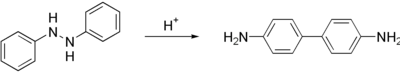| Revision as of 19:25, 7 November 2013 editJZNIOSH (talk | contribs)1,184 edits Updated appearance, added density, boiling pt to Chembox← Previous edit | Latest revision as of 15:20, 8 October 2024 edit undoJWBE (talk | contribs)Extended confirmed users10,128 edits removed Category:Anilines; added Category:4-Aminophenyl compounds using HotCat | ||
| (41 intermediate revisions by 27 users not shown) | |||
| Line 1: | Line 1: | ||
| {{ |
{{Chembox | ||
| | Verifiedimages = changed | | Verifiedimages = changed | ||
| | Verifiedfields = changed | |||
| | Watchedfields = changed | |||
| | verifiedrevid = 413886113 | | verifiedrevid = 413886113 | ||
| | ImageFile_Ref = {{chemboximage|correct|??}} | | ImageFile_Ref = {{chemboximage|correct|??}} | ||
| | ImageFile = Benzidine |
| ImageFile = Benzidine 200.svg | ||
| | ImageAlt = Skeletal formula of benzidine | |||
| | ImageSize = | |||
| | ImageFile1 = Benzidine-3D-balls.png | |||
| | IUPACName = 4,4'-diaminobiphenyl | |||
| | ImageSize1 = 210 | |||
| | OtherNames = Benzidine, di-phenylamine, diphenylamine | |||
| | ImageAlt1 = Ball-and-stick model of the benzidine molecule | |||
| ⚫ | | |
||
| | PIN = -4,4′-diamine | |||
| ⚫ | | |
||
| | OtherNames = Benzidine, di-phenylamine, diphenylamine, 4,4'-bianiline, 4,4'-biphenyldiamine, 1,1'-biphenyl-4,4'-diamine, 4,4'-diaminobiphenyl, p-diaminodiphenyl, p-benzidine | |||
| ⚫ | |Section1={{Chembox Identifiers | ||
| ⚫ | | KEGG_Ref = {{keggcite|correct|kegg}} | ||
| | KEGG = C16444 | | KEGG = C16444 | ||
| | InChI = 1/C12H12N2/c13-11-5-1-9(2-6-11)10-3-7-12(14)8-4-10/h1-8H,13-14H2 | | InChI = 1/C12H12N2/c13-11-5-1-9(2-6-11)10-3-7-12(14)8-4-10/h1-8H,13-14H2 | ||
| Line 16: | Line 21: | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = HFACYLZERDEVSX-UHFFFAOYSA-N | | StdInChIKey = HFACYLZERDEVSX-UHFFFAOYSA-N | ||
| | CASNo= 92-87-5 | | CASNo = 92-87-5 | ||
| | |
| CASNo_Ref = {{cascite|correct|CAS}} | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| ⚫ | | |
||
| | UNII = 2X02101HVF | |||
| ⚫ | | PubChem = 7111 | ||
| | ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| | ChEBI = 80495 | |||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | | ChEMBL_Ref = {{ebicite|correct|EBI}} | ||
| | ChEMBL = 15901 | | ChEMBL = 15901 | ||
| | EC_number = 202-199-1 | |||
| | RTECS = DC9625000 | |||
| | UNNumber = 1885 | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID=6844 | | ChemSpiderID =6844 | ||
| | |
| SMILES = c2c(c1ccc(N)cc1)ccc(N)c2 | ||
| }} | }} | ||
| |Section2={{Chembox Properties | |Section2={{Chembox Properties | ||
| | |
| Formula =C<sub>12</sub>H<sub>12</sub>N<sub>2</sub> | ||
| | |
| MolarMass =184.24 g/mol | ||
| | |
| Appearance = Grayish-yellow, reddish-gray, or white crystalline powder<ref name=PGCH>{{PGCH|0051}}</ref> | ||
| | |
| Density =1.25 g/cm<sup>3</sup> | ||
| | |
| MeltingPtC = 122 to 125 | ||
| | MeltingPt_notes = | |||
| | BoilingPt= 400 °C | |||
| | BoilingPtC = 400 | |||
| ⚫ | | |
||
| | BoilingPt_notes = | |||
| ⚫ | | Solubility = 0.94 g/100 mL at 100 °C | ||
| | MagSus = -110.9·10<sup>−6</sup> cm<sup>3</sup>/mol | |||
| }} | }} | ||
| |Section3 |
|Section3={{Chembox Related | ||
| | |
| OtherCompounds = ] | ||
| }} | }} | ||
| |Section4={{Chembox Hazards | |Section4={{Chembox Hazards | ||
| | |
| MainHazards =carcinogenic | ||
| | |
| FlashPt = | ||
| | AutoignitionPt = | |||
| | Autoignition= | |||
| | PEL = occupational carcinogen<ref name=PGCH/> | |||
| ⚫ | |||
| | GHSPictograms = {{GHS07}}{{GHS08}}{{GHS09}} | |||
| | GHSSignalWord = Danger | |||
| | HPhrases = {{H-phrases|302|350|410}} | |||
| | PPhrases = {{P-phrases|201|202|264|270|273|281|301+312|308+313|330|391|405|501}} | |||
| ⚫ | }} | ||
| }} | }} | ||
| '''Benzidine''' (]), also called ''' |
'''Benzidine''' (]), also called '''1,1'-]-4,4'-diamine''' (]), is an ] with the ] (C<sub>6</sub>H<sub>4</sub>NH<sub>2</sub>)<sub>2</sub>. It is an ] ]. It is a component of a test for ]. Related derivatives are used in the ] of ]. Benzidine has been linked to ] and ].<ref>{{cite web | url = http://www.cancer.org/docroot/PED/content/PED_1_3x_Known_and_Probable_Carcinogens.asp?sitearea=PED | title = Known and Probable Carcinogens | publisher = American Cancer Society | date = 2011-06-29 | access-date = 2007-01-12 | archive-date = 2008-03-17 | archive-url = https://web.archive.org/web/20080317051133/http://www.cancer.org/docroot/PED/content/PED_1_3x_Known_and_Probable_Carcinogens.asp?sitearea=PED | url-status = dead }}</ref> | ||
| ⚫ | |||
| ==Synthesis and properties== | ==Synthesis and properties== | ||
| Benzidine is prepared in a two step process from ]. First, the nitrobenzene is converted to 1,2-diphenylhydrazine, usually using iron powder as the reducing agent. Treatment of this hydrazine with mineral acids induces a ] to 4,4'-benzidine. Smaller amounts of other isomers are also formed.<ref name=Ullmann>{{ |
Benzidine is prepared in a two step process from ]. First, the nitrobenzene is converted to ], usually using iron powder as the reducing agent. Treatment of this hydrazine with mineral acids induces a ] to 4,4'-benzidine. Smaller amounts of other isomers are also formed.<ref name=Ullmann>{{Ullmann |author1=Schwenecke, H. |author2=Mayer, D. | title = Benzidine and Benzidine Derivatives | year = 2005 | doi = 10.1002/14356007.a03_539 }}</ref> The '''benzidine rearrangement''', which proceeds intramolecularly, is a classic ] puzzle in ].<ref>{{ cite book | author = March, J. | title = Advanced Organic Chemistry | edition = 5th | publisher = J. Wiley and Sons | year = 1992 | location = New York | isbn = 0-471-60180-2 }}</ref> | ||
| :] | :] | ||
| The conversion is described as a ].<ref>{{ cite journal | |
The conversion is described as a ].<ref>{{ cite journal |author1=Shine, H. J. |author2=Zmuda, H. |author3=Park, K. H. |author4=Kwart, H. |author5=Horgan, A. G. |author6=Collins, C. |author7=Maxwell, B. E. | title = Mechanism of the benzidine rearrangement. Kinetic isotope effects and transition states. Evidence for concerted rearrangement | journal = Journal of the American Chemical Society | year = 1981 | volume = 103 | issue = 4 | pages = 955–956 | doi = 10.1021/ja00394a047 }}.</ref><ref>{{ cite journal |author1=Shine, H. J. |author2=Zmuda, H. |author3=Park, K. H. |author4=Kwart, H. |author5=Horgan, A. G. |author6=Brechbiel, M. | title = Benzidine rearrangements. 16. The use of heavy-atom kinetic isotope effects in solving the mechanism of the acid-catalyzed rearrangement of hydrazobenzene. The concerted pathway to benzidine and the nonconcerted pathway to diphenyline | journal = Journal of the American Chemical Society | year = 1982 | volume = 104 | issue = 9 | pages = 2501–2509 | doi = 10.1021/ja00373a028 }}</ref> | ||
| :] | :] | ||
| Line 57: | Line 76: | ||
| ==Applications== | ==Applications== | ||
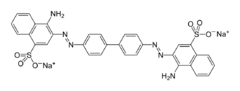
| Conversion of benzidine to the bis(diazonium) salt was once an integral step in the preparation of direct dyes (requiring no mordant). Treatment of this bis(diazonium) salt with ] gives the once popular ] dye. In the past, benzidine was used to test for ]. An ] in blood causes the oxidation of benzidine to a distinctively ]-coloured derivative. The test for ] relies on similar reactivity. Such applications have largely been replaced by methods using ]/] and ]. | |||
| As with some other ]s such as ], benzidine has been significantly withdrawn from use in most industries because it is so carcinogenic. | |||
| ] is derived from benzidine.]] | |||
| In the past, benzidine was used to test for ]. An ] in blood causes the oxidation of benzidine to a distinctively ]-coloured derivative. | |||
| The test for ] relies on similar reactivity. | |||
| Such applications have largely been replaced by methods using ]/] and ]. | |||
| ==Related 4,4’-benzidines== | ==Related 4,4’-benzidines== | ||
| A variety of derivatives of 4,4’-benzidine are commercially produced on the scale of one to a few thousand kilograms per year, mainly as precursors to dyes and pigments.<ref name=Ullmann/> These derivatives include, in order of scale, the following: |
A variety of derivatives of 4,4’-benzidine are commercially produced on the scale of one to a few thousand kilograms per year, mainly as precursors to dyes and pigments.<ref name=Ullmann/> These derivatives include, in order of scale, the following: | ||
| * ] | |||
| ⚫ | * ], 3,3'-dimethyl-4,4’-benzidine | ||
| ⚫ | * ] (3,3'-dimethoxy-4,4’-benzidine, CAS# 119-90-4, m.p. 133 °C) | ||
| ⚫ | * ], precursor to ]. | ||
| ==Safety== | |||
| * 3,3'-dichlorobenzidine (CAS# 91-94-1, m.p. 132–133 °C) | |||
| ⚫ | As with some other ]s such as ], benzidine has been significantly withdrawn from use in most industries because it is so carcinogenic. In August 2010 benzidine dyes were included in the U.S. EPA's List of Chemicals of Concern.<ref>{{ cite web |url=http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/benzidine.html |title=Benzidine Dyes Action Plan Summary |publisher=U. S. Environmental Protection Agency |date=2010-08-18 |url-status=dead |archiveurl=https://web.archive.org/web/20100821232316/http://www.epa.gov/oppt/existingchemicals/pubs/actionplans/benzidine.html |archivedate=2010-08-21 }}</ref> | ||
| ⚫ | * ] |
||
| The manufacture of Benzidine has been illegal in the UK since at least 2002 under the Control of Substances Hazardous to Health Regulations 2002 (COSHH). | |||
| ⚫ | * ''o''-dianisidine ( |
||
| ⚫ | * ] |
||
| ==References== | ==References== | ||
| {{reflist}} | {{reflist}} | ||
| {{Organic reactions}} | |||
| {{Authority control}} | |||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
Latest revision as of 15:20, 8 October 2024

| |

| |
| Names | |
|---|---|
| Preferred IUPAC name -4,4′-diamine | |
| Other names Benzidine, di-phenylamine, diphenylamine, 4,4'-bianiline, 4,4'-biphenyldiamine, 1,1'-biphenyl-4,4'-diamine, 4,4'-diaminobiphenyl, p-diaminodiphenyl, p-benzidine | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.000 |
| EC Number |
|
| KEGG | |
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 1885 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C12H12N2 |
| Molar mass | 184.24 g/mol |
| Appearance | Grayish-yellow, reddish-gray, or white crystalline powder |
| Density | 1.25 g/cm |
| Melting point | 122 to 125 °C (252 to 257 °F; 395 to 398 K) |
| Boiling point | 400 °C (752 °F; 673 K) |
| Solubility in water | 0.94 g/100 mL at 100 °C |
| Magnetic susceptibility (χ) | -110.9·10 cm/mol |
| Related compounds | |
| Related compounds | biphenyl |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | carcinogenic |
| GHS labelling: | |
| Pictograms |   
|
| Signal word | Danger |
| Hazard statements | H302, H350, H410 |
| Precautionary statements | P201, P202, P264, P270, P273, P281, P301+P312, P308+P313, P330, P391, P405, P501 |
| NIOSH (US health exposure limits): | |
| PEL (Permissible) | occupational carcinogen |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Benzidine (trivial name), also called 1,1'-biphenyl-4,4'-diamine (systematic name), is an organic compound with the formula (C6H4NH2)2. It is an aromatic amine. It is a component of a test for cyanide. Related derivatives are used in the production of dyes. Benzidine has been linked to bladder and pancreatic cancer.
Synthesis and properties
Benzidine is prepared in a two step process from nitrobenzene. First, the nitrobenzene is converted to 1,2-diphenylhydrazine, usually using iron powder as the reducing agent. Treatment of this hydrazine with mineral acids induces a rearrangement reaction to 4,4'-benzidine. Smaller amounts of other isomers are also formed. The benzidine rearrangement, which proceeds intramolecularly, is a classic mechanistic puzzle in organic chemistry.
The conversion is described as a sigmatropic reaction.
In terms of its physical properties, 4,4'-benzidine is poorly soluble in cold water but can be recrystallized from hot water, where it crystallises as the monohydrate. It is dibasic, the deprotonated species has Ka values of 9.3 × 10 and 5.6 × 10. Its solutions react with oxidizing agents to give deeply coloured quinone-related derivatives.
Applications
Conversion of benzidine to the bis(diazonium) salt was once an integral step in the preparation of direct dyes (requiring no mordant). Treatment of this bis(diazonium) salt with 1-aminonaphthalene-4-sulfonic acid gives the once popular congo red dye. In the past, benzidine was used to test for blood. An enzyme in blood causes the oxidation of benzidine to a distinctively blue-coloured derivative. The test for cyanide relies on similar reactivity. Such applications have largely been replaced by methods using phenolphthalein/hydrogen peroxide and luminol.
Related 4,4’-benzidines
A variety of derivatives of 4,4’-benzidine are commercially produced on the scale of one to a few thousand kilograms per year, mainly as precursors to dyes and pigments. These derivatives include, in order of scale, the following:
- 3,3'-Dichlorobenzidine
- o-tolidine, 3,3'-dimethyl-4,4’-benzidine
- o-dianisidine (3,3'-dimethoxy-4,4’-benzidine, CAS# 119-90-4, m.p. 133 °C)
- 3,3',4,4'-Tetraamino-diphenyl, precursor to polybenzimidazole fiber.
Safety
As with some other aromatic amines such as 2-naphthylamine, benzidine has been significantly withdrawn from use in most industries because it is so carcinogenic. In August 2010 benzidine dyes were included in the U.S. EPA's List of Chemicals of Concern. The manufacture of Benzidine has been illegal in the UK since at least 2002 under the Control of Substances Hazardous to Health Regulations 2002 (COSHH).
References
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0051". National Institute for Occupational Safety and Health (NIOSH).
- "Known and Probable Carcinogens". American Cancer Society. 2011-06-29. Archived from the original on 2008-03-17. Retrieved 2007-01-12.
- ^ Schwenecke, H.; Mayer, D. (2005). "Benzidine and Benzidine Derivatives". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a03_539. ISBN 978-3527306732.
- March, J. (1992). Advanced Organic Chemistry (5th ed.). New York: J. Wiley and Sons. ISBN 0-471-60180-2.
- Shine, H. J.; Zmuda, H.; Park, K. H.; Kwart, H.; Horgan, A. G.; Collins, C.; Maxwell, B. E. (1981). "Mechanism of the benzidine rearrangement. Kinetic isotope effects and transition states. Evidence for concerted rearrangement". Journal of the American Chemical Society. 103 (4): 955–956. doi:10.1021/ja00394a047..
- Shine, H. J.; Zmuda, H.; Park, K. H.; Kwart, H.; Horgan, A. G.; Brechbiel, M. (1982). "Benzidine rearrangements. 16. The use of heavy-atom kinetic isotope effects in solving the mechanism of the acid-catalyzed rearrangement of hydrazobenzene. The concerted pathway to benzidine and the nonconcerted pathway to diphenyline". Journal of the American Chemical Society. 104 (9): 2501–2509. doi:10.1021/ja00373a028.
- "Benzidine Dyes Action Plan Summary". U. S. Environmental Protection Agency. 2010-08-18. Archived from the original on 2010-08-21.