounds, as well as to nitrogen derivatives, and I beg to reserve this field for further work." It is true that nineteenth-century chemists did not intrude on each other's research; to his dismay, Gomberg found out that this was not the case in the twentieth century. | |||
| The class of tri]-methyl radicals have applications in the synthesis of organic ]s.<ref name="Organic magnet">{{cite journal |doi=10.1070/RC2006v075n10ABEH003621 |title=From the Gomberg radical to organic magnets |date=2006 |last1=Shishlov |first1=Nikolay M. |journal=Russian Chemical Reviews |volume=75 |issue=10 |pages=863–884 }}</ref> | |||
| == History == | |||
| The radical was discovered by ] in 1900 at the ].<ref>{{cite journal | title = An instance of trivalent carbon: triphenylmethyl | authorlink = Moses Gomberg|first=M. |last=Gomberg | journal = ] | year = 1900 | volume = 22 | issue = 11 | pages = 757–771 | doi = 10.1021/ja02049a006| url = https://zenodo.org/record/1428920}}</ref><ref>{{cite journal | title = On trivalent carbon|first=M. |last=Gomberg | journal = Journal of the American Chemical Society | year = 1901 | volume = 23 | issue = 7 | pages = 496–502 | doi = 10.1021/ja02033a015}} (Note: radical is also called a ''cadicle''.)</ref><ref>{{cite journal | title = On trivalent carbon |first=M. |last=Gomberg | journal = Journal of the American Chemical Society | year = 1902 | volume = 24 | issue = 7 | pages = 597–628 | doi = 10.1021/ja02021a001| url = https://zenodo.org/record/1428904}}</ref> He tried to prepare ] from triphenylmethyl chloride and zinc in benzene in a ] and found that the product, based on its behaviour towards iodine and oxygen, was far more reactive than anticipated. The discovered structure was used in the development of ] spectroscopy and confirmed by it.<ref>{{cite journal | title = Electron distribution in triphenylmethyl: Hyperfine structure of the paramagnetic resonance absorption of (C<sub>6</sub>H<sub>5</sub>)<sub>3</sub>C<sup>13*</sup>| first1= S. I. |last1=Weissman |first2=John C. |last2=Sowden | journal = ]| volume = 75 | issue = 2|year = 1953|pages = 503| doi = 10.1021/ja01098a522}}</ref><ref>{{cite journal | title = Electron spin resonance studies of substituted triphenylmethyl radicals | first1= J. |last1= Sinclair |first2= D.|last2= Kivelson | journal = Journal of the American Chemical Society| volume = 90 | issue = 19 |year = 1968|pages = 5074–5080 | doi = 10.1021/ja01021a004}}</ref><ref>{{cite web |url= http://www.chm.bris.ac.uk/motm/triphenylmethyl/tripesr1.html|title= ESR spectrum of the triphenylmethyl radical|last= |first= |date= |website= |publisher= School of Chemistry, University of Bristol|access-date= August 5, 2018}}</ref> The triphenylmethyl radical, and the larger class of triarylmethyl radicals, are called ''Gomberg radicals''.<ref name="Red photoluminescence"/><ref name="Organic magnet"/> | |||
| ⚫ | The correct ] structure for the dimer was suggested as early as 1904 but this structure was soon after abandoned by the scientific community in favor of hexaphenylethane ('''4''').<ref>{{cite journal | title = The hexaphenylethane riddle | first= J. M. |last=McBride | journal = ] | volume = 30 | issue = 14 | year = 1974 | pages = 2009–2022 | doi = 10.1016/S0040-4020(01)97332-6}}</ref> It subsequently took until 1968 for its rediscovery when researchers at the ] published ] data.<ref>{{cite journal | title = A new interpretation of the monomer–dimer equilibrium of triphenylmethyl- and alkyl-substituted-diphenyl methyl-radicals in solution | first1= H. |last1=Lankamp |first2=W. Th. |last2=Nauta |first3= C.|last3= MacLean | journal = ] | volume = 9 | issue = 2 |year = 1968 | pages = 249–254 | doi = 10.1016/S0040-4039(00)75598-5}}</ref> | ||
| ==See also== | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| ==References== | ==References== | ||
| {{reflist}} | {{reflist|2}} | ||
| ==External links== | ==External links== | ||
| * |
* , June 1997 | ||
| * |
* | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 17:52, 25 November 2024

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name Triphenylmethyl | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C19H15 |
| Molar mass | 243.329 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
The triphenylmethyl radical (often shortened to trityl radical after 1927 suggestion by Helferich et al.) is an organic compound with the formula (C6H5)3C. It is a persistent radical. It was the first radical ever to be described in organic chemistry. Because of its accessibility, the trityl radical has been heavily exploited.
Preparation and properties
The triphenylmethyl radical can be prepared by homolysis of triphenylmethyl chloride 1 by a metal like silver or zinc in benzene or diethyl ether. The radical 2 forms a chemical equilibrium with the quinoid-type dimer 3 (Gomberg's dimer). In benzene the concentration of the radical is 2%.
Solutions containing the radical are yellow; when the temperature of the solution is raised, the yellow color becomes more intense as the equilibrium is shifted in favor of the radical rather than the colorless dimer, in accordance with Le Chatelier's principle.
The triphenylmethyl radical exhibits green photoluminescence. Further reaction of the quinoid dimer with another triphenylmethyl radical produces a quinoid radical that exhibits red photoluminescence.
When exposed to air, the radical rapidly oxidizes to the peroxide, and the color of the solution changes from yellow to colorless. Likewise, the radical reacts with iodine to triphenylmethyl iodide.
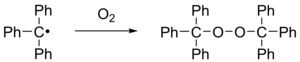
While the triphenyl radical itself forms a quinoid dimer, derivatives of the triphenyl radical with certain substituted phenyl groups do form dimers with a hexaphenylethane-like structure. For example, the tris(3,5-di-tert-butylphenyl) radical dimerizes to give hexakis(3,5-di-t-butylphenyl)ethane, with a bond length of 1.67 Å for the central carbon–carbon bond. Theoretical calculations on a very high level of theory indicate that van der Waals attraction between the tert-butyl groups create a potential minimum that is absent in the unsubstituted molecule. Other derivatives have been reported as the quinoid dimer
The class of triaryl-methyl radicals have applications in the synthesis of organic magnets.
History
The radical was discovered by Moses Gomberg in 1900 at the University of Michigan. He tried to prepare hexaphenylethane from triphenylmethyl chloride and zinc in benzene in a Wurtz reaction and found that the product, based on its behaviour towards iodine and oxygen, was far more reactive than anticipated. The discovered structure was used in the development of ESR spectroscopy and confirmed by it. The triphenylmethyl radical, and the larger class of triarylmethyl radicals, are called Gomberg radicals.
The correct quinoid structure for the dimer was suggested as early as 1904 but this structure was soon after abandoned by the scientific community in favor of hexaphenylethane (4). It subsequently took until 1968 for its rediscovery when researchers at the Vrije Universiteit Amsterdam published proton NMR data.
See also
References
- Helferich, B.; Bredereck, H.; Schneidmüller, A. (1927). "Acylwanderung an partiell acylierten Methyl‐glucosiden". Justus Liebigs Annalen der Chemie (in German). 458 (1): 111–116. doi:10.1002/jlac.19274580108. ISSN 0075-4617.
- Tidwell, Thomas T. (2010). "Triarylmethyl and Related Radicals". Stable Radicals. pp. 1–31. doi:10.1002/9780470666975.ch1. ISBN 9780470666975.
- Smith, Michael B.; March, Jerry (2007), Advanced Organic Chemistry: Reactions, Mechanisms, and Structure (6th ed.), New York: Wiley-Interscience, ISBN 978-0-471-72091-1
- ^ Bulgakov, Ramil G.; Kuleshov, Sergei P.; Valiullina, Zemfira S. (2000). "Red photoluminescence in the synthesis of triphenylmethyl radicals by the Gomberg method". Mendeleev Communications. 10: 22–23. doi:10.1070/MC2000v010n01ABEH001115.
- Lewars, Errol (2008), "8. Hexaphenylethane", Modeling Marvels, Springer, Bibcode:2008moma.book.....L
- Grimme, Stefan; Schreiner, Peter R. (2011). "Steric crowding can stabilize a labile molecule: Solving the hexaphenylethane riddle". Angewandte Chemie International Edition. 50 (52): 12639–12642. doi:10.1002/anie.201103615. PMID 22025456.
- Uchimura, Y.; Takeda, T.; Katoono, R.; Fujiwara, K.; Suzuki, T. (2015). "New Insights into the Hexaphenylethane Riddle: Formation of an α,o-Dimer". Angewandte Chemie International Edition. 54 (13): 4010–4013. doi:10.1002/anie.201500122. PMID 25704856.
- ^ Shishlov, Nikolay M. (2006). "From the Gomberg radical to organic magnets". Russian Chemical Reviews. 75 (10): 863–884. doi:10.1070/RC2006v075n10ABEH003621.
- Gomberg, M. (1900). "An instance of trivalent carbon: triphenylmethyl". Journal of the American Chemical Society. 22 (11): 757–771. doi:10.1021/ja02049a006.
- Gomberg, M. (1901). "On trivalent carbon". Journal of the American Chemical Society. 23 (7): 496–502. doi:10.1021/ja02033a015. (Note: radical is also called a cadicle.)
- Gomberg, M. (1902). "On trivalent carbon". Journal of the American Chemical Society. 24 (7): 597–628. doi:10.1021/ja02021a001.
- Weissman, S. I.; Sowden, John C. (1953). "Electron distribution in triphenylmethyl: Hyperfine structure of the paramagnetic resonance absorption of (C6H5)3C". Journal of the American Chemical Society. 75 (2): 503. doi:10.1021/ja01098a522.
- Sinclair, J.; Kivelson, D. (1968). "Electron spin resonance studies of substituted triphenylmethyl radicals". Journal of the American Chemical Society. 90 (19): 5074–5080. doi:10.1021/ja01021a004.
- "ESR spectrum of the triphenylmethyl radical". School of Chemistry, University of Bristol. Retrieved August 5, 2018.
- McBride, J. M. (1974). "The hexaphenylethane riddle". Tetrahedron. 30 (14): 2009–2022. doi:10.1016/S0040-4020(01)97332-6.
- Lankamp, H.; Nauta, W. Th.; MacLean, C. (1968). "A new interpretation of the monomer–dimer equilibrium of triphenylmethyl- and alkyl-substituted-diphenyl methyl-radicals in solution". Tetrahedron Letters. 9 (2): 249–254. doi:10.1016/S0040-4039(00)75598-5.