| Revision as of 16:58, 27 November 2011 editThe chemistds (talk | contribs)Extended confirmed users5,761 edits added CSID, (Std)InChI & (Std)InChIKey← Previous edit | Latest revision as of 00:07, 30 June 2024 edit undoCitation bot (talk | contribs)Bots5,419,186 edits Altered title. Add: chapter, chapter-url, authors 1-1. Removed or converted URL. Removed parameters. Some additions/deletions were parameter name changes. | Use this bot. Report bugs. | Suggested by Abductive | Category:Foul-smelling chemicals | #UCB_Category 6/127 | ||
| (28 intermediate revisions by 20 users not shown) | |||
| Line 1: | Line 1: | ||
| {{DISPLAYTITLE:''tert''-Butyl isocyanide}} | {{DISPLAYTITLE:''tert''-Butyl isocyanide}} | ||
| {{chembox | {{chembox | ||
| | Watchedfields = changed | |||
| | verifiedrevid = |
| verifiedrevid = 462756293 | ||
| | ImageFileL1 = tert-butylisocyanide.png | | ImageFileL1 = tert-butylisocyanide.png | ||
| ⚫ | | ImageFileR1 = Tert-Butyl-isocyanide_3d_structure.png | ||
| | ImageSizeL1 = 120px | |||
| ⚫ | | Name = ''tert''-Butyl isocyanide | ||
| ⚫ | | ImageFileR1= Tert-Butyl-isocyanide_3d_structure.png | ||
| | PIN = 2-Isocyano-2-methylpropane | |||
| | ImageSizeR2 = 120px | |||
| | OtherNames = t-BuNC | |||
| ⚫ | | |
||
| ⚫ | |Section1={{Chembox Identifiers | ||
| | OtherNames = t-BuNC, propane, 2-isocyano-2-methyl | |||
| ⚫ | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| ⚫ | | |
||
| ⚫ | | |
||
| | CASNo = 7188-38-7 | | CASNo = 7188-38-7 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| ⚫ | | |
||
| | UNII = 22S4Z857K3 | |||
| ⚫ | | |
||
| ⚫ | | PubChem = 23577 | ||
| ⚫ | | |
||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ⚫ | | |
||
| ⚫ | | ChemSpiderID = 22045 | ||
| ⚫ | | |
||
| ⚫ | | SMILES = CC(C)(C)# | ||
| ⚫ | | |
||
| ⚫ | | InChI = 1/C5H9N/c1-5(2,3)6-4/h1-3H3 | ||
| ⚫ | | |
||
| ⚫ | | InChIKey = FAGLEPBREOXSAC-UHFFFAOYAL | ||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| ⚫ | | StdInChI = 1S/C5H9N/c1-5(2,3)6-4/h1-3H3 | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| ⚫ | | StdInChIKey = FAGLEPBREOXSAC-UHFFFAOYSA-N | ||
| }} | }} | ||
| | |
|Section2={{Chembox Properties | ||
| | |
| Formula = C<sub>5</sub>H<sub>9</sub>N | ||
| | |
| MolarMass = 83.13 g/mol | ||
| | |
| Appearance = Colorless liquid | ||
| | |
| Density = 0.735 g/cm<sup>3</sup>, liquid | ||
| | |
| Solubility = N/A | ||
| | |
| MeltingPt = | ||
| | |
| BoilingPtC = 91 | ||
| }} | }} | ||
| }} | }} | ||
| '''''tert''-Butyl isocyanide''' is an ] with the ] Me<sub>3</sub>CNC (Me = methyl, CH<sub>3</sub>). It is an ], commonly called isonitrile or carbylamine, as defined by the ] C≡N-R. ''tert''-Butyl isocyanide, like most alkyl isocyanides, is a reactive colorless liquid with an extremely unpleasant odor. It forms stable ] with ]s and can insert into metal-carbon ].<ref>Malatesta |
'''''tert''-Butyl isocyanide''' is an ] with the ] Me<sub>3</sub>CNC (Me = methyl, CH<sub>3</sub>). It is an ], commonly called isonitrile or carbylamine, as defined by the ] C≡N-R. ''tert''-Butyl isocyanide, like most alkyl isocyanides, is a reactive colorless liquid with an extremely unpleasant odor. It forms stable ] with ]s and can insert into metal-carbon ].<ref>{{Cite book |last=Malatesta |first=L. |title=Progress in Inorganic Chemistry |chapter-url=https://onlinelibrary.wiley.com/doi/10.1002/9780470166024.ch5 |chapter=Isocyanide Complexes of Metals |date=January 1959 |publisher=Wiley |isbn=978-0-470-17589-7 |editor-last=Cotton |editor-first=F. Albert |edition=1 |volume=1 |pages=283–379 |language=en |doi=10.1002/9780470166024.ch5}}</ref> | ||
| ''tert''-Butyl isocyanide is prepared by a Hofmann carbylamine ]. In this conversion, a dichloromethane solution of ] is treated with ] and aqueous ] in the presence of ] amount of the ] benzyltriethylammonium ].<ref>{{ |
''tert''-Butyl isocyanide is prepared by a Hofmann carbylamine ]. In this conversion, a dichloromethane solution of ] is treated with ] and aqueous ] in the presence of ] amount of the ] benzyltriethylammonium ].<ref>{{cite journal| author = Gokel, G.W.; Widera, R.P.; Weber, W.P. | title = Phase-transfer Hofmann carbylamine reaction: tert-butyl isocyanide| journal = Organic Syntheses| volume=55|doi=10.15227/orgsyn.055.0096| pages = 232| year = 1988 }}</ref> | ||
| :Me<sub>3</sub>CNH<sub>2</sub> + CHCl<sub>3</sub> + 3 NaOH → Me<sub>3</sub>CNC + 3 NaCl + 3 H<sub>2</sub>O | :Me<sub>3</sub>CNH<sub>2</sub> + CHCl<sub>3</sub> + 3 NaOH → Me<sub>3</sub>CNC + 3 NaCl + 3 H<sub>2</sub>O | ||
| ''tert''-Butyl isocyanide is isomeric with ], also known as ''tert''-butyl cyanide. | ''tert''-Butyl isocyanide is isomeric with ], also known as ''tert''-butyl cyanide. The difference, as with all carbylamine analogs of nitriles, is that the bond joining the CN functional group to the parent molecule is made on the nitrogen, not the carbon. | ||
| ==Coordination chemistry== | ==Coordination chemistry== | ||
| {{main|Transition metal isocyanide complexes}} | |||
| By virtue of the lone electron pair on ], isocyanides serves as ] in ], especially with metals in the 0, +1, and +2 ] states. ''tert''-Butyl isocyanide has been shown to stabilize metals in unusual oxidation states, such as Pd(I).<ref> |
By virtue of the lone electron pair on ], isocyanides serves as ] in ], especially with metals in the 0, +1, and +2 ] states. ''tert''-Butyl isocyanide has been shown to stabilize metals in unusual oxidation states, such as Pd(I).<ref>{{Cite book |last1=Rettig |first1=M. F. |url=https://onlinelibrary.wiley.com/doi/10.1002/9780470132593.ch29 |title=Tetrakis( tert -Butyl Isocyanide)Di-μ-Chlorodipalladium(I) |last2=Maitlis |first2=P. M. |last3=Cotton |first3=F. A. |last4=Webb |first4=T. R. |date=January 1990 |publisher=Wiley |isbn=978-0-471-52619-3|editor1-link=Robert Angelici|editor-last=Angelici |editor-first=Robert J. |edition=1 |volume=28 |pages=110–113 |language=en |doi=10.1002/9780470132593.ch29}}</ref> | ||
| : Pd(dba)<sub>2</sub> + PdCl<sub>2</sub>(])<sub>2</sub> + 4 ''t''-BuNC → <sub>2</sub> + 2 dba + 2 C<sub>6</sub>H<sub>5</sub>CN | : Pd(dba)<sub>2</sub> + PdCl<sub>2</sub>(])<sub>2</sub> + 4 ''t''-BuNC → <sub>2</sub> + 2 dba + 2 C<sub>6</sub>H<sub>5</sub>CN | ||
| ''tert''-Butyl isocyanide can form hepta-coordinate ] complexes, despite having a large t-Bu group, which is held far away from the ] center because of the linearity of the M-C≡N-C linkages.<ref>Carnahan |
''tert''-Butyl isocyanide can form hepta-coordinate ] complexes, despite having a large t-Bu group, which is held far away from the ] center because of the linearity of the M-C≡N-C linkages.<ref>{{Cite journal |last1=Carnahan |first1=Edmund M. |last2=Protasiewicz |first2=John D. |last3=Lippard |first3=Stephen J. |date=1993-03-01 |title=The 15 years of reductive coupling: what have we learned? |url=https://pubs.acs.org/doi/abs/10.1021/ar00027a003 |journal=Accounts of Chemical Research |language=en |volume=26 |issue=3 |pages=90–97 |doi=10.1021/ar00027a003 |issn=0001-4842}}</ref> | ||
| ''tert''-Butyl isocycanide forms complexes that are stoichiometrically analogous to certain binary metal ] complexes, such as Fe<sub>2</sub>(CO)<sub>9</sub> and Fe<sub>2</sub>(tBuNC)<sub>9</sub>.<ref>{{Cite journal |last1=Bassett |first1=Jean-Marie |last2=Barker |first2=Geoffrey K. |last3=Green |first3=Michael |last4=Howard |first4=Judith A. K. |last5=Stone |first5=F. Gordon A. |last6=Wolsey |first6=Wayne C. |date=1981 |title=Chemistry of low-valent metal isocyanide complexes. Part 3. The synthesis, structure, and dynamic behaviour of nonakis(ethyl and isopropyl isocyanide)di-iron and -ruthenium complexes. Crystal structure of |url=http://xlink.rsc.org/?DOI=DT9810000219 |journal=J. Chem. Soc., Dalton Trans. |language=en |issue=1 |pages=219–227 |doi=10.1039/DT9810000219 |issn=0300-9246}}</ref> | |||
| ===Similarity to metal carbonyls=== | |||
| ''tert''-Butyl isocycanide forms complexes that are stoichiometrically analogous to certain binary metal ] complexes, such as Fe<sub>2</sub>(CO)<sub>9</sub> and Fe<sub>2</sub>(tBuNC)<sub>9</sub>.<ref>Bassett, J.M.; Barker, G.K.; Green, M.; Howard, J.A.; Stone, G.A.; Wolsey, W.C. "Chemistry of low-valent metal isocyanide complexes" ''J.C.S. Dalton'', 1981, 219-227.</ref> Although structurally similar, the analogous carbonyls differ in several ways, mainly because t-BuNC is a better donor ligand than CO. Thus, Fe(tBuNC)<sub>5</sub> is easily protonated, whereas its counterpart Fe(CO)<sub>5</sub> is not.<ref>Bassett, J.M.; Farrugia, L.J.; Stone, G.A. "Protonation of pentakis(t-butyl isocyanide)iron" ''J.C.S. Dalton'', 1980, 1789-1790.</ref> | |||
| ===Insertion into metal-carbon bonds=== | |||
| Under certain circumstances, ''tert''-butyl isocyanide has been shown to insert into metal-carbon bonds to form iminoacyls. The insertion of isocyanides into metal-carbon bonds is of potential relevance in ].<ref>Vicente, J; Abad, J.A.; Fortsch, W.; Lopez-Saez, M.J. Reactivity of ortho-palladated phenol derivatives with unsaturated molecules. ''Organometallics'', 2004, 23, 4414-4429. DOI 10.1021/om0496131.</ref> | |||
| ==Safety== | ==Safety== | ||
| ''tert''-Butyl isocyanide is toxic. Its behavior is similar to that of its close electronic relative ]. | |||
| In keeping with its similarity to carbon monoxide, ''tert''-butyl isocyanide is toxic. | |||
| ==References== | ==References== | ||
| Line 56: | Line 58: | ||
| {{DEFAULTSORT:Butyl isocyanide, tert-}} | {{DEFAULTSORT:Butyl isocyanide, tert-}} | ||
| ] | ] | ||
| ] | |||
| ] | ] | ||
| ] | |||
Latest revision as of 00:07, 30 June 2024
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name 2-Isocyano-2-methylpropane | |||
| Other names t-BuNC | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.027.776 | ||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C5H9N | ||
| Molar mass | 83.13 g/mol | ||
| Appearance | Colorless liquid | ||
| Density | 0.735 g/cm, liquid | ||
| Boiling point | 91 °C (196 °F; 364 K) | ||
| Solubility in water | N/A | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
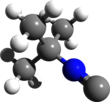
tert-Butyl isocyanide is an organic compound with the formula Me3CNC (Me = methyl, CH3). It is an isocyanide, commonly called isonitrile or carbylamine, as defined by the functional group C≡N-R. tert-Butyl isocyanide, like most alkyl isocyanides, is a reactive colorless liquid with an extremely unpleasant odor. It forms stable complexes with transition metals and can insert into metal-carbon bonds.
tert-Butyl isocyanide is prepared by a Hofmann carbylamine reaction. In this conversion, a dichloromethane solution of tert-butylamine is treated with chloroform and aqueous sodium hydroxide in the presence of catalytic amount of the phase transfer catalyst benzyltriethylammonium chloride.
- Me3CNH2 + CHCl3 + 3 NaOH → Me3CNC + 3 NaCl + 3 H2O
tert-Butyl isocyanide is isomeric with pivalonitrile, also known as tert-butyl cyanide. The difference, as with all carbylamine analogs of nitriles, is that the bond joining the CN functional group to the parent molecule is made on the nitrogen, not the carbon.
Coordination chemistry
Main article: Transition metal isocyanide complexesBy virtue of the lone electron pair on carbon, isocyanides serves as ligands in coordination chemistry, especially with metals in the 0, +1, and +2 oxidation states. tert-Butyl isocyanide has been shown to stabilize metals in unusual oxidation states, such as Pd(I).
- Pd(dba)2 + PdCl2(C6H5CN)2 + 4 t-BuNC → 2 + 2 dba + 2 C6H5CN
tert-Butyl isocyanide can form hepta-coordinate homoleptic complexes, despite having a large t-Bu group, which is held far away from the metal center because of the linearity of the M-C≡N-C linkages.
tert-Butyl isocycanide forms complexes that are stoichiometrically analogous to certain binary metal carbonyl complexes, such as Fe2(CO)9 and Fe2(tBuNC)9.
Safety
tert-Butyl isocyanide is toxic. Its behavior is similar to that of its close electronic relative carbon monoxide.
References
- Malatesta, L. (January 1959). "Isocyanide Complexes of Metals". In Cotton, F. Albert (ed.). Progress in Inorganic Chemistry. Vol. 1 (1 ed.). Wiley. pp. 283–379. doi:10.1002/9780470166024.ch5. ISBN 978-0-470-17589-7.
- Gokel, G.W.; Widera, R.P.; Weber, W.P. (1988). "Phase-transfer Hofmann carbylamine reaction: tert-butyl isocyanide". Organic Syntheses. 55: 232. doi:10.15227/orgsyn.055.0096.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Rettig, M. F.; Maitlis, P. M.; Cotton, F. A.; Webb, T. R. (January 1990). Angelici, Robert J. (ed.). Tetrakis( tert -Butyl Isocyanide)Di-μ-Chlorodipalladium(I). Vol. 28 (1 ed.). Wiley. pp. 110–113. doi:10.1002/9780470132593.ch29. ISBN 978-0-471-52619-3.
- Carnahan, Edmund M.; Protasiewicz, John D.; Lippard, Stephen J. (1993-03-01). "The 15 years of reductive coupling: what have we learned?". Accounts of Chemical Research. 26 (3): 90–97. doi:10.1021/ar00027a003. ISSN 0001-4842.
- Bassett, Jean-Marie; Barker, Geoffrey K.; Green, Michael; Howard, Judith A. K.; Stone, F. Gordon A.; Wolsey, Wayne C. (1981). "Chemistry of low-valent metal isocyanide complexes. Part 3. The synthesis, structure, and dynamic behaviour of nonakis(ethyl and isopropyl isocyanide)di-iron and -ruthenium complexes. Crystal structure of [Fe 2 (µ-CNEt) 3 (CNEt) 6 ]". J. Chem. Soc., Dalton Trans. (1): 219–227. doi:10.1039/DT9810000219. ISSN 0300-9246.