| Revision as of 12:46, 22 June 2012 editPhiJ (talk | contribs)Extended confirmed users578 edits The chembox was about the conjugate acid so I've completely rewritten it.← Previous edit | Latest revision as of 22:18, 18 August 2023 edit undoOAbot (talk | contribs)Bots440,440 editsm Open access bot: doi updated in citation with #oabot. | ||
| (23 intermediate revisions by 18 users not shown) | |||
| Line 1: | Line 1: | ||
| {{orphan|date=December 2008}} | |||
| {{chembox | {{chembox | ||
| | verifiedrevid = 443656014 | | verifiedrevid = 443656014 | ||
| | ImageFile=Phenylperi acid.png | |||
| |ImageSize=200px | | ImageSize=200px | ||
| |IUPACName=8-(phenylamino)-1-naphthalenesulfonate | |||
| | PIN = 8-Anilinonaphthalene-1-sulfonic acid | |||
| | OtherNames = 8-(Phenylamino)naphthalene-1-sulfonic acid | |||
| |Section1={{Chembox Identifiers | |Section1={{Chembox Identifiers | ||
| | KEGG_Ref = {{keggcite|correct|kegg}} | |||
| | KEGG = C11326 | | KEGG = C11326 | ||
| | |
| InChI = 1/C16H13NO3S/c18-21(19,20)15-11-5-7-12-6-4-10-14(16(12)15)17-13-8-2-1-3-9-13/h1-11,17H,(H,18,19,20) | ||
| | |
| InChIKey = FWEOQOXTVHGIFQ-UHFFFAOYAB | ||
| | SMILES1 = c1ccc(cc1)Nc2cccc3c2c(ccc3)S(=O)(=O)O | |||
| ⚫ | | |
||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ⚫ | | ChemSpiderID = |
||
| | ChEMBL = 285527 | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChI = 1S/C16H13NO3S/c18-21(19,20)15-11-5-7-12-6-4-10-14(16(12)15)17-13-8-2-1-3-9-13/h1-11,17H,(H,18,19,20) | |||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChIKey = FWEOQOXTVHGIFQ-UHFFFAOYSA-N | |||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | CASNo=82-76-8 | |||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | UNII = 630I4V6051 | |||
| ⚫ | | PubChem=1369 | ||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ⚫ | | ChemSpiderID = 1328 | ||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | ChEBI = 39708 | |||
| | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| | DrugBank = DB04474 | | DrugBank = DB04474 | ||
| | SMILES = |
| SMILES = O=S(=O)(O)c2c1c(cccc1ccc2)Nc3ccccc3 | ||
| }} | }} | ||
| |Section2={{Chembox Properties | |Section2={{Chembox Properties | ||
| | |
| C=16 | H=13 | N=1 | O=3 | S=1 | ||
| | |
| Appearance= | ||
| | |
| Density= | ||
| | |
| MeltingPt= | ||
| | |
| BoilingPt= | ||
| | |
| Solubility= | ||
| }} | }} | ||
| |Section3={{Chembox Hazards | |Section3={{Chembox Hazards | ||
| | |
| MainHazards= | ||
| | |
| FlashPt= | ||
| | AutoignitionPt = | |||
| | Autoignition= | |||
| }} | }} | ||
| }} | }} | ||
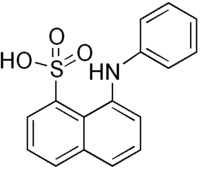
| '''8-Anilinonaphthalene-1- |
'''8-Anilinonaphthalene-1-sulfonic acid''' ('''ANS'''), also called 1-anilino-8-naphthalenesulfonate, is an ] containing both a ] and an ] group. This compound is used as a ] ].<ref>{{cite journal | doi = 10.1046/j.1432-1327.1999.00290.x | title = Fluorescence measurements detect changes in scallop myosin regulatory domain | date = 1999 | author = Andras Malnasi-Csizmadia | author2 = György Hegyi | author3 = Ferenc Tölgyesi | author4 = Andrew G. Szent-Györgyi | author5 = László Nyitray | name-list-style = amp | journal = European Journal of Biochemistry | volume = 261 | pages = 452–8 | pmid = 10215856 | issue = 2 | doi-access = }}</ref> For example, ANS can be used to study conformational changes induced by ] binding in ], as ANS's fluorescent properties will change as it binds to ] regions on the protein surface. Comparison of the fluorescence in the presence and absence of a particular ligand can thus give information about how the binding of the ligand changes the surface of the protein. Its permeability to ] membranes makes it particularly useful.<ref>{{cite journal |author=Gains N |author2=Dawson AP |title=8-Anilinonaphthalene-1-sulphonate interaction with whole and disrupted mitochondria: a re-evaluation of the use of double-reciprocal plots in the derivation of binding parameters for fluorescent probes binding to mitochondrial membranes |journal=Biochem. J. |volume=148 |issue=1 |pages=157–60 |date=April 1975 |doi=10.1042/bj1480157 |pmid=1156395 |pmc=1165518 }}</ref> | ||
| == References == | == References == | ||
| Line 34: | Line 51: | ||
| {{DEFAULTSORT:Anilinonaphthalene-1-sulfonate, 8-}} | {{DEFAULTSORT:Anilinonaphthalene-1-sulfonate, 8-}} | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| {{molecular-cell-biology-stub}} | {{molecular-cell-biology-stub}} | ||
| {{organic-compound-stub}} | {{organic-compound-stub}} | ||
| ] | |||
Latest revision as of 22:18, 18 August 2023
| |
| Names | |
|---|---|
| Preferred IUPAC name 8-Anilinonaphthalene-1-sulfonic acid | |
| Other names 8-(Phenylamino)naphthalene-1-sulfonic acid | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.001.308 |
| KEGG | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C16H13NO3S |
| Molar mass | 299.34 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
8-Anilinonaphthalene-1-sulfonic acid (ANS), also called 1-anilino-8-naphthalenesulfonate, is an organic compound containing both a sulfonic acid and an amine group. This compound is used as a fluorescent molecular probe. For example, ANS can be used to study conformational changes induced by ligand binding in proteins, as ANS's fluorescent properties will change as it binds to hydrophobic regions on the protein surface. Comparison of the fluorescence in the presence and absence of a particular ligand can thus give information about how the binding of the ligand changes the surface of the protein. Its permeability to mitochondrial membranes makes it particularly useful.
References
- Andras Malnasi-Csizmadia; György Hegyi; Ferenc Tölgyesi; Andrew G. Szent-Györgyi & László Nyitray (1999). "Fluorescence measurements detect changes in scallop myosin regulatory domain". European Journal of Biochemistry. 261 (2): 452–8. doi:10.1046/j.1432-1327.1999.00290.x. PMID 10215856.
- Gains N; Dawson AP (April 1975). "8-Anilinonaphthalene-1-sulphonate interaction with whole and disrupted mitochondria: a re-evaluation of the use of double-reciprocal plots in the derivation of binding parameters for fluorescent probes binding to mitochondrial membranes". Biochem. J. 148 (1): 157–60. doi:10.1042/bj1480157. PMC 1165518. PMID 1156395.
This molecular or cell biology article is a stub. You can help Misplaced Pages by expanding it. |
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |