| Revision as of 08:58, 14 February 2014 edit93.128.103.41 (talk) →In the brain: hypothalamus first occurence← Previous edit | Latest revision as of 21:14, 14 December 2024 edit undoBD2412 (talk | contribs)Autopatrolled, IP block exemptions, Administrators2,448,258 editsm Clean up spacing around commas and other punctuation fixes, replaced: ],, [Tag: AWB | ||
| Line 1: | Line 1: | ||
| {{short description|Organic chemical that functions both as a hormone and a neurotransmitter}} | |||
| {{Use dmy dates|date=February 2014}} | |||
| {{About|the neurotransmitter|medical uses|Dopamine (medication)|other uses}} | |||
| {{Other uses}} | |||
| {{cs1 config|name-list-style=vanc|display-authors=6}} | |||
| {{chembox | |||
| {{Good article}} | |||
| | verifiedrevid = 464188976 | |||
| {{Use dmy dates|date=November 2018}} | |||
| | ImageFile_Ref = {{chemboximage|correct|??}} | |||
| {{Infobox drug | |||
| | ImageFile=Dopamine2.svg | |||
| | drug_name = | |||
| |ImageSize=200px | |||
| | IUPAC_name = 4-(2-Aminoethyl)benzene-1,2-diol | |||
| |ImageFile2=Dopamine-3d-CPK.png | |||
| | synonyms = {{ubl|DA, |2-(3,4-Dihydroxyphenyl)ethylamine, |3,4-Dihydroxyphenethylamine, |3-Hydroxytyramine, |Oxytyramine, | Intropin,| Revivan}} | |||
| |ImageSize2=180px | |||
| | image = Dopamine.svg | |||
| |IUPACName=4-(2-aminoethyl)benzene-1,2-diol | |||
| | alt = Dopamine structure | |||
| |OtherNames=2-(3,4-dihydroxyphenyl)ethylamine;<br/> 3,4-dihydroxyphenethylamine;<br/> 3-hydroxytyramine; DA; Intropin; Revivan; Oxytyramine | |||
| | caption = ] of dopamine | |||
| |Section1= {{Chembox Identifiers | |||
| | image2 = Dopamine-based-on-xtal-3D-bs-17.png | |||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | caption2 = ] of the dopamine molecule as found in solution. In the solid state, dopamine adopts a ]ic form.<ref>{{cite journal |title=CSD Entry TIRZAX: 5-(2-Ammonioethyl)-2-hydroxyphenolate, Dopamine|journal=Cambridge Structural Database: Access Structures |year=2013|publisher=]|doi=10.5517/cc10m9nl |doi-access=free |vauthors=Cruickshank L, Kennedy AR, Shankland N}}</ref><ref>{{ cite journal | title = Tautomeric and ionisation forms of dopamine and tyramine in the solid state | vauthors = Cruickshank L, Kennedy AR, Shankland N | journal = ] | volume = 1051 | pages = 132–36 | year = 2013 | doi = 10.1016/j.molstruc.2013.08.002 | bibcode = 2013JMoSt1051..132C }}</ref> | |||
| | UNII = VTD58H1Z2X | |||
| | source_tissues = ]; ]; many others | |||
| | InChI = 1/C8H11NO2/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,5,10-11H,3-4,9H2 | |||
| | target_tissues = System-wide | |||
| | InChIKey = VYFYYTLLBUKUHU-UHFFFAOYAA | |||
| | receptors = ], ], ], ], ], ]<ref name="DA IUPHAR"/> | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | agonists = Direct: ], ]<br/>]: ], ], ] | |||
| | StdInChI = 1S/C8H11NO2/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,5,10-11H,3-4,9H2 | |||
| | antagonists = ]s, ], ] | |||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | precursor = ], ], and ] | |||
| | StdInChIKey = VYFYYTLLBUKUHU-UHFFFAOYSA-N | |||
| | biosynthesis = ] | |||
| | CASNo=51-61-6 | |||
| | metabolism = ], ]<ref name="DA IUPHAR"/> | |||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | |
| CAS_number = 51-61-6 | ||
| | |
| CAS_supplemental = {{CAS|62-31-7}} (hydrochloride) | ||
| | |
| UNII_Ref = {{fdacite|correct|FDA}} | ||
| | UNII = VTD58H1Z2X | |||
| | PubChem=681 | |||
| | PubChem = 681 | |||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| | ChemSpiderID = 661 | |||
| | ChEMBL = 59 | |||
| | IUPHAR_ligand = 940 | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | KEGG = C03758 | |||
| | ChemSpiderID = 661 | |||
| | DrugBank = DB00988 | |||
| | KEGG_Ref = {{keggcite|correct|kegg}} | |||
| | C = 8 | |||
| | KEGG = D07870 | |||
| | H = 11 | |||
| | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| | N = 1 | |||
| | DrugBank = DB00988 | |||
| | O = 2 | |||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | SMILES = NCCc1cc(O)c(O)cc1 | |||
| | ChEBI = 18243 | |||
| | StdInChI = 1S/C8H11NO2/c9-4-3-6-1-2-7(10)8(11)5-6/h1-2,5,10-11H,3-4,9H2 | |||
| | SMILES = c1cc(c(cc1CCN)O)O | |||
| | StdInChI_comment = | |||
| | ATCCode_prefix = C01 | |||
| | StdInChIKey = VYFYYTLLBUKUHU-UHFFFAOYSA-N | |||
| | ATCCode_suffix = CA04 | |||
| }} | |||
| |Section2= {{Chembox Properties | |||
| | Formula=C<sub>8</sub>H<sub>11</sub>NO<sub>2</sub> | |||
| | MolarMass=153.18 g/mol | |||
| | Appearance=colorless solid | |||
| | Density= 1.26 g/cm<sup>3</sup> | |||
| | MeltingPtC= 128 | |||
| | BoilingPt= decomposes | |||
| | Solubility=60.0 g/100 ml | |||
| }} | |||
| }} | }} | ||
| '''Dopamine''' ('''DA''', a contraction of '''3,4-<u>d</u>ihydr<u>o</u>xy<u>p</u>henethyl<u>amine</u>''') is a ] ] that plays several important roles in cells. It is an ] of the ] and ] families. Dopamine constitutes about 80% of the catecholamine content in the brain. It is an ] synthesized by removing a ] from a molecule of its ], ], which is ] in the brain and kidneys. Dopamine is also synthesized in plants and most animals. In the brain, dopamine functions as a ]—a chemical released by ]s (nerve cells) to send signals to other nerve cells. Neurotransmitters are synthesized in specific regions of the brain but affect many regions systemically. The brain includes several distinct ], one of which plays a major role in the motivational component of ]. The anticipation of most types of rewards increases the level of dopamine in the brain,<ref>{{cite journal |vauthors=Berridge KC |date=April 2007 |title=The debate over dopamine's role in reward: the case for incentive salience |journal=Psychopharmacology |language=en-US |volume=191 |issue=3 |pages=391–431 |doi=10.1007/s00213-006-0578-x |pmid=17072591 |s2cid=468204}}</ref> and many ] ] increase dopamine release or block its ] into neurons following release.<ref name="Wise2020">{{cite journal |vauthors=Wise RA, Robble MA |date=January 2020 |title=Dopamine and Addiction |journal=Annual Review of Psychology |language=en-US |volume=71 |issue=1 |pages=79–106 |doi=10.1146/annurev-psych-010418-103337 |pmid=31905114 |s2cid=210043316 |doi-access=free}}</ref> Other brain dopamine pathways are involved in ] and in controlling the release of various hormones. These pathways and ] form a dopamine system which is ].<ref name="Wise2020"/> | |||
| '''Dopamine''' (or '''3,4-]]]]''') is a neurotransmitter in the ] and ] families that plays a number of important roles in the brains and bodies of animals. Its name derives from its chemical structure: it is an ] that is formed by removing a ] from a molecule of ]. | |||
| In ] and media, dopamine is often portrayed as the main chemical of pleasure, but the current opinion in pharmacology is that dopamine instead confers ];<ref name="NAcc function" /><ref name="pmid24107968">{{cite journal |vauthors=Baliki MN, Mansour A, Baria AT, Huang L, Berger SE, Fields HL, Apkarian AV |date=October 2013 |title=Parceling human accumbens into putative core and shell dissociates encoding of values for reward and pain |journal=The Journal of Neuroscience |language=en-US |volume=33 |issue=41 |pages=16383–93 |doi=10.1523/JNEUROSCI.1731-13.2013 |pmc=3792469 |pmid=24107968 |quote=<!--Recent evidence indicates that inactivation of D2 receptors, in the indirect striatopallidal pathway in rodents, is necessary for both acquisition and expression of aversive behavior, and direct pathway D1 receptor activation controls reward-based learning (Hikida et al., 2010; Hikida et al., 2013). It seems we can conclude that direct and indirect pathways of the NAc, via D1 and D2 receptors, subserve distinct anticipation and valuation roles in the shell and core of NAc, which is consistent with observations regarding spatial segregation and diversity of responses of midbrain dopaminergic neurons for rewarding and aversive conditions, some encoding motivational value, others motivational salience, each connected with distinct brain networks and having distinct roles in motivational control (Bromberg-Martin et al., 2010; Cohen et al., 2012; Lammel et al., 2013). ... Thus, the previous results, coupled with the current observations, imply that the NAc pshell response reflects a prediction/anticipation or salience signal, and the NAc pcore response is a valuation response (reward predictive signal) that signals the negative reinforcement value of cessation of pain (i.e., anticipated analgesia). -->}}</ref><ref name="Aversion neurons">{{cite journal |vauthors=Wenzel JM, Rauscher NA, Cheer JF, Oleson EB |date=January 2015 |title=A role for phasic dopamine release within the nucleus accumbens in encoding aversion: a review of the neurochemical literature |journal=ACS Chemical Neuroscience |language=en-US |volume=6 |issue=1 |pages=16–26 |doi=10.1021/cn500255p |pmc=5820768 |pmid=25491156 |quote=Thus, fear-evoking stimuli are capable of differentially altering phasic dopamine transmission across NAcc subregions. The authors propose that the observed enhancement in NAcc shell dopamine likely reflects general motivational salience, perhaps due to relief from a CS-induced fear state when the US (foot shock) is not delivered. This reasoning is supported by a report from Budygin and colleagues<sup>112</sup> showing that, in anesthetized rats, the termination of tail pinch results in augmented dopamine release in the shell.}}</ref> in other words, dopamine signals the perceived motivational prominence (i.e., the desirability or aversiveness) of an outcome, which in turn propels the organism's behavior toward or away from achieving that outcome.<ref name="Aversion neurons" /><ref name="Motivational salience">{{cite journal | vauthors = Puglisi-Allegra S, Ventura R | title = Prefrontal/accumbal catecholamine system processes high motivational salience | journal = Front. Behav. Neurosci. | volume = 6 | page = 31 | date = June 2012 | pmid = 22754514 | pmc = 3384081 | doi = 10.3389/fnbeh.2012.00031 | quote = <!--Motivational salience regulates the strength of goal seeking, the amount of risk taken, and the energy invested from mild to extreme. ... Motivation can be conceptually described as a continuum along which stimuli can either reinforce or punish responses to other stimuli. Behaviorally, stimuli that reinforce are called rewarding and those that punish aversive (Skinner, 1953). Reward and aversion describe the impact a stimulus has on behavior, and provided of motivational properties, thus able to induce attribution of motivational salience. ... Attribution of motivational salience is related to the salience of an UCS (Dallman et al., 2003; Pecina et al., 2006). Thus, the more salient an UCS the more likely a neutral (to-be-conditioned) stimulus will be associated with it through motivational salience attribution. Prior experience is a major determinant of the motivational impact of any given stimulus (Borsook et al., 2007) and emotional arousal induced by motivational stimuli increases the attention given to stimuli influencing both the initial perceptual encoding and the consolidation process (Anderson et al., 2006; McGaugh, 2006).-->| doi-access = free }}</ref> | |||
| In the ], dopamine functions as a ]—a chemical released by nerve cells to send signals to other nerve cells. The brain includes several distinct dopamine systems, one of which plays a major role in reward-motivated behavior. Every type of reward that has been studied increases the level of dopamine in the brain, and a variety of addictive drugs, including stimulants such as ], ], and ], act by amplifying the effects of dopamine. Other brain dopamine systems are involved in motor control and in controlling the release of several important hormones. | |||
| Outside the central nervous system, dopamine functions primarily as a local ] messenger. In blood vessels, it inhibits ] release and acts as a ]; in the kidneys, it increases sodium excretion and urine output; in the pancreas, it reduces insulin production; in the digestive system, it reduces ] and protects ]; and in the immune system, it reduces the activity of ]. With the exception of the blood vessels, dopamine in each of these peripheral systems is synthesized locally and exerts its effects near the cells that release it. | |||
| Several important diseases of the nervous system are associated with dysfunctions of the dopamine system. ], a degenerative condition causing tremor and motor impairment, is caused by loss of dopamine-secreting neurons in the midbrain area called the ]. There is evidence that ] involves altered levels of dopamine activity, and the antipsychotic drugs that are frequently used to treat it have a primary effect of attenuating dopamine activity. ] (ADHD) and ] (RLS) are also believed to be associated with decreased dopamine activity. | |||
| Several important diseases of the nervous system are associated with dysfunctions of the dopamine system, and some of the key medications used to treat them work by altering the effects of dopamine. ], a degenerative condition causing ] and motor impairment, is caused by a loss of dopamine-secreting neurons in an area of the ] called the ]. Its metabolic precursor L-DOPA can be manufactured; ''Levodopa'', a pure form of L-DOPA, is the most widely used treatment for Parkinson's. There is evidence that ] involves altered levels of dopamine activity, and most ] used to treat this are ]s which reduce dopamine activity.<ref>{{cite book | vauthors = Moncrieff J | title =The myth of the chemical cure. A critique of psychiatric drug treatment | year = 2008 | publisher = Palgrave MacMillan | location = Basingstoke, UK | isbn = 978-0-230-57432-8 }}</ref> Similar dopamine antagonist drugs are also some of the most effective ]. ] and ] (ADHD) are associated with decreased dopamine activity.<ref>{{cite journal | vauthors = Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, Fowler JS, Zhu W, Logan J, Ma Y, Pradhan K, Wong C, Swanson JM | title = Evaluating dopamine reward pathway in ADHD: clinical implications | journal = JAMA | volume = 302 | issue = 10 | pages = 1084–91 | date = September 2009 | pmid = 19738093 | pmc = 2958516 | doi = 10.1001/jama.2009.1308 }}</ref> ] ] can be addictive in high doses, but some are used at lower doses to treat ADHD. ] itself is available as a manufactured medication for ]. It is useful in the treatment of ] or ].<ref name="NHS2021">{{cite web |title=Dopamine infusion |url=https://www.bsuh.nhs.uk/library/wp-content/uploads/sites/8/2021/08/dopamine-infusion-August-2021-final.pdf |access-date=13 October 2023}}</ref> In newborn babies it may be used for ] and ].<ref name="medscape2021">{{cite web |title=Shock and Hypotension in the Newborn Medication: Alpha/Beta Adrenergic Agonists, Vasodilators, Inotropic agents, Volume Expanders, Antibiotics, Other |url=https://emedicine.medscape.com/article/979128-medication?form=fpf |access-date=13 October 2023 |website=emedicine.medscape.com |language=en-US}}</ref> | |||
| Outside the nervous system, dopamine functions in several parts of the body as a local chemical messenger. In the blood vessels, it inhibits norepinephrine release and acts as a vasodilator; in the kidneys, it increases sodium excretion and urine output; in the pancreas, it reduces insulin production; in the digestive system, it reduces ] and protects intestinal mucosa; and in the immune system, it reduces the activity of lymphocytes. With the exception of the blood vessels, dopamine in each of these peripheral systems has a "]" function: it is synthesized locally and exerts its effects on cells that are located near the cells that release it. | |||
| {{TOC limit|3}} | |||
| ==Structure== | |||
| A variety of important drugs work by altering the way the body makes or uses dopamine. Dopamine itself is available for intravenous injection: although it cannot reach the brain from the bloodstream, its peripheral effects make it useful in the treatment of heart failure or shock, especially in newborn babies. ], the metabolic precursor of dopamine, does reach the brain and is the most widely used treatment for Parkinson's disease. Dopamine-activating stimulants such as cocaine, amphetamine, and ] (Ritalin){{citation needed|date=September 2013}} are addictive in high doses, but are used at lower doses to treat ADHD. Conversely, many antipsychotic drugs act by suppressing the effects of dopamine. Drugs that act against dopamine by a different mechanism are also some of the most effective anti-nausea agents. | |||
| A dopamine molecule consists of a ] structure (a ] ring with two ] side groups) with one ] group attached via an ] chain.<ref name="PubChem">{{cite web |title=Dopamine |url=https://pubchem.ncbi.nlm.nih.gov/compound/dopamine |access-date=21 September 2015 |publisher=PubChem |language=en-US}}</ref> As such, dopamine is the simplest possible ], a family that also includes the ]s ] and ].<ref name=Catecholamine>{{cite encyclopedia |url=https://www.britannica.com/science/catecholamine |title=Catecholamine |encyclopedia=Britannica |access-date=21 September 2015}}</ref> The presence of a benzene ring with this amine attachment makes it a ], a family that includes numerous ]s.<ref name="Phenethylamine">{{cite web |title=Phenylethylamine |url=http://www.chemicalland21.com/lifescience/phar/PHENYLETHYLAMINE.htm |access-date=21 September 2015 |publisher=ChemicalLand21.com |language=en-US}}</ref> | |||
| Like most ]s, dopamine is an ].<ref name=Carter>{{cite journal |vauthors=Carter JE, Johnson JH, Baaske DM |year=1982 |title=Dopamine Hydrochloride |journal=Analytical Profiles of Drug Substances |volume=11 |pages=257–72|doi=10.1016/S0099-5428(08)60266-X |isbn=978-0122608117 }}</ref> As a ], it is generally ] in ]ic environments (in an ]).<ref name=Carter/> The protonated form is highly water-soluble and relatively stable, but can become ] if exposed to oxygen or other ].<ref name=Carter/> In basic environments, dopamine is not protonated.<ref name=Carter/> In this ] form, it is less water-soluble and also more highly reactive.<ref name=Carter/> Because of the increased stability and water-solubility of the protonated form, dopamine is supplied for chemical or pharmaceutical use as dopamine ]—that is, the ] ] that is created when dopamine is combined with ].<ref name=Carter/> In dry form, dopamine hydrochloride is a fine powder which is white to yellow in color.<ref>{{Cite web |title=Specification Sheet |url=https://www.sigmaaldrich.com/catalog/DataSheetPage.do?brandKey=SIGMA&symbol=H8502 |access-date=2019-09-13 |website=www.sigmaaldrich.com |language=en-US}}</ref> | |||
| ==Dopaminergic systems of the body== | |||
| {{multiple image | |||
| <!-- Essential parameters --> | |||
| | align = center | |||
| | direction = horizontal | |||
| <!-- Extra parameters --> | |||
| | header = | |||
| | header_align = | |||
| | header_background = | |||
| | footer = | |||
| | footer_align = | |||
| | footer_background = | |||
| | background color = | |||
| | image1=Dopamine2.svg | |||
| | caption1=Dopamine structure | |||
| | alt1=Chemical diagram of the structure of a dopamine molecule. | |||
| | width1=250 | |||
| | image2=Fenyloetyloamina.svg | |||
| | caption2=] structure | |||
| | alt2=Chemical diagram of a phenethylamine structure. | |||
| | width2=256 | |||
| | image3=Brenzcatechin.svg | |||
| | caption3=Catechol structure | |||
| | alt3=Chemical diagram of a catechol structure. | |||
| | width3=117 | |||
| }} | |||
| == |
==Biochemistry== | ||
| {{Catecholamine and trace amine biosynthesis|align=right|caption=In humans, ]s and phenethylaminergic ]s are derived from the amino acid ]. It is well established that dopamine is produced from <small>L</small>-tyrosine via <small>L</small>-DOPA; however, recent evidence has shown that CYP2D6 is expressed in the human brain and catalyzes the biosynthesis of dopamine from <small>L</small>-tyrosine via ''p''-tyramine.<ref name="CYP2D6 tyramine-dopamine metabolism" />}} | |||
| ] | |||
| ===Synthesis=== | |||
| {{Main|Dopaminergic pathways}} | |||
| Dopamine is ] in a restricted set of cell types, mainly neurons and cells in the ] of the ]s.<ref name=Seeman/> The primary and minor ]s respectively are: | |||
| :Primary: <small>L</small>-Phenylalanine → <small>L</small>-Tyrosine → <small>L</small>-DOPA → Dopamine<ref name="Trace amine template 1" /><ref name="Trace amine template 2" /> | |||
| Inside the brain, dopamine plays important roles in ], ], ], cognition, and ], as well as a number of basic lower-level functions including ], ], and ]. | |||
| :Minor: <small>L</small>-Phenylalanine → <small>L</small>-Tyrosine → ''p''-Tyramine → Dopamine<ref name="Trace amine template 1" /><ref name="Trace amine template 2" /><ref name="CYP2D6 tyramine-dopamine metabolism" /> | |||
| :Minor: <small>L</small>-Phenylalanine → ] → ] → Dopamine<ref name="CYP2D6 tyramine-dopamine metabolism" /><ref name="Tyrosine 3-hydroxylase m-tyrosine synthesis">{{cite encyclopedia|title=EC 1.14.16.2 – Tyrosine 3-monooxygenase (Homo sapiens)|url=http://www.brenda-enzymes.org/enzyme.php?ecno=1.14.16.2&Suchword=&reference=&organism%5B%5D=Homo+sapiens&show_tm=0| encyclopedia =BRENDA|publisher=Technische Universität Braunschweig|access-date=7 October 2016|date=July 2016|quote = Substrate: L-phenylalanine + tetrahydrobiopterin + O2<br />Product: L-tyrosine + 3-hydroxyphenylalanine + dihydropteridine + H2O<br />Organism: Homo sapiens}}<br /></ref><ref name="AADC m-tyramine synthesis">{{cite encyclopedia|title=EC 4.1.1.28 – Aromatic-L-amino-acid decarboxylase (Homo sapiens)|url=http://www.brenda-enzymes.org/enzyme.php?ecno=4.1.1.28&Suchword=&reference=&organism%5B%5D=Homo+sapiens&show_tm=0| encyclopedia =BRENDA|publisher=Technische Universität Braunschweig|access-date=7 October 2016|date=July 2016|quote = Substrate: m-tyrosine<br />Product: m-tyramine + CO2<br />Organism: Homo sapiens}}<br /></ref> | |||
| The direct precursor of dopamine, ], can be synthesized indirectly from the ] ] or directly from the non-essential amino acid ].<ref name=Musacchio/> These ]s are found in nearly every protein and so are readily available in food, with tyrosine being the most common. Although dopamine is also found in many types of food, it is incapable of crossing the ] that surrounds and protects the brain.<ref name="Nice-pharma"/> It must therefore be synthesized inside the brain to perform its ].<ref name="Nice-pharma"/> | |||
| ] neurons (i.e., neurons whose primary neurotransmitter is dopamine) are comparatively few in number — a total of around 400,000 in the human brain<ref name=SchultzAnnRev>{{cite journal |author=Schultz W |title=Multiple dopamine functions at different time courses |journal=Annu. Rev. Neurosci. |volume=30 |issue= |pages=259–88 |year=2007 |pmid=17600522 |doi=10.1146/annurev.neuro.28.061604.135722}}</ref> — and their cell bodies are confined to a few relatively small brain areas, but they send projections to many other brain areas and exert powerful effects on their targets. These ] were first mapped in 1964 by Annica Dahlström and Kjell Fuxe, who assigned them labels starting with the letter "A" (for "aminergic").<ref>{{cite journal |title=Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons |journal=Acta physiologica Scandinavica. Supplementum |year=1964 |volume=232 |pages=1–55 |pmid=14229500 |author=A. Dahlström and K. Fuxe}}</ref> In their scheme, areas A1 through A7 contain the neurotransmitter ], whereas A8 through A14 contain dopamine. Here is a list of the dopaminergic areas they identified: | |||
| <small>L</small>-Phenylalanine is converted into <small>L</small>-tyrosine by the ] ], with ] (O<sub>2</sub>) and ] as ]. <small>L</small>-Tyrosine is converted into <small>L</small>-DOPA by the enzyme ], with tetrahydrobiopterin, O<sub>2</sub>, and iron (Fe<sup>2+</sup>) as cofactors.<ref name=Musacchio>{{cite book |title=Biochemistry of Biogenic Amines |chapter=Chapter 1: Enzymes involved in the biosynthesis and degradation of catecholamines | vauthors= Musacchio JM | veditors = Iverson L |publisher=Springer | isbn = 978-1-4684-3171-1 |year=2013 |pages=1–35}}</ref> <small>L</small>-DOPA is converted into dopamine by the enzyme ] (also known as DOPA decarboxylase), with ] as the cofactor.<ref name=Musacchio/> | |||
| *The ], a small midbrain area that forms a component of the ]. The dopamine neurons are found mainly in a part of this structure called the ''pars compacta'' (cell group A8) and nearby (group A9).<ref name=Bjorklund>{{cite journal |author=Björklund A, Dunnett SB |title=Dopamine neuron systems in the brain: an update |journal=Trends Neurosci. |volume=30 |issue=5 |pages=194–202 |date=May 2007 |pmid=17408759 |doi=10.1016/j.tins.2007.03.006}}</ref> In rodents, their most important projections go to the ], ], and ], all of which also belong to the basal ganglia, and play important roles in motor control. The name ''substantia nigra'' is Latin for "dark substance", and refers to the fact that the dopaminergic neurons there are darkly pigmented. These neurons are especially vulnerable to damage. When a large fraction of them die, the result is a ].<ref>{{cite journal |author=Christine CW, Aminoff MJ |title=Clinical differentiation of parkinsonian syndromes: prognostic and therapeutic relevance |journal=Am. J. Med. |volume=117 |issue=6 |pages=412–9 |date=September 2004 |pmid=15380498 |doi=10.1016/j.amjmed.2004.03.032}}</ref> | |||
| Dopamine itself is used as precursor in the synthesis of the neurotransmitters norepinephrine and epinephrine.<ref name=Musacchio/> Dopamine is converted into norepinephrine by the enzyme ], with O<sub>2</sub> and ] as cofactors.<ref name=Musacchio/> Norepinephrine is converted into epinephrine by the enzyme ] with ] as the cofactor.<ref name=Musacchio/> | |||
| *The ] (VTA), another midbrain area. This cell group (A10) is the largest group of dopaminergic cells in the human brain, though still quite small in absolute terms. Projections from these dopaminergic neurons go to the ] and the ] as well as several other areas.<ref name=Bjorklund/> These neurons play a central role in reward and other aspects of motivation. The nucleus accumbens is often considered to be the "limbic" part of the ]. As such, it is the part of the striatum involved in the highest level aspects of motor control, which include motivation and decision-making. Thus, the role of the VTA in motivation and decision-making is structurally analogous to the role of the substantia nigra in low-level motor control.<ref name=DeLong>{{cite journal |author=DeLong M, Wichmann T |title=Changing views of basal ganglia circuits and circuit disorders |journal=Clin EEG Neurosci |volume=41 |issue=2 |pages=61–7 |date=April 2010 |pmid=20521487 |doi=10.1177/155005941004100204 |url=}}</ref> In primates (i.e. monkeys and humans), the dopamine neurons from the regions of the substantia nigra and VTA project throughout most of the cortical mantle, with particularly dense innervation of the motor and premotor cortices. Thus, there are major species differences in cortical dopamine projections.<ref>{{cite journal |author=Robbins TW, Arnsten AF. |title=The neuropsychopharmacology of fronto-executive function: monoaminergic modulation |journal=Annu Rev Neurosci. |volume=32 |pages=267–87 |year=2009 |doi=10.1146/annurev.neuro.051508.135535 |pmid=19555290 |pmc=2863127}}</ref> | |||
| Some of the cofactors also require their own synthesis.<ref name=Musacchio/> Deficiency in any required amino acid or cofactor can impair the synthesis of dopamine, norepinephrine, and epinephrine.<ref name=Musacchio/> | |||
| *The posterior ]. These dopaminergic cells (group A11) project to the spinal cord, and their function is not well established. There is some evidence that pathology in this area plays a role in ], a condition in which people have difficulty sleeping due to an overwhelming compulsion to constantly move parts of the body, especially the legs.<ref>{{cite journal |author=Paulus W, Schomburg ED |title=Dopamine and the spinal cord in restless legs syndrome: does spinal cord physiology reveal a basis for augmentation? |journal=Sleep Med Rev |volume=10 |issue=3 |pages=185–96 |date=June 2006 |pmid=16762808 |doi=10.1016/j.smrv.2006.01.004}}</ref> | |||
| ===Degradation=== | |||
| *The ] (cell group A12) and ] (cell group A14) of the hypothalamus. An important projection from these dopaminergic neurons goes to the ], where it influences the secretion of the hormone ]. Dopamine is the primary ] inhibitor of the secretion of ] from the ] gland. Dopamine produced by neurons in the arcuate nucleus is secreted into the ] of the ], which supply the ]. The lactotrope cells that produce ], in the absence of dopamine, secrete prolactin continuously; dopamine inhibits this secretion. Thus, in the context of regulating prolactin secretion, dopamine is occasionally called prolactin-inhibiting factor (PIF), prolactin-inhibiting hormone (PIH), or prolactostatin.<ref name="prolactininhibition">{{cite journal |author=Ben-Jonathan N, Hnasko R |title=Dopamine as a Prolactin (PRL) Inhibitor |journal=Endocrine Reviews |volume=22 |issue=6 |pages=724–763 |year=2001 |doi=10.1210/er.22.6.724 |url=http://edrv.endojournals.org/cgi/reprint/22/6/724.pdf |format=PDF |pmid=11739329}}</ref> | |||
| Dopamine is broken down into inactive ]s by a set of enzymes—] (MAO), ] (COMT), and ] (ALDH), acting in sequence.<ref name=Eisenhofer>{{cite journal | vauthors = Eisenhofer G, Kopin IJ, Goldstein DS | s2cid = 12825309 | title = Catecholamine metabolism: a contemporary view with implications for physiology and medicine | journal = Pharmacological Reviews | volume = 56 | issue = 3 | pages = 331–49 | date = September 2004 | pmid = 15317907 | doi = 10.1124/pr.56.3.1 }}</ref> Both ] of monoamine oxidase, ] and ], effectively metabolize dopamine.<ref name=Musacchio/> Different breakdown pathways exist but the main end-product is ] (HVA), which has no known biological activity.<ref name=Eisenhofer/> From the bloodstream, homovanillic acid is filtered out by the kidneys and then excreted in the urine.<ref name=Eisenhofer/> The two primary metabolic routes that convert dopamine into HVA are:<ref>{{Cite book | vauthors = Zahoor I, Shafi A, Haq E | chapter = Pharmacological Treatment of Parkinson’s Disease: Figure 1: | veditors = Stoker TB, Greenland JC | title = Parkinson's Disease: Pathogenesis and Clinical Aspects . | location = Brisbane (AU) | publisher = Codon Publications | date = December 2018 | chapter-url = https://www.ncbi.nlm.nih.gov/books/NBK536726/figure/Ch7-f0001/ }}</ref> | |||
| * Dopamine → ] → ] → HVA – catalyzed by MAO, ALDH, and COMT respectively | |||
| *The ]. These cells (group A13) project to several areas of the hypothalamus, and participate in the control of ], which is necessary to activate the development of reproductive systems that occurs following puberty, both in males and females.<ref name=prolactininhibition/> | |||
| * Dopamine → ] → HVA – catalyzed by COMT and MAO+ALDH respectively | |||
| An additional group of dopamine-secreting neurons are located in the ] of the eye. These neurons are ]s, meaning that they have no axons. They release dopamine into the extracellular medium, and are specifically active during daylight hours, becoming silent at night. This retinal dopamine acts to enhance the activity of ]s in the retina while suppressing ]s — the result is to increase sensitivity to color and contrast during bright light conditions, at the cost of reduced sensitivity when the light is dim.<ref>{{cite journal |author=Witkovsky P |title=Dopamine and retinal function |journal=Doc Ophthalmol |volume=108 |issue=1 |pages=17–40 |date=January 2004 |pmid=15104164 |doi=10.1023/B:DOOP.0000019487.88486.0a}}</ref> | |||
| ===Outside the nervous system=== | |||
| Dopamine does not cross the ], so its synthesis and functions in peripheral areas are to a large degree independent of its synthesis and functions in the brain. A substantial amount of dopamine circulates in the bloodstream, but its functions there are not entirely clear. Dopamine is found in blood plasma at levels comparable to those of ], but in humans, over 95% of the dopamine in the plasma is in the form of dopamine sulphate, a conjugate produced by the enzyme ] acting on free dopamine. The bulk of this dopamine sulphate is produced in the ] that surround parts of the digestive system. The production of dopamine sulphate is thought to be a mechanism for detoxifying dopamine that is ingested as food or produced by the digestive process — plasma levels typically rise more than fifty-fold after a meal. Dopamine sulphate has no known biological functions and is excreted in urine.<ref name=Eisenhofer/> | |||
| The relatively small quantity of unconjugated dopamine in the bloodstream may be produced by the ], the digestive system, or possibly other organs. It may act on dopamine receptors in peripheral tissues, or be metabolized, or be converted to ] by the enzyme ], which is released into the bloodstream by the ].<ref name=Eisenhofer/> Some dopamine receptors are located in the walls of arteries, where they act as a ] and an inhibitor of ] release.<ref name=Missale>{{cite journal |last1=Missale |first1=C |author2=Nash, SR; Robinson, SW; Jaber, M; Caron, MG |title=Dopamine receptors: from structure to function |journal=Physiological reviews |year=1998 |volume=78 |issue=1 |pages=189–225 |pmid=9457173}}</ref> These responses might be activated by dopamine released from the ] under conditions of low oxygen, but whether arterial dopamine receptors perform other biologically useful functions is not known. | |||
| Beyond its role in modulating blood flow, there are several peripheral systems in which dopamine circulates within a limited area and performs an ] or ] function.<ref name=Eisenhofer>{{cite journal |author=Eisenhofer G, Kopin IJ, Goldstein DS |title=Catecholamine metabolism: a contemporary view with implications for physiology and medicine |journal=Pharmacol. Rev. |volume=56 |issue=3 |pages=331–49 |date=September 2004 |pmid=15317907 |doi=10.1124/pr.56.3.1 |url=http://intl.pharmrev.org/content/56/3/331.full}}</ref> The peripheral systems in which dopamine plays an important role include: | |||
| *'''The immune system.''' Dopamine acts upon receptors present on immune cells, especially ]s.<ref name=Buttarelli>{{cite journal |author=Buttarelli FR, Fanciulli A, Pellicano C, Pontieri FE |title=The dopaminergic system in peripheral blood lymphocytes: from physiology to pharmacology and potential applications to neuropsychiatric disorders |journal=Curr Neuropharmacol |volume=9 |issue=2 |pages=278–88 |date=June 2011 |pmid=22131937 |pmc=3131719 |doi=10.2174/157015911795596612}}</ref> Dopamine can also affect immune cells in the ], ], and ].<ref>{{cite journal |doi=10.1016/S0165-5728(99)00176-9 |last1=Basu |first1=S |last2=Dasgupta |first2=PS. |year=2000 |title=Dopamine, a neurotransmitter, influences the immune system |url= |journal=J Neuroimmunol |volume=102 |issue=2 |pages=113–24 |pmid=10636479}}</ref> In addition, dopamine can be synthesized and released by immune cells themselves.<ref name=Buttarelli/> The main effect of dopamine on lymphocytes is to reduce their activation level. The functional significance of this system is unclear, but it afford a possible route for interactions between the nervous system and immune system, and may be relevant to some autoimmune disorders.<ref>{{cite journal |last1=Sarkar |first1=C |last2=Basu |first2=B |last3=Chakroborty |first3=D |last4=Dasgupta |first4=PS |last5=Basu |first5=S |title=The immunoregulatory role of dopamine: an update |journal=Brain, behavior, and immunity |volume=24 |issue=4 |pages=525–8 |year=2010 |doi=10.1016/j.bbi.2009.10.015 |pmc=2856781 |pmid=19896530}}</ref> | |||
| *'''The kidneys.''' Multiple types of dopamine receptors are present in cells of the ]s. Dopamine is also synthesized there, by ] cells, and discharged into the ]. Its actions include increasing the blood supply to the kidneys, increasing filtration by the glomeruli, and increasing excretion of sodium in the urine. Defects in renal dopamine function can be produced by high blood pressure or by genetic problems, and can lead to reduced sodium excretion as well as hypertension.<ref>{{cite journal |author=Carey RM |title=Theodore Cooper Lecture: Renal dopamine system: paracrine regulator of sodium homeostasis and blood pressure |journal=Hypertension |volume=38 |issue=3 |pages=297–302 |date=September 2001 |pmid=11566894 |doi=10.1161/hy0901.096422 |url=http://hyper.ahajournals.org/content/38/3/297.long}}</ref> | |||
| *'''The pancreas.''' The role of dopamine here is somewhat complex. The ] consists of two parts, known as ] and ]. The exocrine part synthesizes enzymes and other substances, and secretes them into the ], where food is digested. One of the substances synthesized and secreted by the exocrine pancreas is dopamine. The function of this secreted dopamine after it enters the small intestine is not clearly established — the possibilities include protecting the intestinal mucosa from damage and reducing ] (the rate at which food moves through the intestines).<ref name=Rubi>{{cite journal |author=Rubí B, Maechler P |title=Minireview: new roles for peripheral dopamine on metabolic control and tumor growth: let's seek the balance |journal=Endocrinology |volume=151 |issue=12 |pages=5570–81 |date=December 2010 |pmid=21047943 |doi=10.1210/en.2010-0745 |url=http://endo.endojournals.org/content/151/12/5570.long}}</ref> | |||
| In clinical research on schizophrenia, measurements of homovanillic acid in ] have been used to estimate levels of dopamine activity in the brain. A difficulty in this approach however, is separating the high level of plasma homovanillic acid contributed by the metabolism of norepinephrine.<ref>{{cite journal | vauthors = Amin F, Davidson M, Davis KL | title = Homovanillic acid measurement in clinical research: a review of methodology | journal = Schizophrenia Bulletin | volume = 18 | issue = 1 | pages = 123–48 | year = 1992 | pmid = 1553492 | doi = 10.1093/schbul/18.1.123 | doi-access = free }}</ref><ref>{{cite journal | vauthors = Amin F, Davidson M, Kahn RS, Schmeidler J, Stern R, Knott PJ, Apter S | title = Assessment of the central dopaminergic index of plasma HVA in schizophrenia | journal = Schizophrenia Bulletin | volume = 21 | issue = 1 | pages = 53–66 | date = 1995 | pmid = 7770741 | doi = 10.1093/schbul/21.1.53 | doi-access = free }}<!--|access-date=13 November 2015--></ref> | |||
| :The endocrine part of the pancreas, also known as the ], synthesizes a number of hormones, including ], and secretes them into the bloodstream. There is evidence that the ]s that synthesize insulin contain dopamine receptors, and that dopamine acts to reduce the amount of insulin they release. The source of their dopamine input is not clearly established — it may come from dopamine that circulates in the bloodstream and derives from the sympathetic nervous system, or it may be synthesized locally by other types of pancreatic cells.<ref name=Rubi/> | |||
| Although dopamine is normally broken down by an ] enzyme, it is also susceptible to oxidation by direct reaction with oxygen, yielding ]s plus various ] as products.<ref name=Sulzer>{{cite journal | vauthors = Sulzer D, Zecca L | s2cid = 21892355 | title = Intraneuronal dopamine-quinone synthesis: a review | journal = Neurotoxicity Research | volume = 1 | issue = 3 | pages = 181–95 | date = February 2000 | pmid = 12835101 | doi = 10.1007/BF03033289 }}</ref> The rate of oxidation can be increased by the presence of ] iron or other factors. Quinones and free radicals produced by autoxidation of dopamine can ], and there is evidence that this mechanism may contribute to the cell loss that occurs in ] and other conditions.<ref>{{cite journal | vauthors = Miyazaki I, Asanuma M | title = Dopaminergic neuron-specific oxidative stress caused by dopamine itself | journal = Acta Medica Okayama | volume = 62 | issue = 3 | pages = 141–50 | date = June 2008 | pmid = 18596830 | doi = 10.18926/AMO/30942 | url = http://www.lib.okayama-u.ac.jp/www/acta/pdf/62_3_141.pdf }}</ref> | |||
| ==Cellular effects== | |||
| {{Main|Dopamine receptor}} | |||
| ==Functions== | |||
| {| class="wikitable" style="float: right; text-align: center" border="10" | |||
| ===Cellular effects=== | |||
| <caption>Dopamine receptors in the mammal brain</caption> | |||
| {{Main|Dopamine receptor|TAAR1}} | |||
| {| class="wikitable" style="float:right; margin-left:10px; text-align:center;" | |||
| |+] of dopamine in the human brain<ref name="DA IUPHAR">{{cite web |title=Dopamine: Biological activity |url=http://www.guidetopharmacology.org/GRAC/LigandDisplayForward?tab=biology&ligandId=940 |access-date=29 January 2016 |website=IUPHAR/BPS guide to pharmacology |publisher=International Union of Basic and Clinical Pharmacology |language=en-US}}</ref><ref name="Miller+Grandy 2016">{{cite journal | vauthors = Grandy DK, Miller GM, Li JX | title = "TAARgeting Addiction" – The Alamo Bears Witness to Another Revolution: An Overview of the Plenary Symposium of the 2015 Behavior, Biology and Chemistry Conference | journal = Drug and Alcohol Dependence | volume = 159 | pages = 9–16 | date = February 2016 | pmid = 26644139 | pmc = 4724540 | doi = 10.1016/j.drugalcdep.2015.11.014 | quote = TAAR1 is a high-affinity receptor for METH/AMPH and DA }}</ref> | |||
| |- | |- | ||
| ! Family | ! scope="col" | Family | ||
| ! Receptor | ! scope="col" | Receptor | ||
| ! Gene | ! scope="col" | Gene | ||
| ! Type | ! scope="col" | Type | ||
| ! Mechanism | ! scope="col" | Mechanism | ||
| |- | |- | ||
| | rowspan=2 | ] | | rowspan=2 | ] | ||
| | ] | | ] | ||
| | {{Gene|DRD1}} | | {{Gene|DRD1}} | ||
| | rowspan=2 | ]-coupled. | | rowspan=2 | ]-coupled. | ||
| | rowspan=2 | Increase intracellular levels of ]<br> by activating ]. | | rowspan=2 | Increase intracellular levels of ]<br /> by activating ]. | ||
| |- | |- | ||
| | ] | | ] | ||
| | {{Gene|DRD5}} | | {{Gene|DRD5}} | ||
| |- | |- | ||
| | rowspan=3 | ] | | rowspan=3 | ] | ||
| | ] | | ] | ||
| | {{Gene|DRD2}} | | {{Gene|DRD2}} | ||
| | rowspan=3 | ]-coupled. | | rowspan=3 | ]-coupled. | ||
| | rowspan=3 | Decrease intracellular levels of ]<br> by inhibiting ]. | | rowspan=3 | Decrease intracellular levels of ]<br /> by inhibiting ]. | ||
| |- | |- | ||
| | ] | | ] | ||
| Line 131: | Line 138: | ||
| | ] | | ] | ||
| | {{Gene|DRD4}} | | {{Gene|DRD4}} | ||
| |- | |||
| | ] | |||
| | ] | |||
| | {{Gene|TAAR1}} | |||
| | ]-coupled.<br />]-coupled. | |||
| | Increase intracellular levels of ]<br /> and intracellular calcium concentration. | |||
| |} | |} | ||
| Dopamine exerts its effects by binding to and activating ]s.<ref name=Seeman/> In humans, dopamine has a high ] at ]s and ] (hTAAR1).<ref name="DA IUPHAR" /><ref name="Miller+Grandy 2016" /> In mammals, five subtypes of ]s have been identified, labeled from D<sub>1</sub> to D<sub>5</sub>.<ref name=Seeman>{{cite book| title=The Dopamine Receptors |chapter=Chapter 1: Historical overview: Introduction to the dopamine receptors | vauthors = Seeman P | veditors = Neve K| publisher=Springer |year=2009 |isbn=978-1-60327-333-6 |pages=1–22}}</ref> All of them function as ], ]s, meaning that they exert their effects via a complex ].<ref name=Romanelli>{{cite book| title=The Dopamine Receptors |chapter=Chapter 6: Dopamine receptor signalling: intracellular pathways to behavior | vauthors = Romanelli RJ, Williams JT, Neve KA | veditors = Neve KA| publisher = Springer | year = 2009 | isbn = 978-1-60327-333-6 | pages = 137–74}}</ref> These receptors can be divided into two families, known as ] and ].<ref name=Seeman/> For receptors located on neurons in the nervous system, the ultimate effect of D<sub>1</sub>-like activation (D<sub>1</sub> and D<sub>5</sub>) can be excitation (via opening of ]s) or inhibition (via opening of ]s); the ultimate effect of D<sub>2</sub>-like activation (D<sub>2</sub>, D<sub>3</sub>, and D<sub>4</sub>) is usually inhibition of the target neuron.<ref name=Romanelli/> Consequently, it is incorrect to describe dopamine itself as either excitatory or inhibitory: its effect on a target neuron depends on which types of receptors are present on the membrane of that neuron and on the internal responses of that neuron to the second messenger ].<ref name=Romanelli/> D<sub>1</sub> receptors are the most numerous dopamine receptors in the human nervous system; D<sub>2</sub> receptors are next; D<sub>3</sub>, D<sub>4</sub>, and D<sub>5</sub> receptors are present at significantly lower levels.<ref name=Romanelli/> | |||
| ====Storage, release, and reuptake==== | |||
| The level of extracellular dopamine is modulated by two mechanisms: tonic and phasic dopamine transmission. Tonic dopamine transmission occurs when small amounts of dopamine are released independently of neuronal activity, and is regulated by the activity of other neurons and neurotransmitter reuptake.<ref>{{Cite journal |author=Grace AA, |title=Phasic versus tonic dopamine release and the modulation of dopamine system responsivity: A hypothesis for the eitiology of schizophrenia |journal=Neuroscience |volume=41 |issue=1 |pages=1–24 |year=1991 |pmid=1676137 |doi=10.1016/0306-4522(91)90196-U}}</ref> Phasic dopamine release results from the activity of the dopamine-containing cells themselves. This activity is characterized by irregular ] activity of single spikes, and rapid bursts of typically 2-6 spikes in quick succession.<ref>{{Cite journal |author=Grace AA, Bunney BS |title=The control of firing pattern in nigral dopamine neurons: single spike firing |url=http://www.jneurosci.org/cgi/reprint/4/11/2866 |format=PDF |journal=Journal of Neuroscience |volume=4 |issue=11 |pages=2866–2876 |year=1984 |pmid=6150070}}</ref><ref>{{Cite journal |author=Grace AA, Bunney BS |title=The control of firing pattern in nigral dopamine neurons: burst firing |url=http://www.jneurosci.org/cgi/reprint/4/11/2877 |format=PDF |journal=Journal of Neuroscience |volume=4 |issue=11 |pages=28677–2890 |year=1984 |pmid=6150071}}</ref> | |||
| ]<br /> DOPA: ]<br /> DAT: ]<br /> DDC: ]<br /> VMAT: ]<br /> MAO: ]<br /> COMT: ]<br /> HVA: ]|alt=Cartoon diagram of a dopaminergic synapse, showing the synthetic and metabolic mechanisms as well as the things that can happen after release.]] | |||
| Inside the brain, dopamine functions as a neurotransmitter and ], and is controlled by a set of mechanisms common to all ]s.<ref name=Seeman/> After synthesis, dopamine is transported from the ] into secretory vesicles, including ]s, small and ] by a ]—a ], ].<ref name=Eiden>{{cite journal | vauthors = Eiden LE, Schäfer MK, Weihe E, Schütz B | s2cid = 20764857 | title = The vesicular amine transporter family (SLC18): amine/proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine | journal = Pflügers Archiv | volume = 447 | issue = 5 | pages = 636–40 | date = February 2004 | pmid = 12827358 | doi = 10.1007/s00424-003-1100-5 }}</ref><ref>{{Cite journal |last=Westerink |first=Remco |date=2006-02-01 |title=Targeting Exocytosis: Ins and Outs of the Modulation of Quantal Dopamine Release |url=https://www.eurekaselect.com/article/2545 |journal=CNS & Neurological Disorders - Drug Targets |volume=5 |issue=1 |pages=57–77 |doi=10.2174/187152706784111597 |pmid=16613554 |issn=1871-5273}}</ref> Dopamine is stored in these vesicles until it is ejected into the ]. In most cases, the release of dopamine occurs through a process called ] which is caused by ]s, but it can also be caused by the activity of an intracellular ], ].<ref name="Miller+Grandy 2016" /> TAAR1 is a high-affinity receptor for dopamine, ]s, and certain ]s that is located along membranes in the intracellular milieu of the presynaptic cell;<ref name="Miller+Grandy 2016" /> activation of the receptor can regulate dopamine signaling by inducing dopamine ] and ] as well as by inhibiting neuronal firing through a diverse set of mechanisms.<ref name="Miller+Grandy 2016" /><ref name="Miller" /> | |||
| Once in the synapse, dopamine binds to and activates dopamine receptors.<ref name="D2 Long and short" /> These can be ] dopamine receptors, which are located on ]s (the postsynaptic neuron), or presynaptic ]s (e.g., the ] and presynaptic D<sub>3</sub> receptors), which are located on the membrane of an ] (the presynaptic neuron).<ref name=Seeman/><ref name="D2 Long and short">{{cite journal | vauthors = Beaulieu JM, Gainetdinov RR | s2cid = 2545878 | title = The physiology, signaling, and pharmacology of dopamine receptors | journal = Pharmacological Reviews | volume = 63 | issue = 1 | pages = 182–217 | date = March 2011 | pmid = 21303898 | doi = 10.1124/pr.110.002642 }}</ref> After the postsynaptic neuron elicits an action potential, dopamine molecules quickly become unbound from their receptors. They are then absorbed back into the presynaptic cell, via ] mediated either by the ] or by the ].<ref name=Torres>{{cite journal | vauthors = Torres GE, Gainetdinov RR, Caron MG | s2cid = 21545649 | title = Plasma membrane monoamine transporters: structure, regulation and function | journal = Nature Reviews. Neuroscience | volume = 4 | issue = 1 | pages = 13–25 | date = January 2003 | pmid = 12511858 | doi = 10.1038/nrn1008 }}</ref> Once back in the cytosol, dopamine can either be broken down by a ] or repackaged into vesicles by VMAT2, making it available for future release.<ref name=Eiden/> | |||
| ==The substantia nigra dopamine system and motor control== | |||
| In the brain the level of extracellular dopamine is modulated by two mechanisms: ].<ref name="Rice">{{cite journal | vauthors = Rice ME, Patel JC, Cragg SJ | title = Dopamine release in the basal ganglia | journal = Neuroscience | volume = 198 | pages = 112–37 | date = December 2011 | pmid = 21939738 | pmc = 3357127 | doi = 10.1016/j.neuroscience.2011.08.066 }}</ref> Phasic dopamine release, like most neurotransmitter release in the nervous system, is driven directly by action potentials in the dopamine-containing cells.<ref name=Rice/> Tonic dopamine transmission occurs when small amounts of dopamine are released without being preceded by presynaptic action potentials.<ref name=Rice/> Tonic transmission is regulated by a variety of factors, including the activity of other neurons and neurotransmitter reuptake.<ref name=Rice/> | |||
| ]. The dopaminergic pathway from the ] to the ] is shown in light blue.]] | |||
| ==={{Anchor|Functions in the brain}} Central nervous system=== | |||
| The ] is a component of the ], a group of interconnected structures in the forebrain and midbrain that play a central role in motor control. The precise nature of that role has been difficult to work out, but one popular line of thought describes it as "response selection". The response selection theory proposes that when a person or animal is in a situation where several behaviors are possible, activity in the basal ganglia determines which of them is executed, by releasing that response from inhibition. Thus the basal ganglia are responsible for initiating behaviors but not for determining the details of how they are carried out. | |||
| {{Main|Dopaminergic cell groups|Dopaminergic pathways}} | |||
| {{See also|Hypothalamic–pituitary–prolactin axis}} | |||
| ] (VTA) and is released in the ] and the ]. The motor functions of dopamine are linked to a separate pathway, with cell bodies in the ] that manufacture and release dopamine into the ].|alt=A labelled line drawing of dopamine pathways superimposed on a drawing of the human brain.]] | |||
| Inside the brain, dopamine plays important roles in ]s, ], ], ], ], and ], as well as lower-level functions including ], ], and ]. The ] and ] make up the dopamine system which is ]. | |||
| Dopamine is thought to modulate the response selection process in at least two important ways. First, dopamine sets the "effort threshold" for initiating behaviors. The higher the level of dopamine activity, the lower the impetus required to evoke a given behavior. As a consequence, high levels of dopamine lead to high levels of motor activity and "impulsive" behavior; low levels of dopamine lead to torpor and slowed reactions. ], in which dopamine levels in the ''substantia nigra'' circuit are greatly reduced, is characterized by stiffness and greatly reduced movement—however, when people with the disease are confronted with strong stimuli such as a serious threat, their reactions can be as vigorous as those of a healthy person. In the opposite direction, drugs that increase the effects of dopamine, such as cocaine or amphetamine, produce heightened levels of activity, including at the highest levels ] and stereotyped movements. | |||
| ] neurons (dopamine-producing nerve cells) are comparatively few in number—a total of around 400,000 in the human brain<ref name=SchultzAnnRev>{{cite journal | vauthors = Schultz W | s2cid = 13503219 | title = Multiple dopamine functions at different time courses | journal = Annual Review of Neuroscience | volume = 30 | pages = 259–88 | year = 2007 | pmid = 17600522 | doi = 10.1146/annurev.neuro.28.061604.135722 }}</ref>—and their ] are confined in groups to a few relatively small brain areas.<ref name=Bjorklund/> However their ]s project to many other brain areas, and they exert powerful effects on their targets.<ref name=Bjorklund/> These dopaminergic cell groups were first mapped in 1964 by ] and Kjell Fuxe, who assigned them labels starting with the letter "A" (for "aminergic").<ref name=DahlstromFuxe>{{cite journal | vauthors = Dahlstroem A, Fuxe K | title = Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons | journal = Acta Physiologica Scandinavica. Supplementum | volume = 232 | issue = Suppl | pages = 1–55 | year = 1964 | pmid = 14229500 }}</ref> In their scheme, areas A1 through A7 contain the neurotransmitter norepinephrine, whereas A8 through A14 contain dopamine. The dopaminergic areas they identified are the substantia nigra (groups 8 and 9); the ] (group 10); the posterior ] (group 11); the ] (group 12); the ] (group 13) and the ] (group 14).<ref name=DahlstromFuxe/> | |||
| The second important effect of dopamine is as a "teaching" signal. When a motor response is followed by an increase in dopamine activity, the basal ganglia circuit is altered in a way that makes the same response easier to evoke when similar situations arise in the future. This is a form of ], in which dopamine plays the role of a reward signal. | |||
| The substantia nigra is a small midbrain area that forms a component of the ]. This has two parts—an input area called the ] and an output area called the ]. The dopaminergic neurons are found mainly in the pars compacta (cell group A8) and nearby (group A9).<ref name=Bjorklund>{{cite journal | vauthors = Björklund A, Dunnett SB | s2cid = 14239716 | title = Dopamine neuron systems in the brain: an update | journal = Trends in Neurosciences | volume = 30 | issue = 5 | pages = 194–202 | date = May 2007 | pmid = 17408759 | doi = 10.1016/j.tins.2007.03.006 }}</ref> In humans, the projection of dopaminergic neurons from the substantia nigra pars compacta to the dorsal striatum, termed the '']'', plays a significant role in the control of motor function and in learning new ]s.<ref name="Malenka pathways" /> These neurons are especially vulnerable to damage, and when a large number of them die, the result is a ].<ref>{{cite journal | vauthors = Christine CW, Aminoff MJ | title = Clinical differentiation of parkinsonian syndromes: prognostic and therapeutic relevance | journal = The American Journal of Medicine | volume = 117 | issue = 6 | pages = 412–19 | date = September 2004 | pmid = 15380498 | doi = 10.1016/j.amjmed.2004.03.032 }}</ref> | |||
| ===Anatomy and physiology=== | |||
| The ] (VTA) is another midbrain area. The most prominent group of VTA dopaminergic neurons projects to the prefrontal cortex via the ] and another smaller group projects to the nucleus accumbens via the ]. Together, these two pathways are collectively termed the '']''.<ref name=Bjorklund/><ref name="Malenka pathways" /> The VTA also sends dopaminergic projections to the ], ], ], and ].<ref name=Bjorklund/><ref name="Malenka pathways">{{cite book | vauthors = Malenka RC, Nestler EJ, Hyman SE | veditors = Sydor A, Brown RY | title = Molecular Neuropharmacology: A Foundation for Clinical Neuroscience | year = 2009 | publisher = McGraw-Hill Medical | location = New York | isbn = 978-0-07-148127-4 | pages = 147–48, 154–57 | edition = 2nd | chapter = Chapter 6: Widely Projecting Systems: Monoamines, Acetylcholine, and Orexin }}</ref> Mesocorticolimbic neurons play a central role in reward and other aspects of motivation.<ref name="Malenka pathways" /> Accumulating literature shows that dopamine also plays a crucial role in aversive learning through its effects on a number of brain regions.<ref>{{cite journal | vauthors = Fadok JP, Dickerson TM, Palmiter RD | title = Dopamine is necessary for cue-dependent fear conditioning | journal = The Journal of Neuroscience | volume = 29 | issue = 36 | pages = 11089–97 | date = September 2009 | pmid = 19741115 | pmc = 2759996 | doi = 10.1523/JNEUROSCI.1616-09.2009 }}</ref><ref>{{cite journal | vauthors = Tang W, Kochubey O, Kintscher M, Schneggenburger R | title = A VTA to basal amygdala dopamine projection contributes to signal salient somatosensory events during fear learning | journal = The Journal of Neuroscience | pages = JN–RM–1796-19 | date = April 2020 | volume = 40 | issue = 20 | pmid = 32277045 | doi = 10.1523/JNEUROSCI.1796-19.2020 | pmc = 7219297 }}</ref><ref>{{cite journal | vauthors = Jo YS, Heymann G, Zweifel LS | title = Dopamine Neurons Reflect the Uncertainty in Fear Generalization | language = en | journal = Neuron | volume = 100 | issue = 4 | pages = 916–925.e3 | date = November 2018 | pmid = 30318411 | pmc = 6226002 | doi = 10.1016/j.neuron.2018.09.028 }}</ref> | |||
| The anatomy of the basal ganglia is extraordinarily complex, and the role of dopamine there is correspondingly complex. On a macroscopic scale there is only one major dopamine projection, from the ] to the ], but the dopamine inputs contact multiple types of neurons and have several distinct effects on their targets, activating some via D1 receptors while inhibiting others via D2 receptors. A substantial number of dopamine inputs are delivered to the necks of ]s, where they are well-placed to exert a gating effect on specific synaptic connections, often arising from the cerebral cortex. There are two distinct pathways of signal flow arising from the striatum, known as the ] and ]. Dopamine is thought to promote action by upregulating the direct pathway while suppressing the indirect pathway. | |||
| The posterior hypothalamus has dopamine neurons that project to the spinal cord, but their function is not well established.<ref name=Paulus/> There is some evidence that pathology in this area plays a role in restless legs syndrome, a condition in which people have difficulty sleeping due to an overwhelming compulsion to constantly move parts of the body, especially the legs.<ref name=Paulus>{{cite journal | vauthors = Paulus W, Schomburg ED | title = Dopamine and the spinal cord in restless legs syndrome: does spinal cord physiology reveal a basis for augmentation? | journal = Sleep Medicine Reviews | volume = 10 | issue = 3 | pages = 185–96 | date = June 2006 | pmid = 16762808 | doi = 10.1016/j.smrv.2006.01.004 }}</ref> | |||
| The arcuate nucleus and the periventricular nucleus of the hypothalamus have dopamine neurons that form an important projection—the '']'' which goes to the ], where it influences the secretion of the hormone ].<ref name=BenJonathan/> Dopamine is the primary ] inhibitor of the secretion of ] from the ] gland.<ref name=BenJonathan/> Dopamine produced by neurons in the arcuate nucleus is secreted into the ] of the ], which supplies the ].<ref name=BenJonathan/> The ]s that produce prolactin, in the absence of dopamine, secrete prolactin continuously; dopamine inhibits this secretion.<ref name=BenJonathan>{{cite journal | vauthors = Ben-Jonathan N, Hnasko R | title = Dopamine as a prolactin (PRL) inhibitor | journal = Endocrine Reviews | volume = 22 | issue = 6 | pages = 724–63 | date = December 2001 | pmid = 11739329 | doi = 10.1210/er.22.6.724 | doi-access = free }}</ref> | |||
| ==The ventral tegmental area, reward, and cognition== | |||
| The zona incerta, grouped between the arcuate and periventricular nuclei, projects to several areas of the hypothalamus, and participates in the control of ], which is necessary to activate the development of the ] and ]s, following puberty.<ref name=BenJonathan/> | |||
| The ] (VTA) contains the largest group of dopamine neurons in the human brain. They project to numerous brain areas, but the two largest projections are the ], which targets the ] and other ] structures, and the ], which targets the ] and ] parts of the cerebral cortex. | |||
| An additional group of dopamine-secreting neurons is found in the ] of the eye.<ref name=Witkovsky/> These neurons are ], meaning that they have no axons.<ref name=Witkovsky/> They release dopamine into the extracellular medium, and are specifically active during daylight hours, becoming silent at night.<ref name=Witkovsky/> This retinal dopamine acts to enhance the activity of ]s in the retina while suppressing ]s—the result is to increase sensitivity to color and contrast during bright light conditions, at the cost of reduced sensitivity when the light is dim.<ref name=Witkovsky>{{cite journal | vauthors = Witkovsky P | s2cid = 10354133 | title = Dopamine and retinal function | journal = Documenta Ophthalmologica. Advances in Ophthalmology | volume = 108 | issue = 1 | pages = 17–40 | date = January 2004 | pmid = 15104164 | doi = 10.1023/B:DOOP.0000019487.88486.0a | url = https://zenodo.org/record/891239 }}</ref> | |||
| ===Reward=== | |||
| The VTA dopamine system is strongly associated with the ] of the brain. Dopamine is released in areas such as the ] and ] as a result of rewarding experiences such as food, sex, and ] that become ] with them.<ref name="fn5">{{cite journal |author=Arias-Carrión O, Pöppel E |title=Dopamine, learning and reward-seeking behavior |journal=Act Neurobiol Exp |volume=67 |issue=4 |pages=481–488 |year=2007}}</ref> The source of this dopamine is primarily the VTA, although the substantia nigra may also contribute. Electrical ] can itself serve as a potent reward: animals will quickly learn to press a lever if it results in stimulation of dopamine release, and often will continue pressing the lever for a long time, at steadily increasing rates.<ref name="Wise" /> A variety of drugs that increase dopamine levels are intrinsically rewarding and increase the effects of other types of reward.<ref name="Wise">{{cite journal |author=Wise RA |title=Addictive drugs and brain stimulation reward |journal=Annu. Rev. Neurosci. |volume=19 |issue= |pages=319–40 |year=1996 |pmid=8833446 |doi=10.1146/annurev.ne.19.030196.001535 |url=}}</ref> | |||
| ====Basal ganglia==== | |||
| In spite of the overwhelming evidence showing a strong association between dopamine and reward, there has been a great deal of dispute about whether the function of dopamine should be described as reward ''per se'', or as some more complex construct that relates strongly to reward. The difficulty arises mainly from two observations: (1) in addition to being rewarding, dopamine is also arousing — it produces a general increase in movement of all sorts; (2) dopamine release can be caused by events that do not seem to have anything to do with reward, most notably pain. One of the most popular alternatives to the reward theory is the "]" theory, which argues that the function of dopamine is to increase the effects of motivators of all sorts, both positive and negative.<ref name=Schultz>{{cite journal |author=Schultz W |title=Getting formal with dopamine and reward |journal=Neuron |volume=36 |issue=2 |pages=241–263 |year=2002 |pmid=12383780 |doi=10.1016/S0896-6273(02)00967-4}}</ref> | |||
| ]. The dopaminergic pathway from the ] to the ] is shown in light blue.|alt=At the top, a line drawing of a side view of the human brain, with a cross section pulled out showing the basal ganglia structures in color near the center. At the bottom an expanded line drawing of the basal ganglia structures, showing outlines of each structure and broad arrows for their connection pathways.]] | |||
| The largest and most important sources of dopamine in the vertebrate brain are the substantia nigra and ventral tegmental area.<ref name=Bjorklund/> Both structures are components of the midbrain, closely related to each other and functionally similar in many respects.<ref name=Bjorklund/> The largest component of the basal ganglia is the striatum.<ref name=brs>{{cite book |vauthors=Fix JD| title = Neuroanatomy (Board Review Series) |edition=4th |location=Baltimore |publisher=Wulters Kluwer & Lippincott Williams & Wilkins |chapter=Basal Ganglia and the Striatal Motor System |year=2008 |pages=274–81 |isbn=978-0-7817-7245-7}}</ref> The substantia nigra sends a dopaminergic projection to the ], while the ventral tegmental area sends a similar type of dopaminergic projection to the ].<ref name=Bjorklund/> | |||
| A substantial body of evidence suggests that dopamine encodes not reward itself, but rather ''reward prediction error'', that is, the degree to which reward is surprising. According to this hypothesis, which derives initially from recordings made by ], rewards that are expected do not produce any activation of dopamine cells, but rewards that are greater than expected produce a short-lasting increase in dopamine, whereas the omission of an expected reward actually causes dopamine release to drop below its ordinary background level. The "prediction error" hypothesis has drawn particular interest from computational neuroscientists, because an influential computational-learning method known as ] makes heavy use of a signal that encodes prediction error. This confluence of theory and data has led to a fertile interaction between theoretical and empirical neuroscientists.<ref name=Schultz/> | |||
| Progress in understanding the functions of the basal ganglia has been slow.<ref name="brs"/> The most popular hypotheses, broadly stated, propose that the basal ganglia play a central role in ].<ref name=chakravarthy>{{cite journal | vauthors = Chakravarthy VS, Joseph D, Bapi RS | s2cid = 853119 | title = What do the basal ganglia do? A modeling perspective | journal = Biological Cybernetics | volume = 103 | issue = 3 | pages = 237–53 | date = September 2010 | pmid = 20644953 | doi = 10.1007/s00422-010-0401-y | url = https://www.researchgate.net/publication/45276082 }}</ref> The action selection theory in its simplest form proposes that when a person or animal is in a situation where several behaviors are possible, activity in the basal ganglia determines which of them is executed, by releasing that response from inhibition while continuing to inhibit other motor systems that if activated would generate competing behaviors.<ref name=Floresco>{{cite journal | vauthors = Floresco SB | title = The nucleus accumbens: an interface between cognition, emotion, and action | journal = Annual Review of Psychology | volume = 66 | pages = 25–52 | date = January 2015 | pmid = 25251489 | doi = 10.1146/annurev-psych-010213-115159 | s2cid = 28268183 | url = https://www.researchgate.net/publication/266085689 }}</ref> Thus the basal ganglia, in this concept, are responsible for initiating behaviors, but not for determining the details of how they are carried out. In other words, they essentially form a decision-making system.<ref name=Floresco/> | |||
| Recent research finds that while some dopaminergic neurons react in the way expected of reward neurons, others do not and seem to respond in regard to salience, including aversive stimuli.<ref name="Matsumoto">{{cite journal |doi=10.1038/nature08028 |author=Matsumoto M, Hikosaka O. |title=Two types of dopamine neuron distinctly convey positive and negative motivational signals |journal=Nature |volume=459 |issue=7248 |pages=837–41 |year=2009 |pmid=19448610 |pmc=2739096}}</ref> This research finds the reward neurons predominate in the ventromedial region of the ], as well as in the ]. Neurons in these areas project mainly to the ] and thus might transmit value-related information in regard to reward values.<ref name="Matsumoto"/> The salience neurons are predominate in the dorsolateral area of the substantia nigra pars compacta which projects to the dorsal ] and may relate to orienting behaviour.<ref name="Matsumoto"/> It has been suggested that the difference between these two types of dopaminergic neurons arises from their input: reward-linked ones have input from the ], while the salience-related ones from the ].<ref name="Matsumoto"/> In primates, neurons from the regions of both the substantia nigra and VTA project to the prefrontal cortex;<ref name="Williams">{{cite journal |author=Williams SM, Goldman-Rakic PS. |title=Widespread origin of the primate mesofrontal dopamine system |journal=Cereb Cortex. |volume=8 |issue=4 |pages=321–45 |year=1998 |doi=10.1093/cercor/8.4.321 |pmid=9651129}}</ref> the origins of the dopamine innervation of other cortical areas in primate have not been studied. It has been appreciated for many years that exposure to even mild, uncontrollable stress increases dopamine release in the rodent prefrontal cortex, e.g. reviewed in,<ref name="Deutch">{{cite journal |author=Deutch AY, Roth RH. |title=The determinants of stress-induced activation of the prefrontal cortical dopamine system |journal=Prog Brain Res. |volume=85 |pages=367–402 |year=1990 |doi=10.1016/S0079-6123(08)62691-6 |pmid=2094906 |series=Progress in Brain Research |isbn=9780444811240}}</ref> suggesting that dopamine salience cells have a large influence on this cortical region. | |||
| The basal ganglia can be divided into several sectors, and each is involved in controlling particular types of actions.<ref name=Balleine>{{cite journal |vauthors=Balleine BW, Dezfouli A, Ito M, Doya K |s2cid=53148662 |year=2015 |title=Hierarchical control of goal-directed action in the cortical–basal ganglia network |journal=Current Opinion in Behavioral Sciences |volume=5 |pages=1–7 |doi=10.1016/j.cobeha.2015.06.001}}</ref> The ventral sector of the basal ganglia (containing the ventral striatum and ventral tegmental area) operates at the highest level of the hierarchy, selecting actions at the whole-organism level.<ref name=Floresco/> The dorsal sectors (containing the dorsal striatum and substantia nigra) operate at lower levels, selecting the specific muscles and movements that are used to implement a given behavior pattern.<ref name=Balleine/> | |||
| ===="Seeking" versus "liking"==== | |||
| ] and other researchers have argued for a distinction between reward, which is defined in terms of motivation, and pleasure, which is defined in terms of emotional expression. A simpler way of describing this is as a distinction between "seeking" and "liking". "Seeking" occurs when an animal, given access to some stimulus such as food, executes some type of active behavior in order to acquire it. "Liking" occurs when an animal shows expressions of happiness or satisfaction while consuming something. There is considerable evidence that the dopamine system is part of the brain system that mediates seeking but not part of the system that mediates liking. Drugs that increase the effects of dopamine (most notably stimulants such as methamphetamine or cocaine) produce corresponding increases in seeking behaviors, but do not greatly alter expressions of pleasure. Conversely, opiate drugs such as heroin or morphine produce increases in expressions of pleasure but do not greatly alter seeking behaviors. Animals in which the VTA dopamine system has been rendered inactive do not seek food, and will starve to death if left to themselves, but if food is placed in their mouths they will consume it and show facial expressions indicative of pleasure.<!-- | |||
| --><ref name="fn5">{{cite journal |author=Berridge K, Robinson T |title=What is the role of dopamine in reward: hedonic impact, reward learning, or incentive salience? |journal=Brain Res Brain Res Rev |volume=28 |issue=3 |pages=309–69 |year=1998 |pmid=9858756 |doi=10.1016/S0165-0173(98)00019-8}}</ref><!-- | |||
| --> | |||
| Dopamine contributes to the action selection process in at least two important ways. First, it sets the "threshold" for initiating actions.<ref name=chakravarthy/> The higher the level of dopamine activity, the lower the impetus required to evoke a given behavior.<ref name=chakravarthy/> As a consequence, high levels of dopamine lead to high levels of motor activity and ]; low levels of dopamine lead to ] and slowed reactions.<ref name=chakravarthy/> Parkinson's disease, in which dopamine levels in the substantia nigra circuit are greatly reduced, is characterized by stiffness and difficulty initiating movement—however, when people with the disease are confronted with strong stimuli such as a serious threat, their reactions can be as vigorous as those of a healthy person.<ref name=Jankovic/> In the opposite direction, drugs that increase dopamine release, such as cocaine or amphetamine, can produce heightened levels of activity, including, at the extreme, ] and ].<ref name=Patti>{{cite journal | vauthors = Pattij T, Vanderschuren LJ | title = The neuropharmacology of impulsive behaviour | journal = Trends in Pharmacological Sciences | volume = 29 | issue = 4 | pages = 192–99 | date = April 2008 | pmid = 18304658 | doi = 10.1016/j.tips.2008.01.002 | url = https://www.researchgate.net/publication/5547125 }}</ref> | |||
| ===Role in cognition=== | |||
| Dopamine's effects on higher cognitive function have been studied in monkeys and rodents. This work began with the landmark study of Brozoski et al., 1979 showing that depletion of catecholamines from the dorsolateral prefrontal cortex in monkeys impaired spatial working memory to the same degree as removing the cortex itself.<ref name="Brozoski">{{cite journal |author=Brozoski TJ, Brown RM, Rosvold HE, Goldman PS. |title=Cognitive deficit caused by regional depletion of dopamine in prefrontal cortex of rhesus monkey |journal=Science |volume=205 |issue=4409 |pages=929–32 |year=1979 |bibcode=1979Sci...205..929B |doi=10.1126/science.112679 |pmid=112679}}</ref> It is now known that both dopamine and norepinephrine have essential actions on prefrontal cortical function, and help coordinate cognitive state with arousal state.<ref name="Arnsten">{{cite journal |author=Arnsten AF, Wang MJ, Paspalas CD. |title=Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses |journal=Neuron |volume=76 |issue=1 |pages=223–39 |year=2012 |doi=10.1016/j.neuron.2012.08.038 |pmid=23040817 |pmc=3488343}}</ref> Dopamine has an "inverted U" influence on prefrontal function through its actions on D1 receptors, where either too little or too much impairs working memory function.<ref name="Vijayraghavan">{{cite journal |author=Vijayraghavan S, Wang M, Birnbaum SG, Williams GV, Arnsten AF. |title=Inverted-U dopamine D1 receptor actions on prefrontal neurons engaged in working memory |journal=Nat Neurosci. |volume=10 |issue=3 |pages=376–84 |year=2007 |doi=10.1038/nn1846 |pmid=17277774}}</ref> In the primate prefrontal cortex, dopamine D1 receptor stimulation selectively influences the firing of "Delay" cells (also called "Memory" cells), while dopamine D2 receptors selectively alter the firing of "Response cells".<ref name="Wang">{{cite journal |author1=Wang M, Vijayraghavan S, Goldman-Rakic PS. |title=Selective D2 receptor actions on the functional circuitry of working memory |journal=Science |volume=303 |issue=5659 |pages=853–6 |year=2004 |bibcode=2004Sci...303..853W |doi=10.1126/science.1091162 |pmid=14764884}}</ref> | |||
| The second important effect of dopamine is as a "teaching" signal.<ref name=chakravarthy/> When an action is followed by an increase in dopamine activity, the basal ganglia circuit is altered in a way that makes the same response easier to evoke when similar situations arise in the future.<ref name=chakravarthy/> This is a form of ], in which dopamine plays the role of a reward signal.<ref name=Floresco/> | |||
| ==Diseases and disorders== | |||
| ====Reward==== | |||
| The dopamine system plays a central role in a number of important medical conditions, including Parkinson's disease, attention deficit hyperactivity disorder, schizophrenia, and drug addiction. | |||
| ] | |||
| In the language used to discuss the reward system, ''reward'' is the attractive and motivational property of a stimulus that induces ] (also known as approach behavior) and ].<ref name=Schultz /> A rewarding stimulus is one that can induce the organism to approach it and choose to consume it.<ref name=Schultz /> ], ] (e.g., ] and ]), and approach behavior are the three main functions of reward.<ref name=Schultz /> As an aspect of reward, ''pleasure'' provides a definition of reward;<ref name=Schultz /> however, while all pleasurable stimuli are rewarding, not all rewarding stimuli are pleasurable (e.g., extrinsic rewards like money).<ref name=Schultz /><ref name=Robinson>{{cite journal | vauthors = Robinson TE, Berridge KC | s2cid = 13471436 | title = The neural basis of drug craving: an incentive-sensitization theory of addiction | journal = Brain Research. Brain Research Reviews | volume = 18 | issue = 3 | pages = 247–91 | year = 1993 | pmid = 8401595 | doi = 10.1016/0165-0173(93)90013-p | hdl = 2027.42/30601 | hdl-access = free }}</ref> The motivational or desirable aspect of rewarding stimuli is reflected by the approach behavior that they induce, whereas the pleasure from intrinsic rewards results from consuming them after acquiring them.<ref name=Schultz /> A neuropsychological model which distinguishes these two components of an intrinsically rewarding stimulus is the ] model, where "wanting" or desire (less commonly, "seeking"<ref name=Wright>{{cite journal | vauthors = Wright JS, Panksepp J | s2cid = 145747459 | year = 2012 | title = An evolutionary framework to understand foraging, wanting, and desire: the neuropsychology of the SEEKING system | journal = Neuropsychoanalysis | volume = 14 | issue = 1 | pages = 5–39 | doi = 10.1080/15294145.2012.10773683 | url = https://www.researchgate.net/publication/233748400 |access-date=24 September 2015}}</ref>) corresponds to appetitive or approach behavior while "liking" or pleasure corresponds to consummatory behavior.<ref name=Schultz /><ref name="NAcc function" /><ref name="Berridge2">{{cite journal | vauthors = Berridge KC, Robinson TE, Aldridge JW | title = Dissecting components of reward: 'liking', 'wanting', and learning | journal = Current Opinion in Pharmacology | volume = 9 | issue = 1 | pages = 65–73 | date = February 2009 | pmid = 19162544 | pmc = 2756052 | doi = 10.1016/j.coph.2008.12.014 | quote = <!-- Conversely, amplification of 'wanting' without 'liking' has been produced by the activation of dopamine systems by amphetamine or similar catecholamine-activating drugs given systemically or microinjected directly into the nucleus accumbens, or by genetic mutation that raises extracellular levels of dopamine (via knockdown of dopamine transporters in the synapse) in mesocorticolimbic circuits, and by the near-permanent sensitization of mesocorticolimbic-dopamine-related systems by repeated administration of high-doses of addictive drugs (Figure 3–Figure 5) . We have proposed that in susceptible individuals the neural sensitization of incentive salience by drugs of abuse may generate compulsive 'wanting' to take more drugs, whether or not the same drugs are correspondingly 'liked', and thus contribute to addiction (Figure 5).<br /> --> }}</ref> In human ], "wanting" becomes dissociated with "liking" as the desire to use an addictive drug increases, while the pleasure obtained from consuming it decreases due to ].<ref name="NAcc function" /> | |||
| Within the brain, dopamine functions partly as a global reward signal. An initial dopamine response to a rewarding stimulus encodes information about the ], value, and context of a reward.<ref name=Schultz /> In the context of reward-related learning, dopamine also functions as a ''reward prediction error'' signal, that is, the degree to which the value of a reward is unexpected.<ref name=Schultz/> According to this hypothesis proposed by Montague, Dayan, and Sejnowski,<ref>{{cite journal | vauthors = Montague PR, Dayan P, Sejnowski TJ | title = A framework for mesencephalic dopamine systems based on predictive Hebbian learning | journal = The Journal of Neuroscience | volume = 16 | issue = 5 | pages = 1936–47 | date = March 1996 | pmid = 8774460 | pmc = 6578666 | doi = 10.1523/JNEUROSCI.16-05-01936.1996 | doi-access = free }}</ref> rewards that are expected do not produce a second phasic dopamine response in certain dopaminergic cells, but rewards that are unexpected, or greater than expected, produce a short-lasting increase in synaptic dopamine, whereas the omission of an expected reward actually causes dopamine release to drop below its background level.<ref name=Schultz/> The "prediction error" hypothesis has drawn particular interest from computational neuroscientists, because an influential computational-learning method known as ] makes heavy use of a signal that encodes prediction error.<ref name=Schultz/> This confluence of theory and data has led to a fertile interaction between neuroscientists and computer scientists interested in ].<ref name=Schultz/> | |||
| ===Parkinson's disease=== | |||
| Evidence from ] recordings from the brains of animals shows that dopamine neurons in the ventral tegmental area (VTA) and substantia nigra are strongly activated by a wide variety of rewarding events.<ref name="Schultz">{{cite journal | vauthors = Schultz W | title = Neuronal Reward and Decision Signals: From Theories to Data | journal = Physiological Reviews | volume = 95 | issue = 3 | pages = 853–951 | date = July 2015 | pmid = 26109341 | pmc = 4491543 | doi = 10.1152/physrev.00023.2014 | quote = <!-- Rewards are crucial objects that induce learning, approach behavior, choices, and emotions. Whereas emotions are difficult to investigate in animals, the learning function is mediated by neuronal reward prediction error signals which implement basic constructs of reinforcement learning theory. These signals are found in dopamine neurons, which emit a global reward signal to striatum and frontal cortex, and in specific neurons in striatum, amygdala, and frontal cortex projecting to select neuronal populations ... Figure 12. Reward components inducing the two phasic dopamine response components. The initial component (blue) detects the event before having identified its value. It increases with sensory impact (physical salience), novelty (novelty/surprise salience), generalization to rewarded stimuli, and reward context. This component is coded as temporal event prediction error (389). The second component (red) codes reward value (as reward prediction error) ... The salience of rewards derives from three principal factors, namely, their physical intensity and impact (physical salience), their novelty and surprise (novelty/surprise salience), and their general motivational impact shared with punishers (motivational salience). A separate form not included in this scheme, incentive salience, primarily addresses dopamine function in addiction and refers only to approach behavior (as opposed to learning) --> }}</ref> These reward-responsive dopamine neurons in the VTA and substantia nigra are crucial for reward-related cognition and serve as the central component of the reward system.<ref name="NAcc function">{{cite book |title=Molecular Neuropharmacology: A Foundation for Clinical Neuroscience |vauthors=Malenka RC, Nestler EJ, Hyman SE |publisher=McGraw-Hill Medical |year=2009 |isbn=978-0-07-148127-4 |veditors=Sydor A, Brown RY |edition=2nd |location=New York |pages=147–48, 366–67, 375–76 |language=en-US |quote=<!-- VTA DA neurons play a critical role in motivation, reward-related behavior (Chapter 15), attention, and multiple forms of memory. This organization of the DA system, wide projection from a limited number of cell bodies, permits coordinated responses to potent new rewards. Thus, acting in diverse terminal fields, dopamine confers motivational salience ("wanting") on the reward itself or associated cues (nucleus accumbens shell region), updates the value placed on different goals in light of this new experience (orbital prefrontal cortex), helps consolidate multiple forms of memory (amygdala and hippocampus), and encodes new motor programs that will facilitate obtaining this reward in the future (nucleus accumbens core region and dorsal striatum). In this example, dopamine modulates the processing of sensorimotor information in diverse neural circuits to maximize the ability of the organism to obtain future rewards. ...<br />The brain reward circuitry that is targeted by addictive drugs normally mediates the pleasure and strengthening of behaviors associated with natural reinforcers, such as food, water, and sexual contact. Dopamine neurons in the VTA are activated by food and water, and dopamine release in the NAc is stimulated by the presence of natural reinforcers, such as food, water, or a sexual partner. ...<br />The NAc and VTA are central components of the circuitry underlying reward and memory of reward. As previously mentioned, the activity of dopaminergic neurons in the VTA appears to be linked to reward prediction. The NAc is involved in learning associated with reinforcement and the modulation of motoric responses to stimuli that satisfy internal homeostatic needs. The shell of the NAc appears to be particularly important to initial drug actions within reward circuitry; addictive drugs appear to have a greater effect on dopamine release in the shell than in the core of the NAc. ... If motivational drive is described in terms of wanting, and hedonic evaluation in terms of liking, it appears that wanting can be dissociated from liking and that dopamine may influence these phenomena differently. Differences between wanting and liking are confirmed in reports by human addicts, who state that their desire for drugs (wanting) increases with continued use even when pleasure (liking) decreases because of tolerance. --><!-- ... Addictive drugs are rewarding and reinforcing because they act in brain reward pathways to enhance either dopamine release or the effects of dopamine in the NAc or related structures, or because they produce effects similar to dopamine. -->}}</ref><ref name="Hikosaka">{{cite journal | vauthors = Bromberg-Martin ES, Matsumoto M, Hikosaka O | title = Dopamine in motivational control: rewarding, aversive, and alerting | journal = Neuron | volume = 68 | issue = 5 | pages = 815–34 | date = December 2010 | pmid = 21144997 | pmc = 3032992 | doi = 10.1016/j.neuron.2010.11.022 }}</ref><ref name="Striatum">{{cite journal | vauthors = Yager LM, Garcia AF, Wunsch AM, Ferguson SM | title = The ins and outs of the striatum: Role in drug addiction | journal = Neuroscience | volume = 301 | pages = 529–41 | date = August 2015 | pmid = 26116518 | doi = 10.1016/j.neuroscience.2015.06.033 | pmc=4523218}}</ref> The function of dopamine varies in each ] from the VTA and substantia nigra;<ref name="NAcc function" /> for example, the VTA–] projection assigns incentive salience ("want") to rewarding stimuli and its associated ], the VTA–] projection updates the value of different goals in accordance with their incentive salience, the VTA–amygdala and VTA–hippocampus projections mediate the consolidation of reward-related memories, and both the VTA–] and substantia nigra–dorsal striatum pathways are involved in learning motor responses that facilitate the acquisition of rewarding stimuli.<ref name="NAcc function" /><ref name="NAcc core and shell">{{cite journal | vauthors = Saddoris MP, Cacciapaglia F, Wightman RM, Carelli RM | title = Differential Dopamine Release Dynamics in the Nucleus Accumbens Core and Shell Reveal Complementary Signals for Error Prediction and Incentive Motivation | journal = The Journal of Neuroscience | volume = 35 | issue = 33 | pages = 11572–82 | date = August 2015 | pmid = 26290234 | pmc = 4540796 | doi = 10.1523/JNEUROSCI.2344-15.2015 | quote = <!-- Here, we have found that real-time dopamine release within the nucleus accumbens (a primary target of midbrain dopamine neurons) strikingly varies between core and shell subregions. In the core, dopamine dynamics are consistent with learning-based theories (such as reward prediction error) whereas in the shell, dopamine is consistent with motivation-based theories (e.g., incentive salience). --> }}</ref> Some activity within the VTA dopaminergic projections appears to be associated with reward prediction as well.<ref name="NAcc function" /><ref name="NAcc core and shell" /> | |||
| ] is a disorder characterized by stiffness of the body, slowing of movement, and trembling of limbs when they are not in use. In advanced stages it progresses to dementia and eventually death. The main symptoms are caused by massive loss of dopamine-secreting cells in the ]. These dopamine cells are especially vulnerable to damage, and a variety of insults, including ] (as depicted in the book and movie "]"), repeated sports-related ]s, and some forms of chemical poisoning (ex. ]), can lead to substantial cell loss, producing a ] that is similar in its main features to Parkinson's disease. Most cases of Parkinson's disease, however, are "idiopathic", meaning that the cause of cell death cannot be identified. | |||
| ====Pleasure==== | |||
| The most widely used treatment for Parkinsonism is administration of ], the metabolic precursor for dopamine. This treatment cannot restore the dopamine cells that have been lost, but it causes the remaining cells to produce more dopamine, thereby compensating for the loss to at least some degree. In advanced stages the treatment begins to fail because the cell loss is so severe that the remaining ones cannot produce enough dopamine regardless of L-DOPA levels. As this stage is approached, the metabolic regulatory mechanisms in the dopamine cells, operating far above their normal level, become erratic, producing ], in which patients fluctuate unpredictably between states of hyperactivity and paralysis.<ref name="pmid17988927">{{cite journal |author=Merims D, Giladi N |title=Dopamine dysregulation syndrome, addiction and behavioral changes in Parkinson's disease |journal=Parkinsonism & Related Disorders |volume=14 |issue=4 |pages=273–80 |year=2008 |pmid=17988927 |doi=10.1016/j.parkreldis.2007.09.007 |url=http://linkinghub.elsevier.com/retrieve/pii/S1353-8020(07)00208-8}}</ref> | |||
| While dopamine has a central role in causing "wanting," associated with the appetitive or approach behavioral responses to rewarding stimuli, detailed studies have shown that dopamine cannot simply be equated with hedonic "liking" or pleasure, as reflected in the consummatory behavioral response.<ref name=Robinson/> Dopamine neurotransmission is involved in some but not all aspects of pleasure-related cognition, since ]s have been identified both within the dopamine system (i.e., nucleus accumbens shell) and outside the dopamine system (i.e., ] and ]).<ref name=Robinson/><ref name=Berridge2/><ref name="Pleasure system">{{cite journal | vauthors = Berridge KC, Kringelbach ML | title = Pleasure systems in the brain | journal = Neuron | volume = 86 | issue = 3 | pages = 646–64 | date = May 2015 | pmid = 25950633 | pmc = 4425246 | doi = 10.1016/j.neuron.2015.02.018 }}</ref> For example, ] of dopamine pathways, using electrodes implanted in the brain, is experienced as pleasurable, and many types of animals are willing to work to obtain it.<ref name=Wise/> ]s reduce dopamine levels and tend to cause ], a diminished ability to experience pleasure.<ref name="Wise2">{{cite journal | vauthors = Wise RA | title = Dopamine and reward: the anhedonia hypothesis 30 years on | journal = Neurotoxicity Research | volume = 14 | issue = 2–3 | pages = 169–83 | date = October 2008 | pmid = 19073424 | pmc = 3155128 | doi = 10.1007/BF03033808 }}</ref> Many types of pleasurable experiences—such as sexual intercourse, eating, and playing video games—increase dopamine release.<ref name="fn5">{{cite journal |vauthors=Arias-Carrión O, Pöppel E |title=Dopamine, learning and reward-seeking behavior |journal=Acta Neurobiol Exp |volume=67 |issue=4 |pages=481–88 |year=2007|doi=10.55782/ane-2007-1664 |pmid=18320725 |doi-access=free }}</ref> All addictive drugs directly or indirectly affect dopamine neurotransmission in the nucleus accumbens;<ref name="NAcc function" /><ref name=Wise/> these drugs increase drug "wanting", leading to compulsive drug use, when repeatedly taken in high doses, presumably through the ].<ref name=Berridge2 /> Drugs that increase synaptic dopamine concentrations include ]s such as methamphetamine and cocaine. These produce increases in "wanting" behaviors, but do not greatly alter expressions of pleasure or change levels of satiation.<ref name=Berridge2/><ref name=Wise>{{cite journal | vauthors = Wise RA | title = Addictive drugs and brain stimulation reward | journal = Annual Review of Neuroscience | volume = 19 | pages = 319–40 | year = 1996 | pmid = 8833446 | doi = 10.1146/annurev.ne.19.030196.001535 }}</ref> However, ] drugs such as heroin and morphine produce increases in expressions of "liking" and "wanting" behaviors.<ref name=Berridge2/> Moreover, animals in which the ventral tegmental dopamine system has been rendered inactive do not seek food, and will starve to death if left to themselves, but if food is placed in their mouths they will consume it and show expressions indicative of pleasure.<ref>{{cite journal | vauthors = Ikemoto S | title = Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex | journal = Brain Research Reviews | volume = 56 | issue = 1 | pages = 27–78 | date = November 2007 | pmid = 17574681 | pmc = 2134972 | doi = 10.1016/j.brainresrev.2007.05.004 }}</ref> | |||
| A clinical study from January 2019 that assessed the effect of a dopamine precursor (]), dopamine antagonist (]), and a placebo on reward responses to music – including the degree of pleasure experienced during ]s, as measured by changes in ] as well as subjective ratings – found that the manipulation of dopamine neurotransmission bidirectionally regulates pleasure cognition (specifically, the ]) in human subjects.<ref name="Dopaminergic control of hedonic impact" /><ref name="Secondary source for 'Dopaminergic control of hedonic impact'" /> This research demonstrated that increased dopamine neurotransmission acts as a '']'' condition for pleasurable hedonic reactions to music in humans.<ref name="Dopaminergic control of hedonic impact">{{cite journal | vauthors = Ferreri L, Mas-Herrero E, Zatorre RJ, Ripollés P, Gomez-Andres A, Alicart H, Olivé G, Marco-Pallarés J, Antonijoan RM, Valle M, Riba J, Rodriguez-Fornells A | title = Dopamine modulates the reward experiences elicited by music | journal = Proceedings of the National Academy of Sciences of the United States of America | year = 2019 | volume = 116| issue = 9| pages = 3793–98 | pmid = 30670642 | pmc = 6397525 | doi = 10.1073/pnas.1811878116 | bibcode = 2019PNAS..116.3793F | quote = Listening to pleasurable music is often accompanied by measurable bodily reactions such as goose bumps or shivers down the spine, commonly called "chills" or "frissons." ... Overall, our results straightforwardly revealed that pharmacological interventions bidirectionally modulated the reward responses elicited by music. In particular, we found that risperidone impaired participants' ability to experience musical pleasure, whereas levodopa enhanced it. ... Here, in contrast, studying responses to abstract rewards in human subjects, we show that manipulation of dopaminergic transmission affects both the pleasure (i.e., amount of time reporting chills and emotional arousal measured by EDA) and the motivational components of musical reward (money willing to spend). These findings suggest that dopaminergic signaling is a sine qua non condition not only for motivational responses, as has been shown with primary and secondary rewards, but also for hedonic reactions to music. This result supports recent findings showing that dopamine also mediates the perceived pleasantness attained by other types of abstract rewards (37) and challenges previous findings in animal models on primary rewards, such as food (42, 43).|doi-access = free }}</ref><ref name="Secondary source for 'Dopaminergic control of hedonic impact'">{{cite journal | vauthors = Goupil L, Aucouturier JJ | title = Musical pleasure and musical emotions | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 116 | issue = 9 | pages = 3364–66 | date = February 2019 | pmid = 30770455 | pmc = 6397567 | doi = 10.1073/pnas.1900369116 | bibcode = 2019PNAS..116.3364G | quote = In a pharmacological study published in PNAS, Ferreri et al. (1) present evidence that enhancing or inhibiting dopamine signaling using levodopa or risperidone modulates the pleasure experienced while listening to music. ... In a final salvo to establish not only the correlational but also the causal implication of dopamine in musical pleasure, the authors have turned to directly manipulating dopaminergic signaling in the striatum, first by applying excitatory and inhibitory transcranial magnetic stimulation over their participants' left dorsolateral prefrontal cortex, a region known to modulate striatal function (5), and finally, in the current study, by administrating pharmaceutical agents able to alter dopamine synaptic availability (1), both of which influenced perceived pleasure, physiological measures of arousal, and the monetary value assigned to music in the predicted direction. ... While the question of the musical expression of emotion has a long history of investigation, including in PNAS (6), and the 1990s psychophysiological strand of research had already established that musical pleasure could activate the autonomic nervous system (7), the authors' demonstration of the implication of the reward system in musical emotions was taken as inaugural proof that these were veridical emotions whose study has full legitimacy to inform the neurobiology of our everyday cognitive, social, and affective functions (8). Incidentally, this line of work, culminating in the article by Ferreri et al. (1), has plausibly done more to attract research funding for the field of music sciences than any other in this community.<br />The evidence of Ferreri et al. (1) provides the latest support for a compelling neurobiological model in which musical pleasure arises from the interaction of ancient reward/valuation systems (striatal–limbic–paralimbic) with more phylogenetically advanced perception/predictions systems (temporofrontal).| doi-access = free }}</ref> | |||
| ===Attention deficit hyperactivity disorder=== | |||
| A study published in Nature in 1998 found evidence that playing video games releases dopamine in the human striatum. This dopamine is associated with learning, behavior reinforcement, attention, and ] integration.<ref>{{cite journal | vauthors = Koepp MJ, Gunn RN, Lawrence AD, Cunningham VJ, Dagher A, Jones T, Brooks DJ, Bench CJ, Grasby PM | title = Evidence for striatal dopamine release during a video game | journal = Nature | volume = 393 | issue = 6682 | pages = 266–268 | date = May 1998 | pmid = 9607763 | doi = 10.1038/30498 | bibcode = 1998Natur.393..266K | s2cid = 205000565 }}</ref> Researchers used ] scans and <sup>11</sup>C-labelled ] to track dopamine levels in the brain during goal-directed motor tasks and found that dopamine release was positively correlated with task performance and was greatest in the ]. This was the first study to demonstrate the behavioral conditions under which dopamine is released in humans. It highlights the ability of positron emission tomography to detect ] fluxes during changes in behavior. According to research, potentially problematic video game use is related to personality traits such as low self-esteem and low self-efficacy, anxiety, aggression, and clinical symptoms of depression and anxiety disorders.<ref>{{cite journal | vauthors = von der Heiden JM, Braun B, Müller KW, Egloff B | title = The Association Between Video Gaming and Psychological Functioning | journal = Frontiers in Psychology | volume = 10 | pages = 1731 | year = 2019 | pmid = 31402891 | pmc = 6676913 | doi = 10.3389/fpsyg.2019.01731 | doi-access = free }}</ref> Additionally, the reasons individuals play video games vary and may include ], ], and personal satisfaction. The ] defines Internet Gaming Disorder as a mental disorder closely related to Gambling Disorder. This has been supported by some researchers but has also caused controversy. | |||
| Altered dopamine neurotransmission is implicated in ] (ADHD), a condition associated with impaired ability to regulate attention, behavior, and/or impulses. There are some genetic links between dopamine receptors, the dopamine transporter and ADHD,<ref>{{cite journal |authors=Wu J, Xiao H, Sun H, Zou L, Zhu LQ. |title=Role of dopamine receptors in ADHD: a systematic meta-analysis |journal=Mol Neurobiol. |volume=45 |year=2012 |pages=605-20}}</ref> in addition to links to other neurotransmitter receptors and transporters. The most important relationship between dopamine and ADHD involves the drugs that are used to treat ADHD. Some of the most effective therapeutic agents for ADHD are psychostimulants such as ] (Ritalin) and ], drugs that increase both dopamine and norepinephrine levels in brain.<ref>{{cite journal |authors=Berridge CW, Devilbiss DM. |title=Psychostimulants as cognitive enhancers: the prefrontal cortex, catecholamines, and attention-deficit/hyperactivity disorder |journal=Biol Psychiatry |volume=69 |issue=12 |year=2011 |pages=e101-11}}</ref> | |||
| ===Outside the central nervous system=== | |||
| ===Drug addiction=== | |||
| Dopamine does not cross the blood–brain barrier, so its synthesis and functions in peripheral areas are to a large degree independent of its synthesis and functions in the brain.<ref name="Nice-pharma"/> A substantial amount of dopamine circulates in the bloodstream, but its functions there are not entirely clear.<ref name=Eisenhofer/> Dopamine is found in blood plasma at levels comparable to those of epinephrine, but in humans, over 95% of the dopamine in the plasma is in the form of dopamine ], a conjugate produced by the enzyme ] acting on free dopamine.<ref name=Eisenhofer/> The bulk of this dopamine sulfate is produced in the mesenteric organs.<ref name=Eisenhofer/> The production of dopamine sulfate is thought to be a mechanism for detoxifying dopamine that is ingested as food or produced by the digestive process—levels in the plasma typically rise more than fifty-fold after a meal.<ref name=Eisenhofer/> Dopamine sulfate has no known biological functions and is excreted in urine.<ref name=Eisenhofer/> | |||
| A variety of addictive drugs produce an increase in reward-related dopamine activity. For some addictive drugs such as alcohol or ], activation of the reward system may play only a minor role in addiction, with suppression of suffering being the dominant mechanism, but for other drugs, including ] and ] such as ] and ], increased postsynaptic dopamine receptor activation or increased levels of synaptic dopamine appear to be the primary factor. When people addicted to stimulants go through withdrawal, they do not experience the physical suffering associated with withdrawal from alcohol or opiates; instead they experience apathy, boredom, restlessness, and most importantly an overwhelming urge to consume more of the drug.{{mcn|date=January 2014}} | |||
| The relatively small quantity of unconjugated dopamine in the bloodstream may be produced by the ], the digestive system, or possibly other organs.<ref name=Eisenhofer/> It may act on dopamine receptors in peripheral tissues, or be metabolized, or be converted to norepinephrine by the enzyme ], which is released into the bloodstream by the adrenal medulla.<ref name=Eisenhofer/> Some dopamine receptors are located in the walls of arteries, where they act as a ] and an inhibitor of norepinephrine release from postganglionic sympathetic nerves terminals (dopamine can inhibit norepinephrine release by acting on presynaptic dopamine receptors, and also on presynaptic α-1 receptors, like norepinephrine itself).<ref name=Missale>{{cite journal | vauthors = Missale C, Nash SR, Robinson SW, Jaber M, Caron MG | s2cid = 223462 | title = Dopamine receptors: from structure to function | journal = Physiological Reviews | volume = 78 | issue = 1 | pages = 189–225 | date = January 1998 | pmid = 9457173 | doi = 10.1152/physrev.1998.78.1.189 | url = http://pdfs.semanticscholar.org/a34e/3fc62c3aed64ad85dbaf99a0986b6484225c.pdf | archive-url = https://web.archive.org/web/20190302122655/http://pdfs.semanticscholar.org/a34e/3fc62c3aed64ad85dbaf99a0986b6484225c.pdf | url-status = dead | archive-date = 2019-03-02 }}</ref> These responses might be activated by dopamine released from the ] under conditions of low oxygen, but whether arterial dopamine receptors perform other biologically useful functions is not known.<ref name=Missale/> | |||
| The addiction potential for stimulants is strongly dependent on the level of dopamine increase they produce.{{mcn|date=January 2014}} | |||
| Beyond its role in modulating blood flow, there are several peripheral systems in which dopamine circulates within a limited area and performs an ] or ] function.<ref name=Eisenhofer/> The peripheral systems in which dopamine plays an important role include the ], the ]s and the ]. | |||
| Treatment of stimulant addiction is very difficult, because even if consumption ceases, the "craving" that comes with psychological withdrawal does not. Even still, when the craving seems to be extinct, it may reemerge when the individual experiences environmental stiumli (friends, locations, situations, etc.) that are associated with the drug. The brain mechanisms underlying these cravings have been a topic of extensive research. There is evidence that they are associated with long-lasting changes in the density of dopamine receptors in parts of the brain.{{mcn|date=September 2013}} | |||
| === |
====Immune system==== | ||
| Dopamine has been demonstrated to play a role in ] processing in multiple levels of the ] including the ], ] (PAG), ], ], and ]. Accordingly, decreased levels of dopamine have been associated with painful symptoms that frequently occur in ]. Abnormalities in dopaminergic neurotransmission have also been demonstrated in painful clinical conditions, including ],<ref>{{cite journal |doi=10.1016/S0304-3959(00)00409-7 |last1=Jääskeläinen |first1=SK |last2=Rinne |first2=JO |last3=Forssell |first3=H |last4=Tenovuo |first4=O |last5=Kaasinen |first5=V |last6=Sonninen |first6=P |last7=Bergman |first7=J. |year=2001 |title=Role of the dopaminergic system in chronic pain -- a fluorodopa-PET study |url= |journal=Pain |volume=90 |issue=3 |pages=257–60 |pmid=11207397}}</ref> ], and ]. In general, the analgesic capacity of dopamine occurs as a result of dopamine D2 receptor activation; however, exceptions to this exist in the PAG, in which dopamine D1 receptor activation attenuates pain presumably ''via'' activation of neurons involved in descending inhibition.<ref>{{cite journal |doi=10.1586/14737175.8.5.781 |last1=Wood |first1=PB. |year=2008 |title=Role of central dopamine in pain and analgesia |url= |journal=Expert Rev Neurother |volume=8 |issue=5 |pages=781–97 |pmid=18457535}}</ref> In addition, D1 receptor activation in the insular cortex appears to attenuate subsequent pain-related behavior. | |||
| In the immune system dopamine acts upon receptors present on immune cells, especially ]s.<ref name="Buttarelli">{{cite journal | vauthors = Buttarelli FR, Fanciulli A, Pellicano C, Pontieri FE | title = The dopaminergic system in peripheral blood lymphocytes: from physiology to pharmacology and potential applications to neuropsychiatric disorders | journal = Current Neuropharmacology | volume = 9 | issue = 2 | pages = 278–88 | date = June 2011 | pmid = 22131937 | pmc = 3131719 | doi = 10.2174/157015911795596612 }}</ref> Dopamine can also affect immune cells in the ], ], and ].<ref name="pmid19896530"/> In addition, dopamine can be synthesized and released by immune cells themselves.<ref name=Buttarelli/> The main effect of dopamine on lymphocytes is to reduce their activation level. The functional significance of this system is unclear, but it affords a possible route for interactions between the nervous system and immune system, and may be relevant to some autoimmune disorders.<ref name="pmid19896530">{{cite journal | vauthors = Sarkar C, Basu B, Chakroborty D, Dasgupta PS, Basu S | title = The immunoregulatory role of dopamine: an update | journal = Brain, Behavior, and Immunity | volume = 24 | issue = 4 | pages = 525–28 | date = May 2010 | pmid = 19896530 | pmc = 2856781 | doi = 10.1016/j.bbi.2009.10.015 }}</ref> | |||
| ===Nausea=== | |||
| Nausea and vomiting are largely determined by activity in a brainstem area known as the ]. This area contains a large population of type D2 dopamine receptors. Consequently, drugs that activate D2 receptors have a high potential to cause nausea. This group includes some medications that are administered for ], as well as other dopamine agonists such as ]. In many cases, D2-receptor antagonists such as ] are useful as anti-nausea drugs. | |||
| === |
====Kidneys==== | ||
| {{Main|Dopamine hypothesis of schizophrenia}} | |||
| Abnormally high dopaminergic transmission has been linked to ] and ].<ref>{{cite web |publisher=St. Jude Children's Research Hospital |title=Disruption of gene interaction linked to schizophrenia |accessdate=6 July 2006 |url=http://www.innovations-report.com/html/reports/life_sciences/report-52499.html}}</ref> However, clinical studies relating schizophrenia to brain dopamine metabolism have ranged from controversial to negative, with HVA levels in the CSF the same for schizophrenics and controls.<ref>{{cite journal |last1=Maas |first1=J.W. |author2=Bowden CL, Miller AL, Javors MA, Funderburg LG, Berman N, Weintraub ST. |title=Schizophrenia, psychosis, and cerebral spinal fluid homovanillic acid concentrations |journal=Schizophrenia Bulletin. |volume=23 |issue=1 |pages=147–154 |year=1997 |pmid=9050120 |doi=10.1093/schbul/23.1.147}}</ref> Increased dopaminergic functional activity, specifically in the ], is found in schizophrenic individuals. However, decreased activity in another dopaminergic pathway, the ], may also be involved. The two pathways are thought to be responsible for differing sets of symptoms seen in schizophrenia.{{Citation needed|date=June 2011}} | |||
| The renal dopaminergic system is located in the cells of the ] in the kidney, where all subtypes of dopamine receptors are present.<ref>{{cite journal | vauthors = Hussain T, Lokhandwala MF | s2cid = 10896819 | title = Renal dopamine receptors and hypertension | journal = Experimental Biology and Medicine | volume = 228 | issue = 2 | pages = 134–42 | date = February 2003 | pmid = 12563019 | doi = 10.1177/153537020322800202 }}<!--|access-date=15 January 2016--></ref> Dopamine is also synthesized there, by ] cells, and discharged into the ]. Its actions include increasing the blood supply to the kidneys, increasing the ], and increasing the excretion of sodium in the urine. Hence, defects in renal dopamine function can lead to reduced sodium excretion and consequently result in the development of ]. There is strong evidence that faults in the production of dopamine or in the receptors can result in a number of pathologies including ], ], and either genetic or essential hypertension. Oxidative stress can itself cause hypertension.<ref>{{cite journal | vauthors = Choi MR, Kouyoumdzian NM, Rukavina Mikusic NL, Kravetz MC, Rosón MI, Rodríguez Fermepin M, Fernández BE | title = Renal dopaminergic system: Pathophysiological implications and clinical perspectives | journal = World Journal of Nephrology | volume = 4 | issue = 2 | pages = 196–212 | date = May 2015 | pmid = 25949933 | pmc = 4419129 | doi = 10.5527/wjn.v4.i2.196 | doi-access = free }}</ref> Defects in the system can also be caused by genetic factors or high blood pressure.<ref name="pmid11566894">{{cite journal | vauthors = Carey RM | title = Theodore Cooper Lecture: Renal dopamine system: paracrine regulator of sodium homeostasis and blood pressure | journal = Hypertension | volume = 38 | issue = 3 | pages = 297–302 | date = September 2001 | pmid = 11566894 | doi = 10.1161/hy0901.096422 | doi-access = free }}</ref> | |||
| ] ]s act largely as dopamine antagonists, inhibiting dopamine at the ] level, and thereby blocking the effects of the neurochemical in a dose-dependent manner. The older, so-called ] most commonly act on D2 receptors,<ref>http://www.williams.edu/imput/synapse/pages/IIIB5.htm</ref> while the ] also act on D1, D3 and D4 receptors, though they have a lower affinity for dopamine receptors in general.<ref>http://bjp.rcpsych.org/cgi/content/full/181/4/271</ref><ref>{{cite journal |last1=Durcan |first1=M |last2=Rigdon |first2=GC |last3=Norman |first3=MH |last4=Morgan |first4=PF |title=Is clozapine selective for the dopamine D4 receptor? |journal=Life Sciences |volume=57 |issue=18 |pages=PL275–83 |year=1995 |pmid=7475902 |doi=10.1016/0024-3205(95)02151-8}}</ref> The finding that drugs such as amphetamines, methamphetamine and cocaine, which can increase dopamine levels by more than tenfold,<ref></ref> can temporarily cause psychosis, provides further evidence for this link.<ref>{{cite journal |last1=Lieberman |first1=J.A. |author2=JM Kane, J. Alvir |title=Provocative tests with psychostimulant drugs in schizophrenia |journal=Psychopharmacology (Berl). |volume=91 |issue=4 |pages=415–433 |publisher= |year=1997 |doi=10.1007/BF00216006 |pmid=2884687}}</ref> However, many non-dopaminergic drugs can induce acute and chronic ].<ref name="Cardinal_2011_diagnosis_psychosis">Cardinal, R.N. & Bullmore, E.T., ''The Diagnosis of Psychosis'', ], 2011, ISBN 978-0-521-16484-9</ref> The NMDA antagonists Ketamine and PCP both are used in research to reproduce the positive and negative symptoms commonly associated with schizophrenia.<ref>{{cite doi|10.1016/S0893-133X(98)00060-8}}</ref><ref>{{cite journal |last1=Abi-Saab |first1=WM |author2=D'Souza DC, Moghaddam B, Krystal JH |title=The NMDA antagonist model for schizophrenia: promise and pitfalls |journal=Pharmacopsychiatry |volume=31 |issue=2 |pages=104–109 |year=1998 |pmid=9754841 |doi=10.1055/s-2007-979354}}</ref> | |||
| ====Pancreas==== | |||
| Dopaminergic dysregulation has also been linked to depressive disorders.<ref>{{cite journal |last1=Galani |first1=VJ |author2=Rana DG |title=Depression and antidepressants with dopamine hypothesis-A review |journal=IJPFR |year=2011 |volume=1 |issue=2 |pages=45–60}}</ref> Early research in humans used various methods of analyzing dopamine levels and function in depressed patients. Studies have reported that there is decreased concentration of ], a precursor to dopamine, in the blood plasma, ventricular spinal fluid, and lumbar spinal fluid of depressed patients compared to control subjects.<ref>{{cite journal |last1=Denkert |first1=O |author2=Renz A, Marano C, Matussek N. |title=Altered tyrosine daytime plasma levels in edogenous depressed patients |journal=Arch gen Psychiat |year=1971 |volume=25 |pages=359–363 |doi=10.1001/archpsyc.1971.01750160071013 |pmid=5116991 |issue=4}}</ref><ref>{{cite journal |last1=Birkmayer |first1=W |author2=Linauer W, Storung D |title=Tyrosin and tryptophan- metabolisms in depression patients |journal=Arch Psychiar Nervenkr |year=1970 |volume=213 |pages=377–387 |doi=10.1007/BF00341554 |issue=4}}</ref> One study measured the amount of ], the major metabolite of dopamine in the CSF, as a marker for the dopamine pathway turnover rate, and found decreased concentrations of homovanillic acid in the CSF of depressed patients.<ref>{{cite journal |last1=Bowers |first1=MB |author2=Heninger GR, Gerbode F. |title=Cerebrospinal fluid 5-hydroxyindoleacetic acid and homovanillic acid in psychiatric patients |journal=Int J Neuropharmacol |year=1969 |volume=8 |pages=255–262 |doi=10.1016/0028-3908(69)90046-X |pmid=5796265 |issue=3}}</ref> Postmordem real time ] (RT-PCR) has also been used to find that gene expression of a specific subtype of dopamine receptor was elevated in the ] of people suffering from depression as compared to control subjects.<ref>{{cite journal |last1=Lianbin |first1=X |author2=Katalin S, Attila S, Violetta K, Craig A, Stockmeier C, Beata K, John K, Gregory A, Ordwaya |title=Dopamine receptor gene expression in human amygdaloid nuclei: Elevated D4 receptor mRNA in major depression |journal=Brain Res |year=2008 |volume=1207 |pages=214–224 |doi=10.1016/j.brainres.2008.02.009 |pmid=18371940 |pmc=2577810}}</ref> | |||
| In the pancreas the role of dopamine is somewhat complex. The pancreas consists of two parts, an ] and an ] component. The exocrine part synthesizes and secretes ] and other substances, including dopamine, into the small intestine.<ref name=Rubi/> The function of this secreted dopamine after it enters the small intestine is not clearly established—the possibilities include protecting the intestinal mucosa from damage and reducing ] (the rate at which content moves through the digestive system).<ref name=Rubi>{{cite journal | vauthors = Rubí B, Maechler P | title = Minireview: new roles for peripheral dopamine on metabolic control and tumor growth: let's seek the balance | journal = Endocrinology | volume = 151 | issue = 12 | pages = 5570–81 | date = December 2010 | pmid = 21047943 | doi = 10.1210/en.2010-0745 | doi-access = free }}</ref> | |||
| The action of commonly used antidepressant drugs also has yielded information about possible alterations of the dopaminergic pathway in treating depression. It has been reported that many antidepressant drugs increase extracellular dopamine concentrations in the rat prefrontal cortex,<ref>{{cite journal |last1=Carlson |first1=JN |author2=Visker KE, Nielsen DM, Keller RW, Glick SD |title=Chronic antidepressant drug treatment reduces turning behavior and increases dopamine levels in the medial prefrontal cortex |journal=Brain Res |year=1996 |volume=707 |pages=122–126 |doi=10.1016/0006-8993(95)01341-5 |pmid=8866721 |issue=1}}</ref> but vary greatly in their effects on the ] and ].<ref>{{cite journal |last1=Ainsworth |first1=K |author2=Smith SE, Zetterstrom TS, Pei Q, Franklin M, Sharp T |title=Effect of antidepressant drugs on dopamine D1 and D2 receptor expression and dopamine release in the nucleus accumbens of the rat |journal=Psychopharmacology |year=1998 |volume=140 |pages=470–477 |doi=10.1007/s002130050791 |pmid=9888623 |issue=4}}</ref><ref>{{cite journal |last1=Meltzer |first1=TL |author2=Wiley JN, Williams AE, Heffner TG |title=Evidence for postsynaptic dopamine effects of B-HT 920 in the presence of the dopamine D1 agonist SKF 38393 |journal=Psychopharmacology |year=1988 |volume=95 |pages=329–332 |doi=10.1007/BF00181942 |pmid=2901126 |issue=3}}</ref> This can be compared to ] (ECT), which has been shown to have a multiple fold increase in striatal dopamine levels in rats.<ref>{{cite journal |last1=Nomikos |first1=GG |author2=Damsma G, Wenkstern D, Fibiger HC |title=Acute effects of bupripion on extracellular dopamine concentration in rat striatum and nucleus accumbens studies by in vivo microdialysis study |journal=Neuropsychopharmacol |year=1989 |volume=4 |pages=65–69}}</ref> | |||
| The pancreatic islets make up the endocrine part of the pancreas, and synthesize and secrete hormones including ] into the bloodstream.<ref name=Rubi/> There is evidence that the ]s in the islets that synthesize insulin contain dopamine receptors, and that dopamine acts to reduce the amount of insulin they release.<ref name=Rubi/> The source of their dopamine input is not clearly established—it may come from dopamine that circulates in the bloodstream and derives from the sympathetic nervous system, or it may be synthesized locally by other types of pancreatic cells.<ref name=Rubi/> | |||
| More recent research studies with rodents have found that depression-related behaviors are associated with dopaminergic system dysregulation.<ref name="Tye 2012 537-541">{{cite journal |last1=Deisseroth |author2=Mirzabekov, Warden, Ferenczi, Tsai, Finkelstein, kim, Adhikari, Thompson, Andalman, Gunaydin, Witten & Deisseroth |title=Dopamine neurons modulate neural encoding and expression of depression-related behaviour |journal=Nature |year=2012 |volume=493 |pages=537–541 |bibcode=2013Natur.493..537T |first=Julie J. |doi=10.1038/nature11740 |pmid=23235822 |issue=7433}}</ref> In rodents exposed to chronic mild stress, decreased escape behavior and decreased forced swimming is reversed with activation of the dopaminergic ].<ref name="Tye 2012 537-541"/> Also, rodents that are susceptible to depression-related behavior after ] can have their behavior reversed with dopamine pathway activation.<ref>{{cite journal |last1=Han |first1=D |author2=Walsh, Friedman, Juarez, Ku, Koo, Ferguson, Tsai, Pomeranz, Christoffel, Nectow, Ekstrand, Domingos, Mazei-Robison, Mouzon, Lobo, Neve, Friedman, Russo, Deisseroth, Nestler, Han |title=Rapid regulation of depression-related behaviors by control of midbrain dopamine neurons |journal=Nature |year=2013 |volume=493 |pages=532–536 |bibcode=2013Natur.493..532C |doi=10.1038/nature11713 |pmid=23235832 |issue=7433 |pmc=3554860}}</ref> Depletion of dopamine in the ] and nucleus accumbens has also been reported in cases of ] in animals. These symptoms can be reversed with dopamine agonists and antidepressant administration prior to the learned helplessness protocol.<ref>{{cite journal |last1=Muscat |first1=R |author2=Sampson D, Willner P. |title=Dopaminergic mechanisms of imipramine action in an animal model of depression |journal=Biol Psychiatry |year=1990 |volume=28 |pages=223–230 |doi=10.1016/0006-3223(90)90577-O |pmid=2378927 |issue=3}}</ref> | |||
| ==Medical uses== | |||
| ==Comparative biology and evolution== | |||
| {{Main|Dopamine (medication)}} | |||
| ] | |||
| Dopamine as a manufactured ] is sold under the trade names Intropin, Dopastat, and Revimine, among others. It is on the ].<ref>{{cite web|title=WHO Model List of Essential Medicines| url=http://apps.who.int/iris/bitstream/10665/93142/1/EML_18_eng.pdf |archive-url=https://web.archive.org/web/20140210093816/http://apps.who.int/iris/bitstream/10665/93142/1/EML_18_eng.pdf |archive-date=2014-02-10 |url-status=live| website=World Health Organization| access-date=24 September 2015| date=October 2013}}</ref> It is most commonly used as a stimulant drug in the treatment of severe ], ], and ]. It is especially important in treating these in ].<ref name=Noori>{{cite journal |vauthors=Noori S, Friedlich P, Seri I |year=2003 |title=Pharmacology Review Developmentally Regulated Cardiovascular, Renal, and Neuroendocrine Effects of Dopamine |journal=NeoReviews |volume=4 |issue=10 |pages=e283–e288 |url=https://www.researchgate.net/publication/239322432 |access-date=24 September 2015 |doi=10.1542/neo.4-10-e283|s2cid=71902752 }}</ref><ref name="medscape2021"/> It is given intravenously. Since the half-life of dopamine in plasma is very short—approximately one minute in adults, two minutes in newborn infants and up to five minutes in preterm infants—it is usually given in a continuous intravenous drip rather than a single injection.<ref name=BhattMehta>{{cite journal | vauthors = Bhatt-Mehta V, Nahata MC | title = Dopamine and dobutamine in pediatric therapy | journal = Pharmacotherapy | volume = 9 | issue = 5 | pages = 303–14 | year = 1989 | pmid = 2682552 | doi = 10.1002/j.1875-9114.1989.tb04142.x | s2cid = 25614283 }}</ref> | |||
| ===Microorganisms=== | |||
| Its effects, depending on dosage, include an increase in sodium excretion by the kidneys, an increase in urine output, an increase in ], and an increase in ].<ref name=BhattMehta/> At low doses it acts through the sympathetic nervous system to increase ] and heart rate, thereby increasing ] and blood pressure.<ref name="BryantKnights2010"/> Higher doses also cause ] that further increases blood pressure.<ref name="BryantKnights2010">{{cite book | vauthors = Bronwen JB, Knights KM |title=Pharmacology for Health Professionals |edition=2nd |year=2009 |publisher=Elsevier Australia |isbn=978-0-7295-3929-6 |page=192}}</ref><ref>{{cite journal | vauthors = De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, Brasseur A, Defrance P, Gottignies P, Vincent JL | title = Comparison of dopamine and norepinephrine in the treatment of shock | journal = The New England Journal of Medicine | volume = 362 | issue = 9 | pages = 779–89 | date = March 2010 | pmid = 20200382 | doi = 10.1056/NEJMoa0907118 | url = http://pdfs.semanticscholar.org/9c4a/b0084f4be06a36c826a1e598ec6593827c9d.pdf | url-status = dead | s2cid = 2208904 | archive-url = https://web.archive.org/web/20190228043236/http://pdfs.semanticscholar.org/9c4a/b0084f4be06a36c826a1e598ec6593827c9d.pdf | archive-date = 2019-02-28 }}</ref> Older literature also describes very low doses thought to improve kidney function without other consequences, but recent reviews have concluded that doses at such low levels are not effective and may sometimes be harmful.<ref>{{cite journal | vauthors = Karthik S, Lisbon A | title = Low-dose dopamine in the intensive care unit | journal = Seminars in Dialysis | volume = 19 | issue = 6 | pages = 465–71 | year = 2006 | pmid = 17150046 | doi = 10.1111/j.1525-139X.2006.00208.x | s2cid = 22538344 }}</ref> While some effects result from stimulation of dopamine receptors, the prominent cardiovascular effects result from dopamine acting at ], ], and ] ]s.<ref>{{Cite web|title = Dopamine|url = http://www.fpnotebook.com/cv/pharm/Dpmn.htm|website = Family Practice Notebook|access-date = 1 February 2016| vauthors = Moses S |quote = <!-- Dopamine binds to alpha-1 and beta-1 adrenergic receptors. Mediated through myocardial beta-1 adrenergic receptors, dopamine increase heart rate and force, thereby increasing cardiac output. Alpha-1 adrenergic receptor stimulation on vascular smooth muscle, leads to vasoconstriction and results in an increase in systemic vascular resistance --> }}</ref><ref>{{cite book | title = Clinical Cardiology: Current Practice Guidelines|url = https://books.google.com/books?id=YytoAgAAQBAJ|publisher = OUP Oxford|year= 2013|isbn = 978-0-19-150851-6 | vauthors = Katritsis DG, Gersh BJ, Camm AJ |quote = Dopamine binds to beta-1, beta-2, alpha-1 and dopaminergic receptors }}</ref> | |||
| There are no reports of dopamine in ], but it has been detected in some types of ] and in a type of protozoan called '']''.<ref>{{cite book |author=Roshchina VV |year=2010 |chapter=Evolutionary considerations of neurotransmitters in microbial, plant, and animal cells |title=Microbial Endocrinology |pages=17–52 |editors=Lyte M, Primrose PEPE |publisher=Springer |location=New York |isbn=978-1-4419-5576-0}}</ref> Perhaps more importantly, there are types of bacteria that contain homologs of all the enzymes that animals use to synthesize dopamine. It has even been proposed that animals derived their dopamine-synthesizing machinery from bacteria, via ] that may have occurred relatively late in evolutionary time, perhaps as a result of the symbiotic incorporation of bacteria into ] cells that gave rise to ].<ref>{{cite journal |author=Iyer LM, Aravind L, Coon SL, Klein DC, Koonin EV |title=Evolution of cell-cell signaling in animals: did late horizontal gene transfer from bacteria have a role? |journal=Trends Genet. |volume=20 |issue=7 |pages=292–9 |date=July 2004 |pmid=15219393 |doi=10.1016/j.tig.2004.05.007}}</ref> | |||
| ] of dopamine include negative effects on kidney function and ].<ref name="BryantKnights2010"/> The ], or lethal dose which is expected to prove fatal in 50% of the population, has been found to be: 59 mg/kg (mouse; administered ]); 95 mg/kg (mouse; administered ]); 163 mg/kg (rat; administered intraperitoneally); 79 mg/kg (dog; administered intravenously).<ref>{{cite book | vauthors = Lewis RJ | year = 2004 |title=Sax's Dangerous Properties of Industrial Materials |edition=11th |page=1552 |publisher=Wiley & Sons |location=Hoboken, NJ |isbn=978-0-471-47662-7}}</ref> | |||
| ===Animals=== | |||
| ==Disease, disorders, and pharmacology== | |||
| Dopamine is used as an intercellular messenger in virtually all multicellular animals. In sponges only a single report exists of the presence of dopamine, with no indication of its function;<ref>{{cite journal |year=2004 |title=Isolation of Araguspongine M, a new stereoisomer of an Araguspongine/Xestospongin alkaloid, and dopamine from the marine sponge ''Neopetrosia exigua'' collected in Palau |journal=Marine Drugs |volume=2 |issue=4 |pages=154–163 |author=Liu H, Mishima Y, Fujiwara T, Nagai H, Kitazawa A, Mine Y, ''et al.'' |doi=10.3390/md204154}}</ref> however, dopamine has been reported in the nervous systems of numerous radially symmetric species, including ] (jellyfish, hydra, corals, etc.).<ref>{{cite journal |author=Kass-Simon G, Pierobon P |title=Cnidarian chemical neurotransmission, an updated overview |journal=Comp. Biochem. Physiol., Part a Mol. Integr. Physiol. |volume=146 |issue=1 |pages=9–25 |date=January 2007 |pmid=17101286 |doi=10.1016/j.cbpa.2006.09.008}}</ref> This dates the emergence of dopamine as a neurotransmitter back to the earliest appearance of the nervous system, over 500 million years ago in the ]. Among existing species, dopamine functions as a neurotransmitter in vertebrates, ]s, ]s, ]s, and several types of worms.<ref>{{cite journal |author=Cottrell GA |title=Occurrence of dopamine and noradrenaline in the nervous tissue of some invertebrate species |journal=Br J Pharmacol Chemother |volume=29 |issue=1 |pages=63–9 |date=January 1967 |pmid=19108240 |pmc=1557178 |doi=10.1111/j.1476-5381.1967.tb01939.x}}</ref><ref>{{cite journal |author=Kindt KS, Quast KB, Giles AC, ''et al.'' |title=Dopamine mediates context-dependent modulation of sensory plasticity in C. elegans |journal=Neuron |volume=55 |issue=4 |pages=662–76 |date=August 2007 |pmid=17698017 |doi=10.1016/j.neuron.2007.07.023}}</ref> | |||
| {{See also|List of dopaminergic drugs}} | |||
| The dopamine system plays a central role in several significant medical conditions, including ], ], ], ], ], and ]. Aside from dopamine itself, there are many other important drugs that act on dopamine systems in various parts of the brain or body. Some are used for medical or recreational purposes, but ]s have also developed a variety of research drugs, some of which bind with high affinity to specific types of dopamine receptors and either ] or ] their effects, and many that affect other aspects of dopamine physiology,<ref>{{cite book |title=Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy | vauthors = Standaert DG, Walsh RR |chapter=Pharmacology of dopaminergic neurotransmission |pages=186–206 | veditors = Tashjian AH, Armstrong EJ, Golan DE |isbn=978-1-4511-1805-6 |year=2011 |publisher=Lippincott Williams & Wilkins}}</ref> including ] inhibitors, VMAT inhibitors, and ]. | |||
| In every type of animal that has been examined, dopamine acts to modify motor behavior.<ref name=Barron>{{cite journal |author=Barron AB, Søvik E, Cornish JL |title=The roles of dopamine and related compounds in reward-seeking behavior across animal phyla |journal=Front Behav Neurosci |volume=4 |issue= |pages=163 |year=2010 |pmid=21048897 |pmc=2967375 |doi=10.3389/fnbeh.2010.00163}}</ref> In the much-studied nematode worm '']'', it reduces locomotion and increases food-exploratory movements; in ]n worms it produces "screw-like" movements; in ]es it inhibits swimming and promotes crawling; etc. Across a wide range of vertebrates, dopamine has an "activating" effect on behavior-switching and response selection, comparable to its effect in mammals.<ref name=Barron/> | |||
| === Aging brain === | |||
| Dopamine also consistently plays a role in reward learning, in all animal groups that have been examined except arthropods. In nematodes, planarians, molluscs, and vertebrates, animals can be trained to repeat an action if it is consistently followed by an increase in dopamine levels.<ref name=Barron/> Arthropods are an exception, though. In these species — insects, crustaceans, etc. — dopamine has an aversive effect, and reward is instead mediated by ], a neurotransmitter that is not found in vertebrates but is thought to be closely related to ]. In insects, dopamine increases aversion learning for olfactory stimuli as well as visual stimuli, and reduces approach learning for stimuli that are followed by rewards. It also improves recall for aversive memories and reduces recall for positive memories.<ref name=Barron/> The origin of the striking reversal between dopamine's effects in arthropods versus all other types of animals has not been explained. | |||
| {{Main|Aging brain}} | |||
| A number of studies have reported an age-related decline in dopamine synthesis and dopamine receptor density (i.e., the number of receptors) in the brain.<ref name="Hof 2009">{{cite book | vauthors = Mobbs CV, Hof PR |title=Handbook of the neuroscience of aging |publisher=Elsevier/Academic Press |location=Amsterdam |year=2009 |isbn=978-0-12-374898-0 |oclc= 299710911 }}</ref> This decline has been shown to occur in the striatum and ] regions.<ref>{{cite journal | vauthors = Ota M, Yasuno F, Ito H, Seki C, Nozaki S, Asada T, Suhara T | title = Age-related decline of dopamine synthesis in the living human brain measured by positron emission tomography with L-DOPA | journal = Life Sciences | volume = 79 | issue = 8 | pages = 730–36 | date = July 2006 | pmid = 16580023 | doi = 10.1016/j.lfs.2006.02.017 }}</ref> Decreases in the ], ], and ] receptors are well documented.<ref name="Kaasinen 2000">{{cite journal | vauthors = Kaasinen V, Vilkman H, Hietala J, Någren K, Helenius H, Olsson H, Farde L, Rinne J | s2cid = 40871554 | title = Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain | journal = Neurobiology of Aging | volume = 21 | issue = 5 | pages = 683–68 | year = 2000 | pmid = 11016537 | doi = 10.1016/S0197-4580(00)00149-4 }}</ref><ref name="Wang 1998">{{cite journal | vauthors = Wang Y, Chan GL, Holden JE, Dobko T, Mak E, Schulzer M, Huser JM, Snow BJ, Ruth TJ, Calne DB, Stoessl AJ | title = Age-dependent decline of dopamine D1 receptors in human brain: a PET study | journal = Synapse | volume = 30 | issue = 1 | pages = 56–61 | date = September 1998 | pmid = 9704881 | doi = 10.1002/(SICI)1098-2396(199809)30:1<56::AID-SYN7>3.0.CO;2-J | s2cid = 31445572 }}</ref><ref name="Wong 1984">{{cite journal | vauthors = Wong DF, Wagner HN, Dannals RF, Links JM, Frost JJ, Ravert HT, Wilson AA, Rosenbaum AE, Gjedde A, Douglass KH | s2cid = 24278577 | title = Effects of age on dopamine and serotonin receptors measured by positron tomography in the living human brain | journal = Science | volume = 226 | issue = 4681 | pages = 1393–96 | date = December 1984 | pmid = 6334363 | doi = 10.1126/science.6334363 | bibcode = 1984Sci...226.1393W }}</ref> The reduction of dopamine with aging is thought to be responsible for many neurological symptoms that increase in frequency with age, such as decreased arm swing and increased ].<ref name="Wang Snyder 1998">{{cite book | vauthors = Wang E, Snyder SD |year=1998 |title=Handbook of the aging brain |location=San Diego, California |publisher=Academic Press |isbn=978-0-12-734610-6 |oclc=636693117}}</ref> Changes in dopamine levels may also cause age-related changes in cognitive flexibility.<ref name="Wang Snyder 1998"/> | |||
| === |
=== Multiple sclerosis === | ||
| Studies reported that dopamine imbalance influences the fatigue in ].<ref name="Dopamine Imbalance">{{cite journal | vauthors = Dobryakova E, Genova HM, DeLuca J, Wylie GR | title = The dopamine imbalance hypothesis of fatigue in multiple sclerosis and other neurological disorders | journal = Frontiers in Neurology | volume = 6 | pages = 52 | date = 12 March 2015 | pmid = 25814977 | pmc = 4357260 | doi = 10.3389/fneur.2015.00052 | doi-access = free }}</ref> In patients with multiple sclerosis, dopamine inhibits production of ] and ] by peripheral blood mononuclear cells.<ref>{{cite journal | vauthors = Marino F, Cosentino M | s2cid = 26319461 | title = Multiple sclerosis: Repurposing dopaminergic drugs for MS—the evidence mounts | journal = Nature Reviews. Neurology | volume = 12 | issue = 4 | pages = 191–92 | date = April 2016 | pmid = 27020558 | doi = 10.1038/nrneurol.2016.33 }}</ref> | |||
| ===Parkinson's disease=== | |||
| ] and fruit pulp of ]]] | |||
| Parkinson's disease is an age-related disorder characterized by ]s such as stiffness of the body, slowing of movement, and trembling of limbs when they are not in use.<ref name=Jankovic>{{cite journal | vauthors = Jankovic J | title = Parkinson's disease: clinical features and diagnosis | journal = Journal of Neurology, Neurosurgery, and Psychiatry | volume = 79 | issue = 4 | pages = 368–76 | date = April 2008 | pmid = 18344392 | doi = 10.1136/jnnp.2007.131045 | url = http://jnnp.bmj.com/content/79/4/368.full | doi-access = free }}</ref> In advanced stages it progresses to ] and eventually death.<ref name=Jankovic/> The main symptoms are caused by the loss of dopamine-secreting cells in the substantia nigra.<ref name=Dickson>{{cite book | vauthors = Dickson DV|chapter=Neuropathology of movement disorders | veditors = Tolosa E, Jankovic JJ| title=Parkinson's disease and movement disorders |publisher=Lippincott Williams & Wilkins |location=Hagerstown, MD |year=2007 |pages= 271–83 |isbn=978-0-7817-7881-7}}</ref> These dopamine cells are especially vulnerable to damage, and a variety of insults, including ] (as depicted in the book and movie '']''), repeated sports-related ]s, and some forms of chemical poisoning such as ], can lead to substantial cell loss, producing a ] that is similar in its main features to Parkinson's disease.<ref name=Tuite>{{cite journal | vauthors = Tuite PJ, Krawczewski K | title = Parkinsonism: a review-of-systems approach to diagnosis | journal = Seminars in Neurology | volume = 27 | issue = 2 | pages = 113–22 | date = April 2007 | pmid = 17390256 | doi = 10.1055/s-2007-971174 | s2cid = 260319916 }}</ref> Most cases of Parkinson's disease, however, are ], meaning that the cause of cell death cannot be identified.<ref name=Tuite/> | |||
| Many plants synthesize dopamine to varying degrees, including a variety of food plants. The highest concentrations have been observed in bananas — the fruit pulp of red and yellow bananas contains dopamine at levels of 40 to 50 parts per million by weight. Potatoes, avocados, broccoli, and ]s may also contain dopamine at levels of 1 part per million or more; oranges, tomatoes, spinach, beans, and other plants contain measurable concentrations less than 1 part per million.<ref name=Kulma>{{cite journal |author=Kulma A, Szopa J |title=Catecholamines are active compounds in plants |journal=Plant Science |year=2007 |volume=172 |pages=433–440 |doi=10.1016/j.plantsci.2006.10.013 |issue=3}}</ref> The dopamine in plants is synthesized from the amino acid tyrosine, by biochemical mechanisms similar to those that animals use. It can be metabolized in a number of ways, producing ] and a variety of alkaloids as byproducts.<ref name=Kulma/> The functions of plant catecholamines have not been clearly established, but there is evidence that they play a role in the response to stressors such as bacterial infection, act as growth-promoting factors in some situations, and modify the way that sugars are metabolized. The receptors that mediate these actions have not yet been identified, nor have the intracellular mechanisms that they activate.<ref name=Kulma/> | |||
| The most widely used treatment for parkinsonism is administration of L-DOPA, the metabolic precursor for dopamine.<ref name="Nice-pharma"/> L-DOPA is converted to dopamine in the brain and various parts of the body by the enzyme DOPA decarboxylase.<ref name=Musacchio/> L-DOPA is used rather than dopamine itself because, unlike dopamine, it is capable of crossing the ].<ref name="Nice-pharma">{{cite book| chapter=Symptomatic pharmacological therapy in Parkinson's disease| editor=The National Collaborating Centre for Chronic Conditions| title=Parkinson's Disease| chapter-url=http://guidance.nice.org.uk/CG35/Guidance/pdf/English| access-date=24 September 2015| publisher=Royal College of Physicians| location=London| year=2006| isbn=978-1-86016-283-1| pages=59–100| archive-date=24 September 2010| archive-url=https://web.archive.org/web/20100924153546/http://guidance.nice.org.uk/CG35/Guidance/pdf/English| url-status=dead}}</ref> It is often co-administered with an ] of peripheral ] such as ] or ], to reduce the amount converted to dopamine in the periphery and thereby increase the amount of L-DOPA that enters the brain.<ref name="Nice-pharma"/> When L-DOPA is administered regularly over a long time period, a variety of unpleasant side effects such as ] often begin to appear; even so, it is considered the best available long-term treatment option for most cases of Parkinson's disease.<ref name="Nice-pharma"/> | |||
| Dopamine consumed in food cannot act on the brain, because it cannot cross the ]. However, there are also a variety of plants that contain ], the metabolic precursor of dopamine.<ref name=Ingle>{{cite journal |author=Ingle PK |year=2003 |title=L-DOPA bearing plants |journal=Natural Product Radiance |volume=2 |pages=126–133 |url=http://nopr.niscair.res.in/bitstream/123456789/12261/1/NPR%202%283%29%20126-133.pdf |format=PDF |deadurl=no |accessdate=3 February 2014}}</ref> The highest concentrations are found in the leaves and bean pods of plants of the genus '']'', especially in '']'' (velvet beans), which have been used as a source for L-DOPA as a drug.<ref>{{cite journal |year=1993 |title=Occurrence of L-DOPA and dopamine in plants and cell cultures of ''Mucuna pruriens'' and effects of 2, 4-d and NaCl on these compounds |journal=Plant Cell, Tissue and Organ Culture |volume=33 |issue=3 |pages=259–264 |doi=10.1007/BF02319010 |author=Wichers HJ, Visser JF, Huizing HJ, Pras N}}</ref> Another plant containing substantial amounts of L-DOPA is '']'', the plant that produces fava beans (also known as "broad beans"). The level of L-DOPA in the beans, however, is much lower than in the pod shells and other parts of the plant.<ref>{{cite journal |author=Longo R |year=1974 |title=Distribution of L-dopa and related amino acid in ''Vicia'' |journal=Phytochemistry |volume=13 |pages=167–171 |doi=10.1016/S0031-9422(00)91287-1 |first2=Aldo |first3=Piero |first4=Marcellino}}</ref> The seeds of '']'' and '']'' trees also contain substantial amounts of L-DOPA.<ref name=Ingle/> | |||
| L-DOPA treatment cannot restore the dopamine cells that have been lost, but it causes the remaining cells to produce more dopamine, thereby compensating for the loss to at least some degree.<ref name="Nice-pharma"/> In advanced stages the treatment begins to fail because the cell loss is so severe that the remaining ones cannot produce enough dopamine regardless of L-DOPA levels.<ref name="Nice-pharma"/> Other drugs that enhance dopamine function, such as ] and ], are also sometimes used to treat Parkinsonism, but in most cases L-DOPA appears to give the best trade-off between positive effects and negative side-effects.<ref name="Nice-pharma"/> | |||
| In the marine green alga ], which is a major component of some ]s, dopamine is present in very high concentrations, estimated at 4.4% of dry weight. There is evidence that this dopamine functions as an anti-] defense, reducing consumption by snails and ]s.<ref>{{Cite journal |title=Dopamine functions as an antiherbivore defense in the temperate green alga ''Ulvaria obscura'' |first1=Kathryn L. |last1=Van Alstyne |first2=Amorah V. |last2=Nelson |first3=James R. |last3=Vyvyan |first4=Devon A. |last4=Cancilla |journal=Oecologia |volume=148 |issue=2 |pages=304–311 |doi=10.1007/s00442-006-0378-3 |year=2006 |pmid=16489461}}</ref> | |||
| Dopaminergic medications that are used to treat Parkinson's disease are sometimes associated with the development of a ], which involves the overuse of dopaminergic medication and medication-induced compulsive engagement in ]s like gambling and sexual activity.<ref name="Natural and drug addictions">{{cite journal | vauthors = Olsen CM | title = Natural rewards, neuroplasticity, and non-drug addictions | journal = Neuropharmacology | volume = 61 | issue = 7 | pages = 1109–22 | date = December 2011 | pmid = 21459101 | pmc = 3139704 | doi = 10.1016/j.neuropharm.2011.03.010 | quote = <!-- Notably, sensitization processes can also translate between drug and non-drug rewards (Fiorino and Phillips, 1999; Avena and Hoebel, 2003b; Robinson and Berridge, 2008). In humans, the role of dopamine signaling in incentive-sensitization processes has recently been highlighted by the observation of a dopamine dysregulation syndrome in some patients taking dopaminergic drugs. This syndrome is characterized by a medication-induced increase in (or compulsive) engagement in non-drug rewards such as gambling, shopping, or sex (Evans et al., 2006; Aiken, 2007; Lader, 2008). --> }}</ref><ref name="DDS in PD">{{cite journal | vauthors = Ceravolo R, Frosini D, Rossi C, Bonuccelli U | s2cid = 19277026 | title = Spectrum of addictions in Parkinson's disease: from dopamine dysregulation syndrome to impulse control disorders | journal = Journal of Neurology | volume = 257 | issue = Suppl 2 | pages = S276–83 | date = November 2010 | pmid = 21080189 | doi = 10.1007/s00415-010-5715-0 }}</ref> The latter behaviors are similar to those observed in individuals with a ].<ref name="Natural and drug addictions" /> | |||
| ===As a precursor for melanin=== | |||
| ===Drug addiction and psychostimulants=== | |||
| ]s are a family of dark-pigmented substances that are found in a wide range of organisms. Their physical properties make them difficult to work with experimentally, and consequently a number of aspects of their biochemistry are not well understood. Chemically they are closely related to dopamine, and there is a type of melanin, known as "dopamine-melanin", that can be synthesized by oxidation of dopamine via the enzyme ].<ref>{{cite journal |author=Simon JD, Peles D, Wakamatsu K, Ito S |title=Current challenges in understanding melanogenesis: bridging chemistry, biological control, morphology, and function |journal=Pigment Cell Melanoma Res |volume=22 |issue=5 |pages=563–79 |date=October 2009 |pmid=19627559 |doi=10.1111/j.1755-148X.2009.00610.x}}</ref> The melanin that darkens human skin is not of this type: it is synthesized by a pathway that uses ] as a precursor but not dopamine. However, there is substantial evidence that the "neuromelanin" that gives a dark color to the brain's ] is at least in part dopamine-melanin.<ref>{{cite journal |author=Fedorow H, Tribl F, Halliday G, Gerlach M, Riederer P, Double KL |title=Neuromelanin in human dopamine neurons: comparison with peripheral melanins and relevance to Parkinson's disease |journal=Prog. Neurobiol. |volume=75 |issue=2 |pages=109–24 |date=February 2005 |pmid=15784302 |doi=10.1016/j.pneurobio.2005.02.001}}</ref> | |||
| {{Main|Addiction}} | |||
| ]s (DAT), which transport dopamine back into a synaptic terminal after it has been emitted.|alt=Diagram describes the mechanisms by which cocaine and amphetamines reduce dopamine transporter activity.]] | |||
| ], substituted amphetamines (including ]), ], ] (marketed as ] or ]), and other ] exert their effects primarily or partly by increasing dopamine levels in the brain by a variety of mechanisms.<ref name=Ghodse/> Cocaine and methylphenidate are dopamine transporter blockers or ]s;<ref>{{cite journal | vauthors = Siciliano CA, Jones SR | title = Cocaine Potency at the Dopamine Transporter Tracks Discrete Motivational States During Cocaine Self-Administration | journal = Neuropsychopharmacology | volume = 42 | issue = 9 | pages = 1893–1904 | date = August 2017 | pmid = 28139678 | pmc = 5520781 | doi = 10.1038/npp.2017.24 }}</ref> they ] dopamine reuptake, resulting in increased dopamine concentrations in the synaptic cleft.<ref name=Heal>{{cite journal | vauthors = Heal DJ, Pierce DM | title = Methylphenidate and its isomers: their role in the treatment of attention-deficit hyperactivity disorder using a transdermal delivery system | journal = CNS Drugs | volume = 20 | issue = 9 | pages = 713–38 | year = 2006 | pmid = 16953648 | doi = 10.2165/00023210-200620090-00002 | s2cid = 39535277 }}</ref><ref name=Freye>{{cite book| vauthors = Freye E |title=Pharmacology and abuse of cocaine, amphetamines, ecstasy and related designer drugs a comprehensive review on their mode of action, treatment of abuse and intoxication |year=2009 |publisher=Springer |location=Dordrecht |isbn=978-90-481-2448-0}}</ref>{{rp|54–58}} Like cocaine, substituted amphetamines and amphetamine also increase the concentration of dopamine in the ], but by different mechanisms.<ref name="Miller">{{cite journal | vauthors = Miller GM | title = The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity | journal = Journal of Neurochemistry | volume = 116 | issue = 2 | pages = 164–76 | date = January 2011 | pmid = 21073468 | pmc = 3005101 | doi = 10.1111/j.1471-4159.2010.07109.x }}</ref><ref name=Freye/>{{rp|147–150}} | |||
| Dopamine-derived melanin probably appears in at least some other biological systems as well. Some of the dopamine in plants is likely to be used as a precursor for dopamine-melanin.<ref>{{cite journal |year=1967 |title=Melanins from DOPA-containing plants |journal=Phytochemistry |volume=6 |issue=1 |pages=13–18 |doi=10.1016/0031-9422(67)85002-7 |author=Andrews RS, Pridham JB}}</ref> The complex patterns that appear on butterfly wings, as well as black-and-white stripes on the bodies of insect larvae, are also thought to be caused by spatially structured accumulations of dopamine-melanin.<ref>{{cite journal |author=Beldade P, Brakefield PM |title=The genetics and evo-devo of butterfly wing patterns |journal=Nature Reviews Genetics |volume=3 |issue=6 |pages=442–52 |date=June 2002 |pmid=12042771 |doi=10.1038/nrg818}}</ref> | |||
| The effects of psychostimulants include increases in heart rate, body temperature, and sweating; improvements in alertness, attention, and endurance; increases in pleasure produced by rewarding events; but at higher doses agitation, anxiety, or even ].<ref name=Ghodse>{{cite book | vauthors = Ghodse H|title=Ghodse's Drugs and Addictive Behaviour: A Guide to Treatment |date=2010 |publisher=Cambridge University Press |isbn=978-1-139-48567-8|pages=87–92|edition=4th}}</ref> Drugs in this group can have a high addiction potential, due to their activating effects on the dopamine-mediated reward system in the brain.<ref name=Ghodse/> However some can also be useful, at lower doses, for treating attention deficit hyperactivity disorder (ADHD) and ].<ref name=Kimko/><ref>{{cite journal | vauthors = Mignot EJ | title = A practical guide to the therapy of narcolepsy and hypersomnia syndromes | journal = Neurotherapeutics | volume = 9 | issue = 4 | pages = 739–52 | date = October 2012 | pmid = 23065655 | pmc = 3480574 | doi = 10.1007/s13311-012-0150-9 }}</ref> An important differentiating factor is the onset and duration of action.<ref name=Ghodse/> Cocaine can take effect in seconds if it is injected or inhaled in free base form; the effects last from 5 to 90 minutes.<ref name=Zimmerman>{{cite journal | vauthors = Zimmerman JL | title = Cocaine intoxication | journal = Critical Care Clinics | volume = 28 | issue = 4 | pages = 517–26 | date = October 2012 | pmid = 22998988 | doi = 10.1016/j.ccc.2012.07.003 }}</ref> This rapid and brief action makes its effects easily perceived and consequently gives it high addiction potential.<ref name=Ghodse/> Methylphenidate taken in pill form, in contrast, can take two hours to reach peak levels in the bloodstream,<ref name=Kimko>{{cite journal | vauthors = Kimko HC, Cross JT, Abernethy DR | s2cid = 397390 | title = Pharmacokinetics and clinical effectiveness of methylphenidate | journal = Clinical Pharmacokinetics | volume = 37 | issue = 6 | pages = 457–70 | date = December 1999 | pmid = 10628897 | doi = 10.2165/00003088-199937060-00002 }}</ref> and depending on formulation the effects can last for up to 12 hours.<ref>{{Cite web|title=Quillivant XR – methylphenidate hydrochloride suspension, extended release|url=https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=e0157005-6e3e-4763-b910-9eb0937608c9|access-date=2020-07-11|website=dailymed.nlm.nih.gov}}</ref> These longer acting formulations have the benefit of reducing the potential for abuse, and improving adherence for treatment by using more convenient dosage regimens.<ref>{{cite journal | vauthors = López FA, Leroux JR | title = Long-acting stimulants for treatment of attention-deficit/hyperactivity disorder: a focus on extended-release formulations and the prodrug lisdexamfetamine dimesylate to address continuing clinical challenges | journal = Attention Deficit and Hyperactivity Disorders | volume = 5 | issue = 3 | pages = 249–65 | date = September 2013 | pmid = 23564273 | pmc = 3751218 | doi = 10.1007/s12402-013-0106-x }}</ref> | |||
| ==Pharmacology== | |||
| ] also known as crystal meth|alt=A shiny translucent white crystal of methamphetamine, held between the ends of a finger and thumb]] | |||
| ===Dopamine as an injectable drug=== | |||
| A variety of addictive drugs produce an increase in reward-related dopamine activity.<ref name=Ghodse/> Stimulants such as ], cocaine and methamphetamine promote increased levels of dopamine which appear to be the primary factor in causing addiction. For other addictive drugs such as the ] heroin, the increased levels of dopamine in the reward system may play only a minor role in addiction.<ref name=Nutt>{{cite journal | vauthors = Nutt DJ, Lingford-Hughes A, Erritzoe D, Stokes PR | s2cid = 205511111 | title = The dopamine theory of addiction: 40 years of highs and lows | journal = Nature Reviews. Neuroscience | volume = 16 | issue = 5 | pages = 305–12 | date = May 2015 | pmid = 25873042 | doi = 10.1038/nrn3939 | url = https://kclpure.kcl.ac.uk/ws/files/44680387/Nutt_and_Stokes_Nature_Reviews_Neuroscience_2015_institutional_repository.pdf }}</ref> When people addicted to stimulants go through withdrawal, they do not experience the physical suffering associated with ] or ] from opiates; instead they experience craving, an intense desire for the drug characterized by irritability, restlessness, and other arousal symptoms,<ref name="Sinha">{{cite journal | vauthors = Sinha R | title = The clinical neurobiology of drug craving | journal = Current Opinion in Neurobiology | volume = 23 | issue = 4 | pages = 649–54 | date = August 2013 | pmid = 23764204 | pmc = 3735834 | doi = 10.1016/j.conb.2013.05.001 }}</ref> brought about by ]. | |||
| The dopamine system plays a crucial role in several aspects of addiction. At the earliest stage, genetic differences that alter the expression of dopamine receptors in the brain can predict whether a person will find stimulants appealing or aversive.<ref name="Volkow">{{cite journal | vauthors = Volkow ND, Baler RD | title = Addiction science: Uncovering neurobiological complexity | journal = Neuropharmacology | volume = 76 | issue= Pt B | pages = 235–49 | date = January 2014 | pmid = 23688927 | pmc = 3818510 | doi = 10.1016/j.neuropharm.2013.05.007 }}</ref> Consumption of stimulants produces increases in brain dopamine levels that last from minutes to hours.<ref name=Ghodse/> Finally, the chronic elevation in dopamine that comes with repetitive high-dose stimulant consumption triggers a wide-ranging set of structural changes in the brain that are responsible for the behavioral abnormalities which characterize an addiction.<ref name="Nestler">{{cite journal | vauthors = Nestler EJ | title = Transcriptional mechanisms of drug addiction | journal = Clinical Psychopharmacology and Neuroscience | volume = 10 | issue = 3 | pages = 136–43 | date = December 2012 | pmid = 23430970 | pmc = 3569166 | doi = 10.9758/cpn.2012.10.3.136 }}</ref> Treatment of stimulant addiction is very difficult, because even if consumption ceases, the craving that comes with psychological withdrawal does not.<ref name=Sinha/> Even when the craving seems to be extinct, it may re-emerge when faced with stimuli that are associated with the drug, such as friends, locations and situations.<ref name=Sinha/> ] in the brain are greatly interlinked.<ref>{{cite journal | vauthors = Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, Roffman JL, Smoller JW, Zöllei L, Polimeni JR, Fischl B, Liu H, Buckner RL | title = The organization of the human cerebral cortex estimated by intrinsic functional connectivity | journal = Journal of Neurophysiology | volume = 106 | issue = 3 | pages = 1125–65 | date = September 2011 | pmid = 21653723 | pmc = 3174820 | doi = 10.1152/jn.00338.2011 }}</ref> | |||
| Under the trade names '''Intropin''', '''Dopastat''', '''Revimine''', or other names, dopamine can be used as a ] in ] form. It is most commonly used in the treatment of severe ], ] (slow heart rate), ], or ], especially in newborn infants. Its effects, depending on dosage, include an increase in sodium excretion by the kidneys, an increase in urine output, an increase in heart rate, and an increase in blood pressure. At a "cardiac dose" of 5 to 10 μg/kg/min, dopamine acts through the ] to increase heart muscle contraction force and heart rate, thereby increasing cardiac output and blood pressure. At a "pressor dose" of 10 to 20 μg/kg/min, dopamine also causes ] that further increases blood pressure, but can produce negative side effects such as an impairment of kidney function and ].<ref name="BryantKnights2010">{{cite book |author1=Bronwen Jean Bryant |author2=Kathleen Mary Knights |title=Pharmacology for Health Professionals |url=http://books.google.com/books?id=TQV6sLzYsOYC&pg=PA119 |accessdate=9 June 2011 |edition=2nd |date=15 November 2009 |publisher=Elsevier Australia |isbn=978-0-7295-3929-6 |page=192}}</ref><ref>{{cite journal |title=Comparison of Dopamine and Norepinephrine in the Treatment of Shock |journal=New Engl. J. Med. |volume=362 |pages=779–789 |year=2010 |doi=10.1056/NEJMoa0907118 |author=de Backer D, Biston P, Devriendt J, ''et al.'' |issue=9 |pmid=20200382}}</ref> Older literature also describes a so-called "renal dose" of 2 to 5 μg/kg/min thought to improve kidney function without other consequences, but recent reviews have concluded that doses at this low level are not clinically effective and may sometimes be harmful.<ref>{{cite journal |title=Low-dose dopamine in the intensive care unit |journal=Semin Dial |volume=19 |issue=6 |pages=465–71 |year=2006 |pmid=17150046 |doi=10.1111/j.1525-139X.2006.00208.x |author=Karthik S, Lisbon A}}</ref> | |||
| ===Psychosis and antipsychotic drugs=== | |||
| ===L-DOPA=== | |||
| {{Main|Psychosis}} | |||
| Psychiatrists in the early 1950s discovered that a class of drugs known as ]s (also known as major ]s), were often effective at reducing the ] symptoms of schizophrenia.<ref name=Healy/> The introduction of the first widely used antipsychotic, ] (Thorazine), in the 1950s, led to the release of many patients with schizophrenia from institutions in the years that followed.<ref name=Healy/> By the 1970s researchers understood that these typical antipsychotics worked as ] on the D<sub>2</sub> receptors.<ref name=Healy/><ref name=Brunton>{{cite book | vauthors = Brunton L | title = Goodman and Gilman's The Pharmacological Basis of Therapeutics|publisher=McGraw Hill|pages=417–55|edition=12th}}</ref> This realization led to the so-called ], which postulates that schizophrenia is largely caused by hyperactivity of brain dopamine systems.<ref name="Howes">{{cite journal | vauthors = Howes OD, Kapur S | title = The dopamine hypothesis of schizophrenia: version III—the final common pathway | journal = Schizophrenia Bulletin | volume = 35 | issue = 3 | pages = 549–62 | date = May 2009 | pmid = 19325164 | pmc = 2669582 | doi = 10.1093/schbul/sbp006 }}</ref> The dopamine hypothesis drew additional support from the observation that psychotic symptoms were often intensified by dopamine-enhancing stimulants such as methamphetamine, and that these drugs could also produce psychosis in healthy people if taken in large enough doses.<ref name=Howes/> In the following decades other ] that had fewer serious side effects were developed.<ref name=Healy/> Many of these newer drugs do not act directly on dopamine receptors, but instead produce alterations in dopamine activity indirectly.<ref name=Horacek>{{cite journal | vauthors = Horacek J, Bubenikova-Valesova V, Kopecek M, Palenicek T, Dockery C, Mohr P, Höschl C | s2cid = 18226404 | title = Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia | journal = CNS Drugs | volume = 20 | issue = 5 | pages = 389–409 | year = 2006 | pmid = 16696579 | doi = 10.2165/00023210-200620050-00004 }}</ref> These drugs were also used to treat other psychoses.<ref name=Healy>{{Cite book |title=The Creation of Psychopharmacology | vauthors = Healy D |year=2004 |publisher=Harvard University Press |pages=37–73 |isbn=978-0-674-01599-9 }}</ref> ] have a broadly suppressive effect on most types of active behavior, and particularly reduce the delusional and agitated behavior characteristic of overt psychosis.<ref name=Brunton/> | |||
| Later observations, however, have caused the dopamine hypothesis to lose popularity, at least in its simple original form.<ref name=Howes/> For one thing, patients with schizophrenia do not typically show measurably increased levels of brain dopamine activity.<ref name=Howes/> Even so, many psychiatrists and neuroscientists continue to believe that schizophrenia involves some sort of dopamine system dysfunction.<ref name=Healy/> As the "dopamine hypothesis" has evolved over time, however, the sorts of dysfunctions it postulates have tended to become increasingly subtle and complex.<ref name=Healy/> | |||
| ] is a dopamine precursor used in various forms to treat ] and dopa-responsive ]. It is typically co-administered with an inhibitor of peripheral decarboxylation (DDC, ]), such as ] or ]. Inhibitors of alternative metabolic route for dopamine by ] are also used. These include ] and ]. | |||
| ] ] suggested in a review of 2018 that in many cases of psychosis, including schizophrenia, three interconnected networks based on dopamine, serotonin, and glutamate – each on its own or in various combinations – contributed to an overexcitation of dopamine D<sub>2</sub> receptors in the ].<ref name="pmid29954475">{{cite journal| vauthors = Stahl SM| title=Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate. | journal=CNS Spectr | year= 2018 | volume= 23 | issue= 3 | pages= 187–91 | pmid=29954475 | doi=10.1017/S1092852918001013 | s2cid=49599226 | url=https://www.cambridge.org/core/services/aop-cambridge-core/content/view/3E9E50ED717219011DD1B570365010E8/S1092852918001013a.pdf/beyond_the_dopamine_hypothesis_of_schizophrenia_to_three_neural_networks_of_psychosis_dopamine_serotonin_and_glutamate.pdf |archive-url=https://web.archive.org/web/20200429212444/https://www.cambridge.org/core/services/aop-cambridge-core/content/view/3E9E50ED717219011DD1B570365010E8/S1092852918001013a.pdf/beyond_the_dopamine_hypothesis_of_schizophrenia_to_three_neural_networks_of_psychosis_dopamine_serotonin_and_glutamate.pdf |archive-date=2020-04-29 |url-status=live }}</ref> | |||
| ===Psychomotor stimulants=== | |||
| Cocaine and amphetamines inhibit the ] of dopamine; however, they influence separate mechanisms of action. Cocaine is a ] and ] blocker that competitively inhibits dopamine uptake to increase the lifetime of dopamine and augments an overabundance of dopamine (an increase of up to 150 percent) within the parameters of the dopamine neurotransmitters. Like cocaine, amphetamines increase the concentration of dopamine in the ] gap, but by a different mechanism. Amphetamine and ] are similar in structure to dopamine, and so can enter the terminal bouton of the presynaptic neuron via its dopamine transporters as well as by diffusing through the neural membrane directly.<ref name=Miller>{{cite journal |last=Miller |first=GM |title=The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity |journal=Journal of Neurochemistry |date=January 2011 |volume=116 |issue=2 |pages=164–76 |pmid=21073468 |doi=10.1111/j.1471-4159.2010.07109.x |pmc=3005101}}</ref> Upon entering the presynaptic neuron, amphetamines activate ] which, through ] and ] signaling, induces dopamine efflux and non-competitive reuptake inhibition.<ref name=Miller/> Since amphetamines are structurally similar to ], they are also substrates for monoamine transporters; consequently, they competitively inhibit the reuptake of dopamine and other monoamines as well.<ref name=Miller/> | |||
| ===Attention deficit hyperactivity disorder=== | |||
| ===Antipsychotic drugs=== | |||
| Altered dopamine neurotransmission is implicated in attention deficit hyperactivity disorder (ADHD), a condition associated with impaired ], in turn leading to problems with regulating attention (]), inhibiting behaviors (]), and forgetting things or missing details (]), among other problems.<ref name="Malenka ADHD neurosci">{{cite book | vauthors = Malenka RC, Nestler EJ, Hyman SE | veditors = Sydor A, Brown RY | title = Molecular Neuropharmacology: A Foundation for Clinical Neuroscience | year = 2009 | publisher = McGraw-Hill Medical | location = New York | isbn = 978-0-07-148127-4 | pages = 266, 318–23 | edition = 2nd | chapter = Chapters 10 and 13}}</ref> There are genetic links between dopamine receptors, the dopamine transporter, and ADHD, in addition to links to other neurotransmitter receptors and transporters.<ref name=Wu>{{cite journal | vauthors = Wu J, Xiao H, Sun H, Zou L, Zhu LQ | s2cid = 895006 | title = Role of dopamine receptors in ADHD: a systematic meta-analysis | journal = Molecular Neurobiology | volume = 45 | issue = 3 | pages = 605–20 | date = June 2012 | pmid = 22610946 | doi = 10.1007/s12035-012-8278-5 }}</ref> The most important relationship between dopamine and ADHD involves the drugs that are used to treat ADHD.<ref name=Berridge3/> Some of the most effective therapeutic agents for ADHD are psychostimulants such as methylphenidate (Ritalin, Concerta) and ] (Evekeo, Adderall, Dexedrine), drugs that increase both dopamine and norepinephrine levels in the brain.<ref name="Berridge3">{{cite journal | vauthors = Berridge CW, Devilbiss DM | title = Psychostimulants as cognitive enhancers: the prefrontal cortex, catecholamines, and attention-deficit/hyperactivity disorder | journal = Biological Psychiatry | volume = 69 | issue = 12 | pages = e101–11 | date = June 2011 | pmid = 20875636 | pmc = 3012746 | doi = 10.1016/j.biopsych.2010.06.023 }}</ref> The clinical effects of these psychostimulants in treating ADHD are mediated through the ] of dopamine and norepinephrine receptors, specifically ] and ], in the prefrontal cortex.<ref name="Malenka ADHD neurosci" /><ref name="Unambiguous PFC D1 A2">{{cite journal | vauthors = Spencer RC, Devilbiss DM, Berridge CW | title = The cognition-enhancing effects of psychostimulants involve direct action in the prefrontal cortex | journal = Biological Psychiatry | volume = 77 | issue = 11 | pages = 940–50 | date = June 2015 | pmid = 25499957 | pmc = 4377121 | doi = 10.1016/j.biopsych.2014.09.013 }}</ref><ref name="Cognitive and motivational effects">{{cite journal | vauthors = Ilieva IP, Hook CJ, Farah MJ | s2cid = 15788121 | title = Prescription Stimulants' Effects on Healthy Inhibitory Control, Working Memory, and Episodic Memory: A Meta-analysis | journal = Journal of Cognitive Neuroscience | volume = 27 | issue = 6 | pages = 1069–89 | date = June 2015 | pmid = 25591060 | doi = 10.1162/jocn_a_00776 | url = https://repository.upenn.edu/neuroethics_pubs/130 }}</ref> | |||
| ===Pain=== | |||
| A range of drugs that reduce dopamine activity have been found useful in the treatment of ] and other disorders that produce ]. These ] drugs are also sometimes known as neuroleptics or "major tranquilizers", in contrast to "minor tranquilizers" such as ] that are used to treat anxiety or sleep disorders. These drugs have a broadly suppressive effect on most types of active behavior, and particularly reduce the delusional and agitated behavior characteristic of overt psychosis. The introduction of the first widely used antipsychotic drug, ] (Thorazine), in the 1950s, led to the release of many schizophrenia patients from institutions in the years that followed. | |||
| Dopamine plays a role in ] processing in multiple levels of the central nervous system including the spinal cord, ], ], basal ganglia, and ].<ref name="Wood" /> Decreased levels of dopamine have been associated with painful symptoms that frequently occur in Parkinson's disease.<ref name="Wood" /> Abnormalities in dopaminergic neurotransmission also occur in several painful clinical conditions, including ], ], and restless legs syndrome.<ref name=Wood>{{cite journal | vauthors = Wood PB | s2cid = 24325199 | title = Role of central dopamine in pain and analgesia | journal = Expert Review of Neurotherapeutics | volume = 8 | issue = 5 | pages = 781–97 | date = May 2008 | pmid = 18457535 | doi = 10.1586/14737175.8.5.781 }}</ref> | |||
| ===Nausea=== | |||
| Even so, the widespread use of antipsychotic drugs has long been controversial. There are several reasons for this. First, these drugs are perceived as very aversive by people who have to take them, because they produce a general dullness of thought and suppress the ability to experience pleasure.<ref name="fn2">{{cite journal |author=Lambert M, Schimmelmann B, Karow A, Naber D |title=Subjective well-being and initial dysphoric reaction under antipsychotic drugs - concepts, measurement and clinical relevance |journal=Pharmacopsychiatry |volume=36 |issue=Suppl 3 |pages=S181–90 |year=2003 |pmid=14677077 |doi=10.1055/s-2003-45128}}</ref> Second, it is difficult to show that they act specifically against psychotic behaviors rather than merely suppressing all types of active behavior. Third, they can produce a range of serious side effects, including weight gain, diabetes, fatigue, sexual dysfunction, hormonal changes, and a type of movement disorder known as ]. Some of these side effects may continue long after the cessation of drug use, or even permanently. | |||
| Nausea and ] are largely determined by activity in the ] in the ] of the ], in a region known as the ].<ref name=Flake>{{cite journal | vauthors = Flake ZA, Scalley RD, Bailey AG | title = Practical selection of antiemetics | journal = American Family Physician | volume = 69 | issue = 5 | pages = 1169–74 | date = March 2004 | pmid = 15023018 | url = http://www.aafp.org/afp/2004/0301/p1169.html }}</ref> This area contains a large population of type D<sub>2</sub> dopamine receptors.<ref name=Flake/> Consequently, drugs that activate D<sub>2</sub> receptors have a high potential to cause nausea.<ref name=Flake/> This group includes some medications that are administered for Parkinson's disease, as well as other ] such as ].<ref name=Connolly>{{cite journal | vauthors = Connolly BS, Lang AE | title = Pharmacological treatment of Parkinson disease: a review | journal = JAMA | volume = 311 | issue = 16 | pages = 1670–83 | year = 2014 | pmid = 24756517 | doi = 10.1001/jama.2014.3654 }}</ref> In some cases, D<sub>2</sub>-receptor antagonists such as ] are useful as ].<ref name=Flake/> | |||
| '''Fear and Anxiety''' | |||
| The first drugs introduced specifically for the treatment of psychosis all had strong direct effects on multiple aspects of dopamine function. Drugs of this type are known as "typical antipsychotics". Because of the problems they cause, there has been wide interest in newer types of drugs known as "atypical antipsychotics" or "second-generation antipsychotics", which aim to target the specific types of dopamine receptors involved in psychosis, and thereby reduce psychotic symptoms without producing as many undesirable side effects. There remains substantial dispute, however, about how much of an improvement in the patient experience these drugs produce. | |||
| Simultaneous positron emission tomography (PET) and functional magnetic resonance imaging (fMRI), have shown that the amount of dopamine release is dependent on the strength of conditioned fear response and is linearly coupled to learning-induced activity in the amygdala.<ref>{{cite journal | vauthors = Frick A, Björkstrand J, Lubberink M, Eriksson A, Fredrikson M, Åhs F | title = Dopamine and fear memory formation in the human amygdala | journal = Molecular Psychiatry | volume = 27 | issue = 3 | pages = 1704–1711 | date = March 2022 | pmid = 34862441 | doi = 10.1038/s41380-021-01400-x | pmc = 9095491 }}</ref> Dopamine is generally linked to reward learning, but it also plays a key role in fear learning and extinction by helping to form, store and update fear memories through its interaction with other brain regions like amygdala, ventromedial prefrontal cortex and striatum.<ref>{{cite journal | vauthors = Hamati R, Ahrens J, Shvetz C, Holahan MR, Tuominen L | title = 65 years of research on dopamine's role in classical fear conditioning and extinction: A systematic review | journal = The European Journal of Neuroscience | volume = 59 | issue = 6 | pages = 1099–1140 | date = March 2024 | pmid = 37848184 | doi = 10.1111/ejn.16157 | doi-access = free }}</ref> | |||
| ===Toxicity=== | |||
| The LD<sub>50</sub>, or dose which is expected to be lethal in 50% of the population, has been found to be: 59 mg/kg (mouse; administered i.v.); 950 mg/kg (mouse; administered i.p.); 163 mg/kg (rat; administered i.p.); 79 mg/kg (dog; administered i.v.)<ref>R. J. Lewis (Ed.) (2004), Sax's Dangerous Properties of Industrial Materials, 11th Ed., p. 1552, Wiley & Sons, Hoboken, NJ.</ref>{{clarify|date=October 2012}} | |||
| ==Comparative biology and evolution== | |||
| '''<big>Binding profile of dopamine</big>'''<ref>{{cite web |title=PDSP K<sub>i</sub> Database |work=Psychoactive Drug Screening Program (PDSP) |author=Roth, BL; Driscol, J |url=http://pdsp.med.unc.edu/pdsp.php |publisher=University of North Carolina at Chapel Hill and the United States National Institute of Mental Health |accessdate=15 November 2013 |date=12 January 2011}}</ref> | |||
| ===Microorganisms=== | |||
| There are no reports of dopamine in ], but it has been detected in some types of ] and in the ]n called '']''.<ref>{{cite book | vauthors = Roshchina VV |year=2010 |chapter=Evolutionary considerations of neurotransmitters in microbial, plant, and animal cells |title=Microbial Endocrinology |pages=17–52 | veditors = Lyte M, Primrose PE |publisher=Springer |location=New York |isbn=978-1-4419-5576-0}}</ref> Perhaps more importantly, there are types of bacteria that contain ] of all the enzymes that animals use to synthesize dopamine.<ref name=Iyer/> It has been proposed that animals derived their dopamine-synthesizing machinery from bacteria, via ] that may have occurred relatively late in evolutionary time, perhaps as a result of the ] incorporation of bacteria into ] cells that gave rise to ].<ref name=Iyer>{{cite journal | vauthors = Iyer LM, Aravind L, Coon SL, Klein DC, Koonin EV | title = Evolution of cell-cell signaling in animals: did late horizontal gene transfer from bacteria have a role? | journal = Trends in Genetics | volume = 20 | issue = 7 | pages = 292–99 | date = July 2004 | pmid = 15219393 | doi = 10.1016/j.tig.2004.05.007 }}</ref> | |||
| ===Animals=== | |||
| {| class="wikitable" | |||
| Dopamine is used as a neurotransmitter in most multicellular animals.<ref name=Barron/> In ]s there is only a single report of the presence of dopamine, with no indication of its function;<ref>{{cite journal |year=2004 |title=Isolation of Araguspongine M, a new stereoisomer of an Araguspongine/Xestospongin alkaloid, and dopamine from the marine sponge ''Neopetrosia exigua'' collected in Palau |journal=Marine Drugs |volume=2 |issue=4 |pages=154–63 |vauthors=Liu H, Mishima Y, Fujiwara T, Nagai H, Kitazawa A, Mine Y, etal |doi=10.3390/md204154|pmc=3783253 |doi-access=free }}</ref> however, dopamine has been reported in the nervous systems of many other ] species, including the ] ], ] and some ]s.<ref>{{cite journal | vauthors = Kass-Simon G, Pierobon P | title = Cnidarian chemical neurotransmission, an updated overview | journal = Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology | volume = 146 | issue = 1 | pages = 9–25 | date = January 2007 | pmid = 17101286 | doi = 10.1016/j.cbpa.2006.09.008 }}</ref> This dates the emergence of dopamine as a neurotransmitter back to the earliest appearance of the nervous system, over 500 million years ago in the ] Period. Dopamine functions as a neurotransmitter in ]s, ]s, ]s, ], and several types of ].<ref name="Cottrell">{{cite journal | vauthors = Cottrell GA | title = Occurrence of dopamine and noradrenaline in the nervous tissue of some invertebrate species | journal = British Journal of Pharmacology and Chemotherapy | volume = 29 | issue = 1 | pages = 63–69 | date = January 1967 | pmid = 19108240 | pmc = 1557178 | doi = 10.1111/j.1476-5381.1967.tb01939.x }}</ref><ref>{{cite journal | vauthors = Kindt KS, Quast KB, Giles AC, De S, Hendrey D, Nicastro I, Rankin CH, Schafer WR | s2cid = 2092645 | title = Dopamine mediates context-dependent modulation of sensory plasticity in C. elegans | journal = Neuron | volume = 55 | issue = 4 | pages = 662–76 | date = August 2007 | pmid = 17698017 | doi = 10.1016/j.neuron.2007.07.023 | doi-access = free }}</ref> | |||
| ! Macromolecule !! K<sub>i</sub> (nM) | |||
| |- | |||
| | ] || 8248 | |||
| |- | |||
| | ] || >10000 | |||
| |- | |||
| | ] || 130 | |||
| |- | |||
| | ] || 598 | |||
| |- | |||
| | ] || 32.5 | |||
| |- | |||
| | ] || 182.6 | |||
| |- | |||
| | ] || 228 | |||
| |- | |||
| | ] || 67 | |||
| |- | |||
| | ] || 323 | |||
| |- | |||
| | ] || 422 | |||
| |} | |||
| In every type of animal that has been examined, dopamine has been seen to modify motor behavior.<ref name="Barron">{{cite journal | vauthors = Barron AB, Søvik E, Cornish JL | title = The roles of dopamine and related compounds in reward-seeking behavior across animal phyla | journal = Frontiers in Behavioral Neuroscience | volume = 4 | pages = 163 | year = 2010 | pmid = 21048897 | pmc = 2967375 | doi = 10.3389/fnbeh.2010.00163 | doi-access = free }}</ref> In the ], ] '']'', it reduces ] and increases food-exploratory movements; in ]s it produces "screw-like" movements; in ]es it inhibits swimming and promotes crawling. Across a wide range of vertebrates, dopamine has an "activating" effect on behavior-switching and response selection, comparable to its effect in mammals.<ref name=Barron/><ref>{{cite journal | vauthors = Kalivas PW, Stewart J | s2cid = 10775295 | title = Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity | journal = Brain Research. Brain Research Reviews | volume = 16 | issue = 3 | pages = 223–44 | date = 1 September 1991 | pmid = 1665095 | doi = 10.1016/0165-0173(91)90007-U }}</ref> | |||
| ==Biochemical mechanisms== | |||
| Dopamine has also consistently been shown to play a role in reward learning, in all animal groups.<ref name=Barron/> As in all vertebrates – ]s such as ], ]s, ]s and ] can all be trained to repeat an action if it is consistently followed by an increase in dopamine levels.<ref name="Barron"/> In ], distinct elements for reward learning suggest a modular structure to the insect reward processing system that broadly parallels that in the mammalian one.<ref>{{cite journal | vauthors = Perry CJ, Barron AB | s2cid = 19678766 | title = Neural mechanisms of reward in insects | journal = Annual Review of Entomology | volume = 58 | issue = 1 | pages = 543–62 | date = 2013 | pmid = 23020615 | doi = 10.1146/annurev-ento-120811-153631 | url = http://pdfs.semanticscholar.org/5526/26c5fe0572b4d6419555a1976877c757b0da.pdf | archive-url = https://web.archive.org/web/20200605184440/http://pdfs.semanticscholar.org/5526/26c5fe0572b4d6419555a1976877c757b0da.pdf | url-status = dead | archive-date = 2020-06-05 }}</ref> For example, dopamine regulates short- and long-term learning in monkeys;<ref>{{cite journal | vauthors = Takikawa Y, Kawagoe R, Hikosaka O | title = A possible role of midbrain dopamine neurons in short- and long-term adaptation of saccades to position-reward mapping | journal = Journal of Neurophysiology | volume = 92 | issue = 4 | pages = 2520–29 | date = October 2004 | pmid = 15163669 | doi = 10.1152/jn.00238.2004 | s2cid = 12534057 | url = http://pdfs.semanticscholar.org/b8a6/84a6d815d43db0b2491e4d3db5c664970e6e.pdf | archive-url = https://web.archive.org/web/20190302163535/http://pdfs.semanticscholar.org/b8a6/84a6d815d43db0b2491e4d3db5c664970e6e.pdf | url-status = dead | archive-date = 2019-03-02 }}</ref> in fruit flies, different groups of dopamine neurons mediate reward signals for short- and long-term memories.<ref>{{cite journal | vauthors = Yamagata N, Ichinose T, Aso Y, Plaçais PY, Friedrich AB, Sima RJ, Preat T, Rubin GM, Tanimoto H | title = Distinct dopamine neurons mediate reward signals for short- and long-term memories | journal = Proceedings of the National Academy of Sciences of the United States of America | volume = 112 | issue = 2 | pages = 578–83 | date = January 2015 | pmid = 25548178 | doi = 10.1073/pnas.1421930112 | pmc = 4299218 | bibcode = 2015PNAS..112..578Y | doi-access = free }}</ref> | |||
| Structurally, dopamine belongs to the ] and ] classes. In biological systems, dopamine is synthesized in brain cells and adrenal cells from the precursor ]. In brain cells, it is transported to ] sites and packaged into vesicles for release, which occurs during synaptic transmission. After release, free dopamine is either reabsorbed into the presynaptic terminal for reuse, or broken down by the enzymes ] or COMT, producing a variety of degradation metabolites. | |||
| It had long been believed that arthropods were an exception to this with dopamine being seen as having an adverse effect. Reward was seen to be mediated instead by ], a neurotransmitter closely related to norepinephrine.<ref name=Waddell/> More recent studies, however, have shown that dopamine does play a part in reward learning in fruit flies. It has also been found that the rewarding effect of octopamine is due to its activating a set of dopaminergic neurons not previously accessed in the research.<ref name="Waddell">{{cite journal | vauthors = Waddell S | title = Reinforcement signalling in Drosophila; dopamine does it all after all | journal = Current Opinion in Neurobiology | volume = 23 | issue = 3 | pages = 324–29 | date = June 2013 | pmid = 23391527 | pmc = 3887340 | doi = 10.1016/j.conb.2013.01.005 }}</ref> | |||
| ===Biosynthesis=== | |||
| ===Plants=== | |||
| Dopamine is ] in a restricted set of cell types, mainly ]s and cells in the ] of the ]s. This is the ]: | |||
| ] and fruit pulp of ].|alt=Photo of a bunch of bananas.]] | |||
| * <small>L</small>-Phenylalanine → <small>L</small>-Tyrosine → <small>L</small>-DOPA → Dopamine | |||
| Many plants, including a variety of food plants, synthesize dopamine to varying degrees.<ref name=Kulma/> The highest concentrations have been observed in bananas—the fruit pulp of ] and ] contains dopamine at levels of 40 to 50 parts per million by weight.<ref name=Kulma/> Potatoes, avocados, broccoli, and Brussels sprouts may also contain dopamine at levels of 1 part per million or more; oranges, tomatoes, spinach, beans, and other plants contain measurable concentrations less than 1 part per million.<ref name=Kulma>{{cite journal |vauthors=Kulma A, Szopa J |title=Catecholamines are active compounds in plants |journal=Plant Science |year=2007 |volume=172 |pages=433–40 |doi=10.1016/j.plantsci.2006.10.013 |issue=3|bibcode=2007PlnSc.172..433K }}</ref> The dopamine in plants is synthesized from the amino acid tyrosine, by biochemical mechanisms similar to those that animals use.<ref name=Kulma/> It can be metabolized in a variety of ways, producing ] and a variety of ]s as byproducts.<ref name=Kulma/> The functions of plant catecholamines have not been clearly established, but there is evidence that they play a role in the response to stressors such as bacterial infection, act as growth-promoting factors in some situations, and modify the way that sugars are metabolized. The receptors that mediate these actions have not yet been identified, nor have the intracellular mechanisms that they activate.<ref name=Kulma/> | |||
| Thus the direct precursor of dopamine is ], but this itself can be synthesized from the essential amino acid ] or the non-essential amino acid ]. These amino acids are found in nearly every protein and as such are provided from ingestion of protein-containing food, with tyrosine being the most common. Although dopamine itself is also found in many types of food, it is incapable of crossing the ] that surrounds and protects the brain. It must therefore be synthesized inside the brain in order to perform its neural actions. | |||
| Dopamine consumed in food cannot act on the brain, because it cannot cross the blood–brain barrier.<ref name="Nice-pharma"/> However, there are also a variety of plants that contain L-DOPA, the metabolic precursor of dopamine.<ref name=Ingle>{{cite journal | vauthors = Ingle PK |year=2003 |title=L-DOPA bearing plants |journal=Natural Product Radiance |volume=2 |pages=126–33 |url=http://nopr.niscair.res.in/bitstream/123456789/12261/1/NPR%202%283%29%20126-133.pdf |archive-url=https://web.archive.org/web/20140302114720/http://nopr.niscair.res.in/bitstream/123456789/12261/1/NPR%202%283%29%20126-133.pdf |archive-date=2014-03-02 |url-status=live |access-date=24 September 2015}}</ref> The highest concentrations are found in the leaves and bean pods of plants of the genus '']'', especially in '']'' (velvet beans), which have been used as a source for L-DOPA as a drug.<ref>{{cite journal |year=1993 |title=Occurrence of L-DOPA and dopamine in plants and cell cultures of ''Mucuna pruriens'' and effects of 2, 4-d and NaCl on these compounds |journal=Plant Cell, Tissue and Organ Culture |volume=33 |issue=3 |pages=259–64 |doi=10.1007/BF02319010 | vauthors = Wichers HJ, Visser JF, Huizing HJ, Pras N|s2cid=44814336 }}</ref> Another plant containing substantial amounts of L-DOPA is '']'', the plant that produces fava beans (also known as "broad beans"). The level of L-DOPA in the beans, however, is much lower than in the pod shells and other parts of the plant.<ref>{{cite journal | vauthors = Longo R, Castellani A, Sberze P, Tibolla M | title = Distribution of l-dopa and related amino acids in Vicia | journal = Phytochemistry | year = 1974 | volume = 13 | issue = 1 | pages = 167–71 | doi = 10.1016/S0031-9422(00)91287-1| bibcode = 1974PChem..13..167L }}</ref> The seeds of '']'' and '']'' trees also contain substantial amounts of L-DOPA.<ref name=Ingle/> | |||
| <small>L</small>-Phenylalanine is converted into <small>L</small>-tyrosine by the enzyme ] (TH) (also known as phenylalanine hydroxylase), with molecular ] (O<sub>2</sub>) and ] (THB) as ]s. <small>L</small>-Tyrosine is converted into <small>L</small>-DOPA by the enzyme ] (TH), with ] (THB), O<sub>2</sub>, and ] ] (Fe<sup>2+</sup>) as cofactors. <small>L</small>-DOPA is converted into dopamine by the enzyme ] (AAAD; also known as DOPA decarboxylase (DDC)), with ] (PLP) as the cofactor. | |||
| In a species of ] ] '']'', a major component of some ]s, dopamine is present in very high concentrations, estimated at 4.4% of dry weight. There is evidence that this dopamine functions as an anti-] defense, reducing consumption by snails and ].<ref name="pmid16489461">{{cite journal | vauthors = Van Alstyne KL, Nelson AV, Vyvyan JR, Cancilla DA | s2cid = 5029574 | title = Dopamine functions as an antiherbivore defense in the temperate green alga Ulvaria obscura | journal = Oecologia | volume = 148 | issue = 2 | pages = 304–11 | date = June 2006 | pmid = 16489461 | doi = 10.1007/s00442-006-0378-3 | bibcode = 2006Oecol.148..304V }}</ref> | |||
| Dopamine itself is also used as precursor in the synthesis of the neurotransmitters ] and ]. Dopamine is converted into norepinephrine by the enzyme ] (DBH), with O<sub>2</sub> and ] as cofactors. Norepinephrine is converted into epinephrine by the enzyme ] (PNMT) with ] (SAMe) as the cofactor. | |||
| ===As a precursor for melanin=== | |||
| It should be noted that some of the cofactors also require their own synthesis. Deficiency in any required amino acid or cofactor will result in subsequent dopamine, norepinephrine, and epinephrine biosynthesis impairment and deficiency. | |||
| {{anchor|dopamine-melanin}} | |||
| {{Phenylalanine biosynthesis|caption=This is the biosynthesis of catecholamines, including dopamine, as well as phenethylaminergic trace amines from the amino acid phenylalanine. Abbreviations used:<br />{{nowrap|DBH: Dopamine β-hydroxylase;}}<br />{{nowrap|AADC:Aromatic L-amino acid decarboxylase;}}<br />{{nowrap|AAAH: (Biopterin-dependent) aromatic amino acid hydroxylase;}}<br /> {{nowrap|COMT: Catechol O-methyltransferase;}}<br />{{nowrap|PNMT: Phenylethanolamine N-methyltransferase}}}} | |||
| Melanins are a family of dark-pigmented substances found in a wide range of organisms.<ref name=Simon/> Chemically they are closely related to dopamine, and there is a type of melanin, known as '''dopamine-melanin''', that can be synthesized by oxidation of dopamine via the enzyme ].<ref name=Simon>{{cite journal | vauthors = Simon JD, Peles D, Wakamatsu K, Ito S | title = Current challenges in understanding melanogenesis: bridging chemistry, biological control, morphology, and function | journal = Pigment Cell & Melanoma Research | volume = 22 | issue = 5 | pages = 563–79 | date = October 2009 | pmid = 19627559 | doi = 10.1111/j.1755-148X.2009.00610.x | doi-access = free }}</ref> The melanin that darkens human skin is not of this type: it is synthesized by a pathway that uses L-DOPA as a precursor but not dopamine.<ref name=Simon/> However, there is substantial evidence that the ] that gives a dark color to the brain's substantia nigra is at least in part dopamine-melanin.<ref>{{cite journal | vauthors = Fedorow H, Tribl F, Halliday G, Gerlach M, Riederer P, Double KL | s2cid = 503902 | title = Neuromelanin in human dopamine neurons: comparison with peripheral melanins and relevance to Parkinson's disease | journal = Progress in Neurobiology | volume = 75 | issue = 2 | pages = 109–24 | date = February 2005 | pmid = 15784302 | doi = 10.1016/j.pneurobio.2005.02.001 }}</ref> | |||
| ===Storage, release, and reuptake=== | |||
| Inside the brain dopamine functions as a ], and is controlled by a set of mechanisms that are common to all neurotransmitters. After synthesis, dopamine is transported from the ]{{Citation needed|date=November 2013}} into ]s by the ] (VMAT2). Dopamine is stored in and remains in these vesicles until an ] occurs and causes the contents of the vesicles to be ejected into the ]. | |||
| Dopamine-derived melanin probably appears in at least some other biological systems as well. Some of the dopamine in plants is likely to be used as a precursor for dopamine-melanin.<ref>{{cite journal |year=1967 |title=Melanins from DOPA-containing plants |journal=Phytochemistry |volume=6 |issue=1 |pages=13–18 |doi=10.1016/0031-9422(67)85002-7 | vauthors = Andrews RS, Pridham JB|bibcode=1967PChem...6...13A }}</ref> The complex patterns that appear on butterfly wings, as well as black-and-white stripes on the bodies of insect larvae, are also thought to be caused by spatially structured accumulations of dopamine-melanin.<ref>{{cite journal | vauthors = Beldade P, Brakefield PM | s2cid = 17417235 | title = The genetics and evo-devo of butterfly wing patterns | journal = Nature Reviews. Genetics | volume = 3 | issue = 6 | pages = 442–52 | date = June 2002 | pmid = 12042771 | doi = 10.1038/nrg818 }}</ref> | |||
| Once in the synapse, dopamine binds to and activates ]s, which can be located either on ] target cells or on the membrane of the dopamine-releasing cell itself (i.e., ]s). | |||
| ==History and development== | |||
| After an action potential, the dopamine molecules quickly become unbound from their receptors. They are then absorbed back into the presynaptic cell, via ] mediated either by the high-affinity ] (DAT) or by the low-affinity ] (PMAT). Once back in the cytosol, dopamine is subsequently repackaged into vesicles by VMAT2, making it available for future release. | |||
| {{Main|History of catecholamine research}} | |||
| Dopamine was first synthesized in 1910 by ] and James Ewens at ] Laboratories in London, England<ref name="pmid18781671">{{cite journal | vauthors = Fahn S | title = The history of dopamine and levodopa in the treatment of Parkinson's disease | journal = Movement Disorders | volume = 23 | issue = Suppl 3 | pages = S497–508 | year = 2008 | pmid = 18781671 | doi = 10.1002/mds.22028 | s2cid = 45572523 }}</ref> and first identified in the human brain by ] in 1957. It was named dopamine because it is a ] whose precursor in the Barger-Ewens synthesis is 3,4-'''d'''ihydr'''o'''xy'''p'''henyl'''a'''lanine (levodopa or L-DOPA). Dopamine's function as a neurotransmitter was first recognized in 1958 by ] and ] at the Laboratory for Chemical Pharmacology of the National Heart Institute of ].<ref name="pmid11165672">{{cite journal | vauthors = Benes FM | title = Carlsson and the discovery of dopamine | journal = Trends in Pharmacological Sciences | volume = 22 | issue = 1 | pages = 46–47 | date = January 2001 | pmid = 11165672 | doi = 10.1016/S0165-6147(00)01607-2 }}</ref> Carlsson was awarded the 2000 ] for showing that dopamine is not only a precursor of norepinephrine (noradrenaline) and epinephrine (adrenaline), but is also itself a neurotransmitter.<ref name=Barondes>{{cite book| vauthors = Barondes SH | year=2003| title=Better Than Prozac| pages=| location=New York| publisher=Oxford University Press| isbn=978-0-19-515130-5| url-access=registration| url=https://archive.org/details/betterthanprozac00baro/page/21}}</ref> | |||
| === |
===Polydopamine=== | ||
| Research motivated by ] ]s in ]s led to the discovery in 2007 that a wide variety of materials, if placed in a solution of dopamine at slightly basic ], will become coated with a layer of polymerized dopamine, often referred to as '''polydopamine'''.<ref>{{cite journal | vauthors = Lee H, Dellatore SM, Miller WM, Messersmith PB | title = Mussel-inspired surface chemistry for multifunctional coatings | journal = Science | volume = 318 | issue = 5849 | pages = 426–30 | date = October 2007 | pmid = 17947576 | pmc = 2601629 | doi = 10.1126/science.1147241 | bibcode = 2007Sci...318..426L }}</ref><ref name=Dreyer>{{cite journal | title = Perspectives on poly(dopamine) | vauthors = Dreyer DR, Miller DJ, Freeman BD, Paul DR, Bielawski CW | journal = Chemical Science | year = 2013 | doi = 10.1039/C3SC51501J | volume=4 | issue = 10 | page=3796}}</ref> This polymerized dopamine forms by a spontaneous oxidation reaction, and is formally a type of melanin.<ref name=Lynge>{{cite journal | vauthors = Lynge ME, van der Westen R, Postma A, Städler B | title = Polydopamine—a nature-inspired polymer coating for biomedical science | journal = Nanoscale | volume = 3 | issue = 12 | pages = 4916–28 | date = December 2011 | pmid = 22024699 | doi = 10.1039/c1nr10969c | url = https://www.researchgate.net/publication/51742922 | archive-url = https://web.archive.org/web/20140307205318/http://www.researchgate.net/profile/Almar_Postma/publication/51742922_Polydopamine--a_nature-inspired_polymer_coating_for_biomedical_science/file/d912f50318c9e0c7bb.pdf | bibcode = 2011Nanos...3.4916L | archive-date = 7 March 2014 }}</ref> Furthermore, dopamine self-polymerization can be used to modulate the mechanical properties of peptide-based gels.<ref>{{cite journal | vauthors = Fichman G, Schneider JP | title = Dopamine Self-Polymerization as a Simple and Powerful Tool to Modulate the Viscoelastic Mechanical Properties of Peptide-Based Gels | journal = Molecules | volume = 26 | issue = 5 | pages = 1363 | date = March 2021 | pmid = 33806346 | pmc = 7961423 | doi = 10.3390/molecules26051363 | doi-access = free }}</ref> Synthesis of polydopamine usually involves reaction of dopamine hydrochloride with ] as a base in water. The structure of polydopamine is unknown.<ref name=Dreyer /> | |||
| ] | |||
| Polydopamine coatings can form on objects ranging in size from ]s to large surfaces.<ref name=Lynge/> Polydopamine layers have chemical properties that have the potential to be extremely useful, and numerous studies have examined their possible applications.<ref name=Lynge/> At the simplest level, they can be used for protection against damage by light, or to form capsules for drug delivery.<ref name=Lynge/> At a more sophisticated level, their adhesive properties may make them useful as substrates for ]s or other biologically active macromolecules.<ref name=Lynge/> | |||
| Dopamine is broken down into inactive metabolites by a set of enzymes, ] (MAO), ] (ALDH), and ] (COMT), acting in sequence. Both ]s of MAO, ] and ], are equally effective. | |||
| == See also == | |||
| The metabolites produced by these processes are: | |||
| * ] | |||
| *DOPAL (]) | |||
| * ] | |||
| *DOPAC (]) | |||
| * ] | |||
| *DOPET (3,4-dihydroxyphenylethanol, also known as ]) | |||
| * ] | |||
| *MOPET (3-methoxy-4-hydroxyphenylethanol, also known as ]) | |||
| * |
* ] | ||
| * |
* ] | ||
| == References == | |||
| All of these are intermediate metabolites except MOPET and HVA, which are filtered from the bloodstream by the kidneys and then excreted in the urine. | |||
| {{Reflist|30em}} | |||
| ==Further reading (most recent first)== | |||
| The specific reactions that make up these pathways are: | |||
| *{{cite news | |||
| * Dopamine → DOPAL, mediated by MAO | |||
| | vauthors = Szalavitz M | |||
| * DOPAL → DOPAC, mediated by ] | |||
| |title=A 'Dopamine Fast' Will Not Save You From Addiction | |||
| * DOPAL → DOPET, mediated by ] (minor pathway) | |||
| |date=September 13, 2024 | |||
| * DOPAC → HVA, mediated by COMT | |||
| |newspaper=] | |||
| * DOPET → MOPET, mediated by COMT | |||
| |url=https://www.nytimes.com/2024/09/13/opinion/addiction-dopamine-brain-chemistry.html}} | |||
| * Dopamine → 3-MT, mediated by COMT | |||
| * 3-MT → HVA, mediated by MAO | |||
| * {{cite news | |||
| In most areas of the brain, including the ] and ], dopamine is inactivated by reuptake via the DAT, then enzymatic breakdown by MAO into DOPAC. In the ], however, there are very few DAT proteins, and dopamine is inactivated instead by reuptake via the ] (NET), presumably on neighboring norepinephrine neurons, then enzymatic breakdown by COMT into 3-MT.<ref>{{cite journal |last1=Morón |first1=JA |last2=Brockington |first2=A |last3=Wise |first3=RA |last4=Rocha |first4=BA |last5=Hope |first5=BT |title=Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines |url=http://www.jneurosci.org/cgi/content/abstract/22/2/389 |journal=Journal of Neuroscience |volume=22 |issue=2 |pages=389–95 |year=2002 |pmid=11784783}}</ref> The DAT pathway is roughly an order of magnitude faster than the NET pathway: in mice, dopamine concentrations decay with a half-life of 200 milliseconds in the ] (which uses the DAT pathway) versus 2,000 milliseconds in the prefrontal cortex.<ref>{{cite journal |doi=10.1523/JNEUROSCI.0665-07.2007 |last1=Yavich |first1=L |last2=Forsberg |first2=MM |last3=Karayiorgou |first3=M |last4=Gogos |first4=JA |last5=Männistö |first5=PT |title=Site-specific role of catechol-O-methyltransferase in dopamine overflow within prefrontal cortex and dorsal striatum |url=http://www.jneurosci.org/cgi/content/abstract/27/38/10196 |journal=Journal of Neuroscience |volume=27 |issue=38 |pages=10196–209 |year=2007 |pmid=17881525}}</ref> Dopamine that is not broken down by enzymes is repackaged into vesicles for future release. | |||
| |title=We Have a Dopamine Problem | |||
| | vauthors = Smith DG | |||
| |date=June 30, 2023 | |||
| |newspaper=] | |||
| |url=https://www.nytimes.com/2023/06/30/well/mind/dopamine-brain-behavior.html?pgtype=Article&action=click&module=RelatedLinks}} | |||
| * {{cite book | |||
| ==Chemistry== | |||
| |title=One Man's Endless Hunt for a Dopamine Rush in Virtual Reality | |||
| {{multiple image | |||
| | vauthors = Metz C | |||
| <!-- Essential parameters --> | |||
| |date=September 29, 2021 | |||
| | align = center | |||
| |publisher=] | |||
| | direction = horizontal | |||
| |url=https://www.nytimes.com/2021/09/29/technology/virtual-reality-fascination.html}} | |||
| <!-- Extra parameters --> | |||
| | header = | |||
| | header_align = | |||
| | header_background = | |||
| | footer = | |||
| | footer_align = | |||
| | footer_background = | |||
| | background color = | |||
| |image1=Dopamine2.svg | |||
| |caption1=Dopamine structure | |||
| |alt1=Dopamine structure | |||
| |width1=250 | |||
| |image2=Fenyloetyloamina.svg | |||
| |caption2=Phenethylamine structure | |||
| |alt2=Phenethylamine structure | |||
| |width2=256 | |||
| |image3=Brenzcatechin.svg | |||
| |caption3=Catechol structure | |||
| |alt3=Catechol structure | |||
| |width3=117 | |||
| }} | |||
| * {{cite book | |||
| Chemically, a dopamine molecule consists of a ] structure (a ] ring with two ] side groups) with one ] group attached. As such, dopamine is the simplest possible ], a family that also includes the ]s ] and ]. The presence of a benzene ring with an attached amine group makes it a ], a family that includes numerous psychoactive drugs. | |||
| |title=Dopamine Nation | |||
| | vauthors = Lembke A | |||
| |year=2021 | |||
| |publisher=Headline | |||
| |isbn=9781472294128}} | |||
| ==External links== | |||
| Dopamine, like most ]s, is an ]. At neutral or acidic pH levels it is generally ]. The protonated form is highly water-soluble and relatively stable, though it is capable of oxidizing if exposed to oxygen or other oxidants. At basic pH levels, dopamine becomes deprotonated. In this ] form it is less soluble and also highly reactive and easily oxidized. Because of this pH-dependence, dopamine is supplied for chemical or pharmaceutical use in the form of dopamine hydochloride, that is, the ] salt that is created when dopamine is combined with hydrochloric acid. In dry form, dopamine hydrochloride is a fine colorless powder. When dissolved in distilled water it gives a solution that is mildly acidic and therefore relatively stable. It cannot, however, be combined with alkaline solutions such as a ] buffer without being rendered inactive. | |||
| * {{Wiktionary-inline|Dopamine}} | |||
| * {{Commons category-inline}} | |||
| ===Oxidation=== | |||
| Dopamine in the body is normally broken down by oxidation catalyzed by the enzyme ]. However, dopamine is also capable of ], that is, direct reaction with oxygen, yielding ]s plus various ]s as products.<ref name=Sulzer>{{cite journal |author=Sulzer D, Zecca L |title=Intraneuronal dopamine-quinone synthesis: a review |journal=Neurotox Res |volume=1 |issue=3 |pages=181–95 |date=February 2000 |pmid=12835101 |doi=10.1007/BF03033289 |url=}}</ref> The rate of autoxidation can be increased by the presence of ferrous iron or other factors. The ability of dopamine autoxidation to produce quinones and free radicals makes it a potent cell toxin, and there is evidence that this mechanism may contribute to cell loss that occurs in ] or other conditions.<ref>{{cite journal |author=Miyazaki I, Asanuma M |title=Dopaminergic neuron-specific oxidative stress caused by dopamine itself |journal=Acta Med. Okayama |volume=62 |issue=3 |pages=141–50 |date=June 2008 |pmid=18596830 |doi= |url=http://www.lib.okayama-u.ac.jp/www/acta/pdf/62_3_141.pdf |format=PDF}}</ref> | |||
| === {{anchor|Polydopamine}} Polydopamine=== | |||
| Research motivated by mussel adhesive proteins led to the discovery in 2007 that a wide variety of materials, if placed in a solution of dopamine at slightly basic pH, will become coated with a layer of polymerized dopamine, often referred to as '''polydopamine'''.<ref>''Mussel-Inspired Surface Chemistry for Multifunctional Coatings'' Haeshin Lee, Shara M. Dellatore, William M. Miller, Phillip B. Messersmith Science 19 October 2007: Vol. 318 no. 5849 pp. 426–430 {{DOI|10.1126/science.1147241}}</ref><ref name=Dreyer>''Perspectives on poly(dopamine)'' Daniel R. Dreyer, Daniel J. Miller, Benny D. Freeman, Donald R. Paul and Christopher W. Bielawski Chem" ''Sci'' 2013, Advance Article {{DOI|10.1039/C3SC51501J}}</ref> This polymerized dopamine forms by a spontaneous oxidation reaction, and is formally a type of ].<ref name=Lynge>{{cite journal |author=Lynge ME, van der Westen R, Postma A, Städler B |title=Polydopamine--a nature-inspired polymer coating for biomedical science |journal=Nanoscale |volume=3 |issue=12 |pages=4916–28 |date=December 2011 |pmid=22024699 |doi=10.1039/c1nr10969c |url=http://www.researchgate.net/publication/51742922_Polydopamine--a_nature-inspired_polymer_coating_for_biomedical_science/file/d912f50318c9e0c7bb.pdf}}</ref> Synthesis usually involves reaction of dopamine hydrochloride with ] as a base in water. The structure of polydopamine is unknown.<ref name=Dreyer /> | |||
| Polydopamine coatings can form on objects ranging in size from nanoparticles to large surfaces. Polydopamine layers have chemical properties that have the potential to be extremely useful, and numerous studies have examined their possible applications. At the simplest level, they can be used for protection against damage by light, or to form capsules for drug delivery. At a more sophisticated level, their adhesive properties may make them useful as substrates for biosensors or other biologically active macromolecules.<ref name=Lynge/> | |||
| ==History== | |||
| {{Main|History of catecholamine research}} | |||
| Dopamine was first synthesized in 1910 by ] and ] at ] Laboratories in London, England.<ref></ref> It was named dopamine because it is a ] whose ] in the Barger-Ewens synthesis is 3,4-''d''ihydr''o''xy''p''henyl''a''lanine (levodopamine or ]). Dopamine's function as a neurotransmitter was first recognized in 1958 by ] and ] at the Laboratory for Chemical Pharmacology of the National Heart Institute of ].<ref>{{cite journal |doi=10.1016/S0165-6147(00)01607-2 |last1=Benes |first1=F.M. |year=2001 |title=Carlsson and the discovery of dopamine |url= |journal=Trends in Pharmacological Sciences |volume=22 |issue=1 |pages=46–47 |pmid=11165672}}</ref> Carlsson was awarded the 2000 ] for showing that dopamine is not only a precursor of ] (noradrenaline) and ] (adrenaline), but also a neurotransmitter. | |||
| == See also == | |||
| {{columns-list|3| | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] (N-methyldopamine) | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| }} | |||
| ==References== | |||
| {{Reflist|30em}} | |||
| ==External links== | |||
| {{Wiktionary|Dopamine}} | |||
| * {{DrugBank|APRD00085}} | |||
| * | |||
| * - The Association for Clinical Biochemistry and Laboratory Medicine | |||
| {{Neurotransmitters}} | {{Neurotransmitters}} | ||
| {{Cardiac stimulants}} | |||
| {{Dopaminergics}} | |||
| {{Dopamine receptor modulators}} | |||
| {{Phenethylamines}} | |||
| {{TAAR ligands}} | {{TAAR ligands}} | ||
| {{Monoamine neurotoxins}} | |||
| {{Phenethylamines}} | |||
| {{Emergency medicine}} | |||
| {{Authority control}} | |||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 21:14, 14 December 2024
Organic chemical that functions both as a hormone and a neurotransmitter This article is about the neurotransmitter. For medical uses, see Dopamine (medication). For other uses, see Dopamine (disambiguation).Pharmaceutical compound
 Skeletal formula of dopamine Skeletal formula of dopamine | |
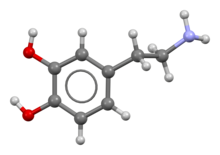 Ball-and-stick model of the dopamine molecule as found in solution. In the solid state, dopamine adopts a zwitterionic form. Ball-and-stick model of the dopamine molecule as found in solution. In the solid state, dopamine adopts a zwitterionic form. | |
| Clinical data | |
|---|---|
| Other names |
|
| Physiological data | |
| Source tissues | Substantia nigra; ventral tegmental area; many others |
| Target tissues | System-wide |
| Receptors | D1, D2, D3, D4, D5, TAAR1 |
| Agonists | Direct: apomorphine, bromocriptine Indirect: cocaine, amphetamine, methylphenidate |
| Antagonists | Neuroleptics, metoclopramide, domperidone |
| Precursor | Phenylalanine, tyrosine, and L-DOPA |
| Biosynthesis | DOPA decarboxylase |
| Metabolism | MAO, COMT |
| Identifiers | |
IUPAC name
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| ECHA InfoCard | 100.000.101 |
| Chemical and physical data | |
| Formula | C8H11NO2 |
| Molar mass | 153.181 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Dopamine (DA, a contraction of 3,4-dihydroxyphenethylamine) is a neuromodulatory molecule that plays several important roles in cells. It is an organic chemical of the catecholamine and phenethylamine families. Dopamine constitutes about 80% of the catecholamine content in the brain. It is an amine synthesized by removing a carboxyl group from a molecule of its precursor chemical, L-DOPA, which is synthesized in the brain and kidneys. Dopamine is also synthesized in plants and most animals. In the brain, dopamine functions as a neurotransmitter—a chemical released by neurons (nerve cells) to send signals to other nerve cells. Neurotransmitters are synthesized in specific regions of the brain but affect many regions systemically. The brain includes several distinct dopamine pathways, one of which plays a major role in the motivational component of reward-motivated behavior. The anticipation of most types of rewards increases the level of dopamine in the brain, and many addictive drugs increase dopamine release or block its reuptake into neurons following release. Other brain dopamine pathways are involved in motor control and in controlling the release of various hormones. These pathways and cell groups form a dopamine system which is neuromodulatory.
In popular culture and media, dopamine is often portrayed as the main chemical of pleasure, but the current opinion in pharmacology is that dopamine instead confers motivational salience; in other words, dopamine signals the perceived motivational prominence (i.e., the desirability or aversiveness) of an outcome, which in turn propels the organism's behavior toward or away from achieving that outcome.
Outside the central nervous system, dopamine functions primarily as a local paracrine messenger. In blood vessels, it inhibits norepinephrine release and acts as a vasodilator; in the kidneys, it increases sodium excretion and urine output; in the pancreas, it reduces insulin production; in the digestive system, it reduces gastrointestinal motility and protects intestinal mucosa; and in the immune system, it reduces the activity of lymphocytes. With the exception of the blood vessels, dopamine in each of these peripheral systems is synthesized locally and exerts its effects near the cells that release it.
Several important diseases of the nervous system are associated with dysfunctions of the dopamine system, and some of the key medications used to treat them work by altering the effects of dopamine. Parkinson's disease, a degenerative condition causing tremor and motor impairment, is caused by a loss of dopamine-secreting neurons in an area of the midbrain called the substantia nigra. Its metabolic precursor L-DOPA can be manufactured; Levodopa, a pure form of L-DOPA, is the most widely used treatment for Parkinson's. There is evidence that schizophrenia involves altered levels of dopamine activity, and most antipsychotic drugs used to treat this are dopamine antagonists which reduce dopamine activity. Similar dopamine antagonist drugs are also some of the most effective anti-nausea agents. Restless legs syndrome and attention deficit hyperactivity disorder (ADHD) are associated with decreased dopamine activity. Dopaminergic stimulants can be addictive in high doses, but some are used at lower doses to treat ADHD. Dopamine itself is available as a manufactured medication for intravenous injection. It is useful in the treatment of severe heart failure or cardiogenic shock. In newborn babies it may be used for hypotension and septic shock.
Structure
A dopamine molecule consists of a catechol structure (a benzene ring with two hydroxyl side groups) with one amine group attached via an ethyl chain. As such, dopamine is the simplest possible catecholamine, a family that also includes the neurotransmitters norepinephrine and epinephrine. The presence of a benzene ring with this amine attachment makes it a substituted phenethylamine, a family that includes numerous psychoactive drugs.
Like most amines, dopamine is an organic base. As a base, it is generally protonated in acidic environments (in an acid-base reaction). The protonated form is highly water-soluble and relatively stable, but can become oxidized if exposed to oxygen or other oxidants. In basic environments, dopamine is not protonated. In this free base form, it is less water-soluble and also more highly reactive. Because of the increased stability and water-solubility of the protonated form, dopamine is supplied for chemical or pharmaceutical use as dopamine hydrochloride—that is, the hydrochloride salt that is created when dopamine is combined with hydrochloric acid. In dry form, dopamine hydrochloride is a fine powder which is white to yellow in color.
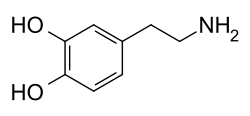 Dopamine structure
Dopamine structure Phenethylamine structure
Phenethylamine structure Catechol structure
Catechol structure
Biochemistry
Biosynthetic pathways for catecholamines and trace amines in the human brain
 L-Phenylalanine
L-Tyrosine
L-DOPA
Epinephrine
Phenethylamine
p-Tyramine
Dopamine
Norepinephrine
N-Methylphenethylamine
N-Methyltyramine
p-Octopamine
Synephrine
3-Methoxytyramine
AADC
AADC
AADC
primary
L-Phenylalanine
L-Tyrosine
L-DOPA
Epinephrine
Phenethylamine
p-Tyramine
Dopamine
Norepinephrine
N-Methylphenethylamine
N-Methyltyramine
p-Octopamine
Synephrine
3-Methoxytyramine
AADC
AADC
AADC
primarypathway PNMT PNMT PNMT PNMT AAAH AAAH brain CYP2D6 minor pathway COMT DBH DBH |
Synthesis
Dopamine is synthesized in a restricted set of cell types, mainly neurons and cells in the medulla of the adrenal glands. The primary and minor metabolic pathways respectively are:
- Primary: L-Phenylalanine → L-Tyrosine → L-DOPA → Dopamine
- Minor: L-Phenylalanine → L-Tyrosine → p-Tyramine → Dopamine
- Minor: L-Phenylalanine → m-Tyrosine → m-Tyramine → Dopamine
The direct precursor of dopamine, L-DOPA, can be synthesized indirectly from the essential amino acid phenylalanine or directly from the non-essential amino acid tyrosine. These amino acids are found in nearly every protein and so are readily available in food, with tyrosine being the most common. Although dopamine is also found in many types of food, it is incapable of crossing the blood–brain barrier that surrounds and protects the brain. It must therefore be synthesized inside the brain to perform its neuronal activity.
L-Phenylalanine is converted into L-tyrosine by the enzyme phenylalanine hydroxylase, with molecular oxygen (O2) and tetrahydrobiopterin as cofactors. L-Tyrosine is converted into L-DOPA by the enzyme tyrosine hydroxylase, with tetrahydrobiopterin, O2, and iron (Fe) as cofactors. L-DOPA is converted into dopamine by the enzyme aromatic L-amino acid decarboxylase (also known as DOPA decarboxylase), with pyridoxal phosphate as the cofactor.
Dopamine itself is used as precursor in the synthesis of the neurotransmitters norepinephrine and epinephrine. Dopamine is converted into norepinephrine by the enzyme dopamine β-hydroxylase, with O2 and L-ascorbic acid as cofactors. Norepinephrine is converted into epinephrine by the enzyme phenylethanolamine N-methyltransferase with S-adenosyl-L-methionine as the cofactor.
Some of the cofactors also require their own synthesis. Deficiency in any required amino acid or cofactor can impair the synthesis of dopamine, norepinephrine, and epinephrine.
Degradation
Dopamine is broken down into inactive metabolites by a set of enzymes—monoamine oxidase (MAO), catechol-O-methyl transferase (COMT), and aldehyde dehydrogenase (ALDH), acting in sequence. Both isoforms of monoamine oxidase, MAO-A and MAO-B, effectively metabolize dopamine. Different breakdown pathways exist but the main end-product is homovanillic acid (HVA), which has no known biological activity. From the bloodstream, homovanillic acid is filtered out by the kidneys and then excreted in the urine. The two primary metabolic routes that convert dopamine into HVA are:
- Dopamine → DOPAL → DOPAC → HVA – catalyzed by MAO, ALDH, and COMT respectively
- Dopamine → 3-Methoxytyramine → HVA – catalyzed by COMT and MAO+ALDH respectively
In clinical research on schizophrenia, measurements of homovanillic acid in plasma have been used to estimate levels of dopamine activity in the brain. A difficulty in this approach however, is separating the high level of plasma homovanillic acid contributed by the metabolism of norepinephrine.
Although dopamine is normally broken down by an oxidoreductase enzyme, it is also susceptible to oxidation by direct reaction with oxygen, yielding quinones plus various free radicals as products. The rate of oxidation can be increased by the presence of ferric iron or other factors. Quinones and free radicals produced by autoxidation of dopamine can poison cells, and there is evidence that this mechanism may contribute to the cell loss that occurs in Parkinson's disease and other conditions.
Functions
Cellular effects
Main articles: Dopamine receptor and TAAR1| Family | Receptor | Gene | Type | Mechanism |
|---|---|---|---|---|
| D1-like | D1 | DRD1 | Gs-coupled. | Increase intracellular levels of cAMP by activating adenylate cyclase. |
| D5 | DRD5 | |||
| D2-like | D2 | DRD2 | Gi-coupled. | Decrease intracellular levels of cAMP by inhibiting adenylate cyclase. |
| D3 | DRD3 | |||
| D4 | DRD4 | |||
| TAAR | TAAR1 | TAAR1 | Gs-coupled. Gq-coupled. |
Increase intracellular levels of cAMP and intracellular calcium concentration. |
Dopamine exerts its effects by binding to and activating cell surface receptors. In humans, dopamine has a high binding affinity at dopamine receptors and human trace amine-associated receptor 1 (hTAAR1). In mammals, five subtypes of dopamine receptors have been identified, labeled from D1 to D5. All of them function as metabotropic, G protein-coupled receptors, meaning that they exert their effects via a complex second messenger system. These receptors can be divided into two families, known as D1-like and D2-like. For receptors located on neurons in the nervous system, the ultimate effect of D1-like activation (D1 and D5) can be excitation (via opening of sodium channels) or inhibition (via opening of potassium channels); the ultimate effect of D2-like activation (D2, D3, and D4) is usually inhibition of the target neuron. Consequently, it is incorrect to describe dopamine itself as either excitatory or inhibitory: its effect on a target neuron depends on which types of receptors are present on the membrane of that neuron and on the internal responses of that neuron to the second messenger cAMP. D1 receptors are the most numerous dopamine receptors in the human nervous system; D2 receptors are next; D3, D4, and D5 receptors are present at significantly lower levels.
Storage, release, and reuptake
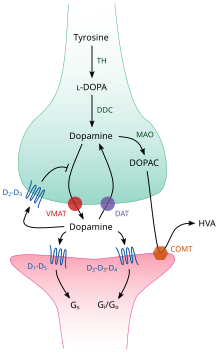
TH: tyrosine hydroxylase
DOPA: L-DOPA
DAT: dopamine transporter
DDC: DOPA decarboxylase
VMAT: vesicular monoamine transporter 2
MAO: Monoamine oxidase
COMT: Catechol-O-methyl transferase
HVA: Homovanillic acid
Inside the brain, dopamine functions as a neurotransmitter and neuromodulator, and is controlled by a set of mechanisms common to all monoamine neurotransmitters. After synthesis, dopamine is transported from the cytosol into secretory vesicles, including synaptic vesicles, small and large dense core vesicles by a solute carrier—a vesicular monoamine transporter, VMAT2. Dopamine is stored in these vesicles until it is ejected into the synaptic cleft. In most cases, the release of dopamine occurs through a process called exocytosis which is caused by action potentials, but it can also be caused by the activity of an intracellular trace amine-associated receptor, TAAR1. TAAR1 is a high-affinity receptor for dopamine, trace amines, and certain substituted amphetamines that is located along membranes in the intracellular milieu of the presynaptic cell; activation of the receptor can regulate dopamine signaling by inducing dopamine reuptake inhibition and efflux as well as by inhibiting neuronal firing through a diverse set of mechanisms.
Once in the synapse, dopamine binds to and activates dopamine receptors. These can be postsynaptic dopamine receptors, which are located on dendrites (the postsynaptic neuron), or presynaptic autoreceptors (e.g., the D2sh and presynaptic D3 receptors), which are located on the membrane of an axon terminal (the presynaptic neuron). After the postsynaptic neuron elicits an action potential, dopamine molecules quickly become unbound from their receptors. They are then absorbed back into the presynaptic cell, via reuptake mediated either by the dopamine transporter or by the plasma membrane monoamine transporter. Once back in the cytosol, dopamine can either be broken down by a monoamine oxidase or repackaged into vesicles by VMAT2, making it available for future release.
In the brain the level of extracellular dopamine is modulated by two mechanisms: phasic and tonic transmission. Phasic dopamine release, like most neurotransmitter release in the nervous system, is driven directly by action potentials in the dopamine-containing cells. Tonic dopamine transmission occurs when small amounts of dopamine are released without being preceded by presynaptic action potentials. Tonic transmission is regulated by a variety of factors, including the activity of other neurons and neurotransmitter reuptake.
Central nervous system
Main articles: Dopaminergic cell groups and Dopaminergic pathways See also: Hypothalamic–pituitary–prolactin axis
Inside the brain, dopamine plays important roles in executive functions, motor control, motivation, arousal, reinforcement, and reward, as well as lower-level functions including lactation, sexual gratification, and nausea. The dopaminergic cell groups and pathways make up the dopamine system which is neuromodulatory.
Dopaminergic neurons (dopamine-producing nerve cells) are comparatively few in number—a total of around 400,000 in the human brain—and their cell bodies are confined in groups to a few relatively small brain areas. However their axons project to many other brain areas, and they exert powerful effects on their targets. These dopaminergic cell groups were first mapped in 1964 by Annica Dahlström and Kjell Fuxe, who assigned them labels starting with the letter "A" (for "aminergic"). In their scheme, areas A1 through A7 contain the neurotransmitter norepinephrine, whereas A8 through A14 contain dopamine. The dopaminergic areas they identified are the substantia nigra (groups 8 and 9); the ventral tegmental area (group 10); the posterior hypothalamus (group 11); the arcuate nucleus (group 12); the zona incerta (group 13) and the periventricular nucleus (group 14).
The substantia nigra is a small midbrain area that forms a component of the basal ganglia. This has two parts—an input area called the pars reticulata and an output area called the pars compacta. The dopaminergic neurons are found mainly in the pars compacta (cell group A8) and nearby (group A9). In humans, the projection of dopaminergic neurons from the substantia nigra pars compacta to the dorsal striatum, termed the nigrostriatal pathway, plays a significant role in the control of motor function and in learning new motor skills. These neurons are especially vulnerable to damage, and when a large number of them die, the result is a parkinsonian syndrome.
The ventral tegmental area (VTA) is another midbrain area. The most prominent group of VTA dopaminergic neurons projects to the prefrontal cortex via the mesocortical pathway and another smaller group projects to the nucleus accumbens via the mesolimbic pathway. Together, these two pathways are collectively termed the mesocorticolimbic projection. The VTA also sends dopaminergic projections to the amygdala, cingulate gyrus, hippocampus, and olfactory bulb. Mesocorticolimbic neurons play a central role in reward and other aspects of motivation. Accumulating literature shows that dopamine also plays a crucial role in aversive learning through its effects on a number of brain regions.
The posterior hypothalamus has dopamine neurons that project to the spinal cord, but their function is not well established. There is some evidence that pathology in this area plays a role in restless legs syndrome, a condition in which people have difficulty sleeping due to an overwhelming compulsion to constantly move parts of the body, especially the legs.
The arcuate nucleus and the periventricular nucleus of the hypothalamus have dopamine neurons that form an important projection—the tuberoinfundibular pathway which goes to the pituitary gland, where it influences the secretion of the hormone prolactin. Dopamine is the primary neuroendocrine inhibitor of the secretion of prolactin from the anterior pituitary gland. Dopamine produced by neurons in the arcuate nucleus is secreted into the hypophyseal portal system of the median eminence, which supplies the pituitary gland. The prolactin cells that produce prolactin, in the absence of dopamine, secrete prolactin continuously; dopamine inhibits this secretion.
The zona incerta, grouped between the arcuate and periventricular nuclei, projects to several areas of the hypothalamus, and participates in the control of gonadotropin-releasing hormone, which is necessary to activate the development of the male and female reproductive systems, following puberty.
An additional group of dopamine-secreting neurons is found in the retina of the eye. These neurons are amacrine cells, meaning that they have no axons. They release dopamine into the extracellular medium, and are specifically active during daylight hours, becoming silent at night. This retinal dopamine acts to enhance the activity of cone cells in the retina while suppressing rod cells—the result is to increase sensitivity to color and contrast during bright light conditions, at the cost of reduced sensitivity when the light is dim.
Basal ganglia

The largest and most important sources of dopamine in the vertebrate brain are the substantia nigra and ventral tegmental area. Both structures are components of the midbrain, closely related to each other and functionally similar in many respects. The largest component of the basal ganglia is the striatum. The substantia nigra sends a dopaminergic projection to the dorsal striatum, while the ventral tegmental area sends a similar type of dopaminergic projection to the ventral striatum.
Progress in understanding the functions of the basal ganglia has been slow. The most popular hypotheses, broadly stated, propose that the basal ganglia play a central role in action selection. The action selection theory in its simplest form proposes that when a person or animal is in a situation where several behaviors are possible, activity in the basal ganglia determines which of them is executed, by releasing that response from inhibition while continuing to inhibit other motor systems that if activated would generate competing behaviors. Thus the basal ganglia, in this concept, are responsible for initiating behaviors, but not for determining the details of how they are carried out. In other words, they essentially form a decision-making system.
The basal ganglia can be divided into several sectors, and each is involved in controlling particular types of actions. The ventral sector of the basal ganglia (containing the ventral striatum and ventral tegmental area) operates at the highest level of the hierarchy, selecting actions at the whole-organism level. The dorsal sectors (containing the dorsal striatum and substantia nigra) operate at lower levels, selecting the specific muscles and movements that are used to implement a given behavior pattern.
Dopamine contributes to the action selection process in at least two important ways. First, it sets the "threshold" for initiating actions. The higher the level of dopamine activity, the lower the impetus required to evoke a given behavior. As a consequence, high levels of dopamine lead to high levels of motor activity and impulsive behavior; low levels of dopamine lead to torpor and slowed reactions. Parkinson's disease, in which dopamine levels in the substantia nigra circuit are greatly reduced, is characterized by stiffness and difficulty initiating movement—however, when people with the disease are confronted with strong stimuli such as a serious threat, their reactions can be as vigorous as those of a healthy person. In the opposite direction, drugs that increase dopamine release, such as cocaine or amphetamine, can produce heightened levels of activity, including, at the extreme, psychomotor agitation and stereotyped movements.
The second important effect of dopamine is as a "teaching" signal. When an action is followed by an increase in dopamine activity, the basal ganglia circuit is altered in a way that makes the same response easier to evoke when similar situations arise in the future. This is a form of operant conditioning, in which dopamine plays the role of a reward signal.
Reward

In the language used to discuss the reward system, reward is the attractive and motivational property of a stimulus that induces appetitive behavior (also known as approach behavior) and consummatory behavior. A rewarding stimulus is one that can induce the organism to approach it and choose to consume it. Pleasure, learning (e.g., classical and operant conditioning), and approach behavior are the three main functions of reward. As an aspect of reward, pleasure provides a definition of reward; however, while all pleasurable stimuli are rewarding, not all rewarding stimuli are pleasurable (e.g., extrinsic rewards like money). The motivational or desirable aspect of rewarding stimuli is reflected by the approach behavior that they induce, whereas the pleasure from intrinsic rewards results from consuming them after acquiring them. A neuropsychological model which distinguishes these two components of an intrinsically rewarding stimulus is the incentive salience model, where "wanting" or desire (less commonly, "seeking") corresponds to appetitive or approach behavior while "liking" or pleasure corresponds to consummatory behavior. In human drug addicts, "wanting" becomes dissociated with "liking" as the desire to use an addictive drug increases, while the pleasure obtained from consuming it decreases due to drug tolerance.
Within the brain, dopamine functions partly as a global reward signal. An initial dopamine response to a rewarding stimulus encodes information about the salience, value, and context of a reward. In the context of reward-related learning, dopamine also functions as a reward prediction error signal, that is, the degree to which the value of a reward is unexpected. According to this hypothesis proposed by Montague, Dayan, and Sejnowski, rewards that are expected do not produce a second phasic dopamine response in certain dopaminergic cells, but rewards that are unexpected, or greater than expected, produce a short-lasting increase in synaptic dopamine, whereas the omission of an expected reward actually causes dopamine release to drop below its background level. The "prediction error" hypothesis has drawn particular interest from computational neuroscientists, because an influential computational-learning method known as temporal difference learning makes heavy use of a signal that encodes prediction error. This confluence of theory and data has led to a fertile interaction between neuroscientists and computer scientists interested in machine learning.
Evidence from microelectrode recordings from the brains of animals shows that dopamine neurons in the ventral tegmental area (VTA) and substantia nigra are strongly activated by a wide variety of rewarding events. These reward-responsive dopamine neurons in the VTA and substantia nigra are crucial for reward-related cognition and serve as the central component of the reward system. The function of dopamine varies in each axonal projection from the VTA and substantia nigra; for example, the VTA–nucleus accumbens shell projection assigns incentive salience ("want") to rewarding stimuli and its associated cues, the VTA–prefrontal cortex projection updates the value of different goals in accordance with their incentive salience, the VTA–amygdala and VTA–hippocampus projections mediate the consolidation of reward-related memories, and both the VTA–nucleus accumbens core and substantia nigra–dorsal striatum pathways are involved in learning motor responses that facilitate the acquisition of rewarding stimuli. Some activity within the VTA dopaminergic projections appears to be associated with reward prediction as well.
Pleasure
While dopamine has a central role in causing "wanting," associated with the appetitive or approach behavioral responses to rewarding stimuli, detailed studies have shown that dopamine cannot simply be equated with hedonic "liking" or pleasure, as reflected in the consummatory behavioral response. Dopamine neurotransmission is involved in some but not all aspects of pleasure-related cognition, since pleasure centers have been identified both within the dopamine system (i.e., nucleus accumbens shell) and outside the dopamine system (i.e., ventral pallidum and parabrachial nucleus). For example, direct electrical stimulation of dopamine pathways, using electrodes implanted in the brain, is experienced as pleasurable, and many types of animals are willing to work to obtain it. Antipsychotic drugs reduce dopamine levels and tend to cause anhedonia, a diminished ability to experience pleasure. Many types of pleasurable experiences—such as sexual intercourse, eating, and playing video games—increase dopamine release. All addictive drugs directly or indirectly affect dopamine neurotransmission in the nucleus accumbens; these drugs increase drug "wanting", leading to compulsive drug use, when repeatedly taken in high doses, presumably through the sensitization of incentive-salience. Drugs that increase synaptic dopamine concentrations include psychostimulants such as methamphetamine and cocaine. These produce increases in "wanting" behaviors, but do not greatly alter expressions of pleasure or change levels of satiation. However, opiate drugs such as heroin and morphine produce increases in expressions of "liking" and "wanting" behaviors. Moreover, animals in which the ventral tegmental dopamine system has been rendered inactive do not seek food, and will starve to death if left to themselves, but if food is placed in their mouths they will consume it and show expressions indicative of pleasure.
A clinical study from January 2019 that assessed the effect of a dopamine precursor (levodopa), dopamine antagonist (risperidone), and a placebo on reward responses to music – including the degree of pleasure experienced during musical chills, as measured by changes in electrodermal activity as well as subjective ratings – found that the manipulation of dopamine neurotransmission bidirectionally regulates pleasure cognition (specifically, the hedonic impact of music) in human subjects. This research demonstrated that increased dopamine neurotransmission acts as a sine qua non condition for pleasurable hedonic reactions to music in humans.
A study published in Nature in 1998 found evidence that playing video games releases dopamine in the human striatum. This dopamine is associated with learning, behavior reinforcement, attention, and sensorimotor integration. Researchers used positron emission tomography scans and C-labelled raclopride to track dopamine levels in the brain during goal-directed motor tasks and found that dopamine release was positively correlated with task performance and was greatest in the ventral striatum. This was the first study to demonstrate the behavioral conditions under which dopamine is released in humans. It highlights the ability of positron emission tomography to detect neurotransmitter fluxes during changes in behavior. According to research, potentially problematic video game use is related to personality traits such as low self-esteem and low self-efficacy, anxiety, aggression, and clinical symptoms of depression and anxiety disorders. Additionally, the reasons individuals play video games vary and may include coping, socialization, and personal satisfaction. The DSM-5 defines Internet Gaming Disorder as a mental disorder closely related to Gambling Disorder. This has been supported by some researchers but has also caused controversy.
Outside the central nervous system
Dopamine does not cross the blood–brain barrier, so its synthesis and functions in peripheral areas are to a large degree independent of its synthesis and functions in the brain. A substantial amount of dopamine circulates in the bloodstream, but its functions there are not entirely clear. Dopamine is found in blood plasma at levels comparable to those of epinephrine, but in humans, over 95% of the dopamine in the plasma is in the form of dopamine sulfate, a conjugate produced by the enzyme sulfotransferase 1A3/1A4 acting on free dopamine. The bulk of this dopamine sulfate is produced in the mesenteric organs. The production of dopamine sulfate is thought to be a mechanism for detoxifying dopamine that is ingested as food or produced by the digestive process—levels in the plasma typically rise more than fifty-fold after a meal. Dopamine sulfate has no known biological functions and is excreted in urine.
The relatively small quantity of unconjugated dopamine in the bloodstream may be produced by the sympathetic nervous system, the digestive system, or possibly other organs. It may act on dopamine receptors in peripheral tissues, or be metabolized, or be converted to norepinephrine by the enzyme dopamine beta hydroxylase, which is released into the bloodstream by the adrenal medulla. Some dopamine receptors are located in the walls of arteries, where they act as a vasodilator and an inhibitor of norepinephrine release from postganglionic sympathetic nerves terminals (dopamine can inhibit norepinephrine release by acting on presynaptic dopamine receptors, and also on presynaptic α-1 receptors, like norepinephrine itself). These responses might be activated by dopamine released from the carotid body under conditions of low oxygen, but whether arterial dopamine receptors perform other biologically useful functions is not known.
Beyond its role in modulating blood flow, there are several peripheral systems in which dopamine circulates within a limited area and performs an exocrine or paracrine function. The peripheral systems in which dopamine plays an important role include the immune system, the kidneys and the pancreas.
Immune system
In the immune system dopamine acts upon receptors present on immune cells, especially lymphocytes. Dopamine can also affect immune cells in the spleen, bone marrow, and circulatory system. In addition, dopamine can be synthesized and released by immune cells themselves. The main effect of dopamine on lymphocytes is to reduce their activation level. The functional significance of this system is unclear, but it affords a possible route for interactions between the nervous system and immune system, and may be relevant to some autoimmune disorders.
Kidneys
The renal dopaminergic system is located in the cells of the nephron in the kidney, where all subtypes of dopamine receptors are present. Dopamine is also synthesized there, by tubule cells, and discharged into the tubular fluid. Its actions include increasing the blood supply to the kidneys, increasing the glomerular filtration rate, and increasing the excretion of sodium in the urine. Hence, defects in renal dopamine function can lead to reduced sodium excretion and consequently result in the development of high blood pressure. There is strong evidence that faults in the production of dopamine or in the receptors can result in a number of pathologies including oxidative stress, edema, and either genetic or essential hypertension. Oxidative stress can itself cause hypertension. Defects in the system can also be caused by genetic factors or high blood pressure.
Pancreas
In the pancreas the role of dopamine is somewhat complex. The pancreas consists of two parts, an exocrine and an endocrine component. The exocrine part synthesizes and secretes digestive enzymes and other substances, including dopamine, into the small intestine. The function of this secreted dopamine after it enters the small intestine is not clearly established—the possibilities include protecting the intestinal mucosa from damage and reducing gastrointestinal motility (the rate at which content moves through the digestive system).
The pancreatic islets make up the endocrine part of the pancreas, and synthesize and secrete hormones including insulin into the bloodstream. There is evidence that the beta cells in the islets that synthesize insulin contain dopamine receptors, and that dopamine acts to reduce the amount of insulin they release. The source of their dopamine input is not clearly established—it may come from dopamine that circulates in the bloodstream and derives from the sympathetic nervous system, or it may be synthesized locally by other types of pancreatic cells.
Medical uses
Main article: Dopamine (medication)
Dopamine as a manufactured medication is sold under the trade names Intropin, Dopastat, and Revimine, among others. It is on the World Health Organization's List of Essential Medicines. It is most commonly used as a stimulant drug in the treatment of severe low blood pressure, slow heart rate, and cardiac arrest. It is especially important in treating these in newborn infants. It is given intravenously. Since the half-life of dopamine in plasma is very short—approximately one minute in adults, two minutes in newborn infants and up to five minutes in preterm infants—it is usually given in a continuous intravenous drip rather than a single injection.
Its effects, depending on dosage, include an increase in sodium excretion by the kidneys, an increase in urine output, an increase in heart rate, and an increase in blood pressure. At low doses it acts through the sympathetic nervous system to increase heart muscle contraction force and heart rate, thereby increasing cardiac output and blood pressure. Higher doses also cause vasoconstriction that further increases blood pressure. Older literature also describes very low doses thought to improve kidney function without other consequences, but recent reviews have concluded that doses at such low levels are not effective and may sometimes be harmful. While some effects result from stimulation of dopamine receptors, the prominent cardiovascular effects result from dopamine acting at α1, β1, and β2 adrenergic receptors.
Side effects of dopamine include negative effects on kidney function and irregular heartbeats. The LD50, or lethal dose which is expected to prove fatal in 50% of the population, has been found to be: 59 mg/kg (mouse; administered intravenously); 95 mg/kg (mouse; administered intraperitoneally); 163 mg/kg (rat; administered intraperitoneally); 79 mg/kg (dog; administered intravenously).
Disease, disorders, and pharmacology
See also: List of dopaminergic drugsThe dopamine system plays a central role in several significant medical conditions, including Parkinson's disease, attention deficit hyperactivity disorder, Tourette syndrome, schizophrenia, bipolar disorder, and addiction. Aside from dopamine itself, there are many other important drugs that act on dopamine systems in various parts of the brain or body. Some are used for medical or recreational purposes, but neurochemists have also developed a variety of research drugs, some of which bind with high affinity to specific types of dopamine receptors and either agonize or antagonize their effects, and many that affect other aspects of dopamine physiology, including dopamine transporter inhibitors, VMAT inhibitors, and enzyme inhibitors.
Aging brain
Main article: Aging brainA number of studies have reported an age-related decline in dopamine synthesis and dopamine receptor density (i.e., the number of receptors) in the brain. This decline has been shown to occur in the striatum and extrastriatal regions. Decreases in the D1, D2, and D3 receptors are well documented. The reduction of dopamine with aging is thought to be responsible for many neurological symptoms that increase in frequency with age, such as decreased arm swing and increased rigidity. Changes in dopamine levels may also cause age-related changes in cognitive flexibility.
Multiple sclerosis
Studies reported that dopamine imbalance influences the fatigue in multiple sclerosis. In patients with multiple sclerosis, dopamine inhibits production of IL-17 and IFN-γ by peripheral blood mononuclear cells.
Parkinson's disease
Parkinson's disease is an age-related disorder characterized by movement disorders such as stiffness of the body, slowing of movement, and trembling of limbs when they are not in use. In advanced stages it progresses to dementia and eventually death. The main symptoms are caused by the loss of dopamine-secreting cells in the substantia nigra. These dopamine cells are especially vulnerable to damage, and a variety of insults, including encephalitis (as depicted in the book and movie Awakenings), repeated sports-related concussions, and some forms of chemical poisoning such as MPTP, can lead to substantial cell loss, producing a parkinsonian syndrome that is similar in its main features to Parkinson's disease. Most cases of Parkinson's disease, however, are idiopathic, meaning that the cause of cell death cannot be identified.
The most widely used treatment for parkinsonism is administration of L-DOPA, the metabolic precursor for dopamine. L-DOPA is converted to dopamine in the brain and various parts of the body by the enzyme DOPA decarboxylase. L-DOPA is used rather than dopamine itself because, unlike dopamine, it is capable of crossing the blood–brain barrier. It is often co-administered with an enzyme inhibitor of peripheral decarboxylation such as carbidopa or benserazide, to reduce the amount converted to dopamine in the periphery and thereby increase the amount of L-DOPA that enters the brain. When L-DOPA is administered regularly over a long time period, a variety of unpleasant side effects such as dyskinesia often begin to appear; even so, it is considered the best available long-term treatment option for most cases of Parkinson's disease.
L-DOPA treatment cannot restore the dopamine cells that have been lost, but it causes the remaining cells to produce more dopamine, thereby compensating for the loss to at least some degree. In advanced stages the treatment begins to fail because the cell loss is so severe that the remaining ones cannot produce enough dopamine regardless of L-DOPA levels. Other drugs that enhance dopamine function, such as bromocriptine and pergolide, are also sometimes used to treat Parkinsonism, but in most cases L-DOPA appears to give the best trade-off between positive effects and negative side-effects.
Dopaminergic medications that are used to treat Parkinson's disease are sometimes associated with the development of a dopamine dysregulation syndrome, which involves the overuse of dopaminergic medication and medication-induced compulsive engagement in natural rewards like gambling and sexual activity. The latter behaviors are similar to those observed in individuals with a behavioral addiction.
Drug addiction and psychostimulants
Main article: Addiction
Cocaine, substituted amphetamines (including methamphetamine), Adderall, methylphenidate (marketed as Ritalin or Concerta), and other psychostimulants exert their effects primarily or partly by increasing dopamine levels in the brain by a variety of mechanisms. Cocaine and methylphenidate are dopamine transporter blockers or reuptake inhibitors; they non-competitively inhibit dopamine reuptake, resulting in increased dopamine concentrations in the synaptic cleft. Like cocaine, substituted amphetamines and amphetamine also increase the concentration of dopamine in the synaptic cleft, but by different mechanisms.
The effects of psychostimulants include increases in heart rate, body temperature, and sweating; improvements in alertness, attention, and endurance; increases in pleasure produced by rewarding events; but at higher doses agitation, anxiety, or even loss of contact with reality. Drugs in this group can have a high addiction potential, due to their activating effects on the dopamine-mediated reward system in the brain. However some can also be useful, at lower doses, for treating attention deficit hyperactivity disorder (ADHD) and narcolepsy. An important differentiating factor is the onset and duration of action. Cocaine can take effect in seconds if it is injected or inhaled in free base form; the effects last from 5 to 90 minutes. This rapid and brief action makes its effects easily perceived and consequently gives it high addiction potential. Methylphenidate taken in pill form, in contrast, can take two hours to reach peak levels in the bloodstream, and depending on formulation the effects can last for up to 12 hours. These longer acting formulations have the benefit of reducing the potential for abuse, and improving adherence for treatment by using more convenient dosage regimens.

A variety of addictive drugs produce an increase in reward-related dopamine activity. Stimulants such as nicotine, cocaine and methamphetamine promote increased levels of dopamine which appear to be the primary factor in causing addiction. For other addictive drugs such as the opioid heroin, the increased levels of dopamine in the reward system may play only a minor role in addiction. When people addicted to stimulants go through withdrawal, they do not experience the physical suffering associated with alcohol withdrawal or withdrawal from opiates; instead they experience craving, an intense desire for the drug characterized by irritability, restlessness, and other arousal symptoms, brought about by psychological dependence.
The dopamine system plays a crucial role in several aspects of addiction. At the earliest stage, genetic differences that alter the expression of dopamine receptors in the brain can predict whether a person will find stimulants appealing or aversive. Consumption of stimulants produces increases in brain dopamine levels that last from minutes to hours. Finally, the chronic elevation in dopamine that comes with repetitive high-dose stimulant consumption triggers a wide-ranging set of structural changes in the brain that are responsible for the behavioral abnormalities which characterize an addiction. Treatment of stimulant addiction is very difficult, because even if consumption ceases, the craving that comes with psychological withdrawal does not. Even when the craving seems to be extinct, it may re-emerge when faced with stimuli that are associated with the drug, such as friends, locations and situations. Association networks in the brain are greatly interlinked.
Psychosis and antipsychotic drugs
Main article: PsychosisPsychiatrists in the early 1950s discovered that a class of drugs known as typical antipsychotics (also known as major tranquilizers), were often effective at reducing the psychotic symptoms of schizophrenia. The introduction of the first widely used antipsychotic, chlorpromazine (Thorazine), in the 1950s, led to the release of many patients with schizophrenia from institutions in the years that followed. By the 1970s researchers understood that these typical antipsychotics worked as antagonists on the D2 receptors. This realization led to the so-called dopamine hypothesis of schizophrenia, which postulates that schizophrenia is largely caused by hyperactivity of brain dopamine systems. The dopamine hypothesis drew additional support from the observation that psychotic symptoms were often intensified by dopamine-enhancing stimulants such as methamphetamine, and that these drugs could also produce psychosis in healthy people if taken in large enough doses. In the following decades other atypical antipsychotics that had fewer serious side effects were developed. Many of these newer drugs do not act directly on dopamine receptors, but instead produce alterations in dopamine activity indirectly. These drugs were also used to treat other psychoses. Antipsychotic drugs have a broadly suppressive effect on most types of active behavior, and particularly reduce the delusional and agitated behavior characteristic of overt psychosis.
Later observations, however, have caused the dopamine hypothesis to lose popularity, at least in its simple original form. For one thing, patients with schizophrenia do not typically show measurably increased levels of brain dopamine activity. Even so, many psychiatrists and neuroscientists continue to believe that schizophrenia involves some sort of dopamine system dysfunction. As the "dopamine hypothesis" has evolved over time, however, the sorts of dysfunctions it postulates have tended to become increasingly subtle and complex.
Psychopharmacologist Stephen M. Stahl suggested in a review of 2018 that in many cases of psychosis, including schizophrenia, three interconnected networks based on dopamine, serotonin, and glutamate – each on its own or in various combinations – contributed to an overexcitation of dopamine D2 receptors in the ventral striatum.
Attention deficit hyperactivity disorder
Altered dopamine neurotransmission is implicated in attention deficit hyperactivity disorder (ADHD), a condition associated with impaired cognitive control, in turn leading to problems with regulating attention (attentional control), inhibiting behaviors (inhibitory control), and forgetting things or missing details (working memory), among other problems. There are genetic links between dopamine receptors, the dopamine transporter, and ADHD, in addition to links to other neurotransmitter receptors and transporters. The most important relationship between dopamine and ADHD involves the drugs that are used to treat ADHD. Some of the most effective therapeutic agents for ADHD are psychostimulants such as methylphenidate (Ritalin, Concerta) and amphetamine (Evekeo, Adderall, Dexedrine), drugs that increase both dopamine and norepinephrine levels in the brain. The clinical effects of these psychostimulants in treating ADHD are mediated through the indirect activation of dopamine and norepinephrine receptors, specifically dopamine receptor D1 and adrenoceptor α2, in the prefrontal cortex.
Pain
Dopamine plays a role in pain processing in multiple levels of the central nervous system including the spinal cord, periaqueductal gray, thalamus, basal ganglia, and cingulate cortex. Decreased levels of dopamine have been associated with painful symptoms that frequently occur in Parkinson's disease. Abnormalities in dopaminergic neurotransmission also occur in several painful clinical conditions, including burning mouth syndrome, fibromyalgia, and restless legs syndrome.
Nausea
Nausea and vomiting are largely determined by activity in the area postrema in the medulla of the brainstem, in a region known as the chemoreceptor trigger zone. This area contains a large population of type D2 dopamine receptors. Consequently, drugs that activate D2 receptors have a high potential to cause nausea. This group includes some medications that are administered for Parkinson's disease, as well as other dopamine agonists such as apomorphine. In some cases, D2-receptor antagonists such as metoclopramide are useful as anti-nausea drugs.
Fear and Anxiety
Simultaneous positron emission tomography (PET) and functional magnetic resonance imaging (fMRI), have shown that the amount of dopamine release is dependent on the strength of conditioned fear response and is linearly coupled to learning-induced activity in the amygdala. Dopamine is generally linked to reward learning, but it also plays a key role in fear learning and extinction by helping to form, store and update fear memories through its interaction with other brain regions like amygdala, ventromedial prefrontal cortex and striatum.
Comparative biology and evolution
Microorganisms
There are no reports of dopamine in archaea, but it has been detected in some types of bacteria and in the protozoan called Tetrahymena. Perhaps more importantly, there are types of bacteria that contain homologs of all the enzymes that animals use to synthesize dopamine. It has been proposed that animals derived their dopamine-synthesizing machinery from bacteria, via horizontal gene transfer that may have occurred relatively late in evolutionary time, perhaps as a result of the symbiotic incorporation of bacteria into eukaryotic cells that gave rise to mitochondria.
Animals
Dopamine is used as a neurotransmitter in most multicellular animals. In sponges there is only a single report of the presence of dopamine, with no indication of its function; however, dopamine has been reported in the nervous systems of many other radially symmetric species, including the cnidarian jellyfish, hydra and some corals. This dates the emergence of dopamine as a neurotransmitter back to the earliest appearance of the nervous system, over 500 million years ago in the Cambrian Period. Dopamine functions as a neurotransmitter in vertebrates, echinoderms, arthropods, molluscs, and several types of worm.
In every type of animal that has been examined, dopamine has been seen to modify motor behavior. In the model organism, nematode Caenorhabditis elegans, it reduces locomotion and increases food-exploratory movements; in flatworms it produces "screw-like" movements; in leeches it inhibits swimming and promotes crawling. Across a wide range of vertebrates, dopamine has an "activating" effect on behavior-switching and response selection, comparable to its effect in mammals.
Dopamine has also consistently been shown to play a role in reward learning, in all animal groups. As in all vertebrates – invertebrates such as roundworms, flatworms, molluscs and common fruit flies can all be trained to repeat an action if it is consistently followed by an increase in dopamine levels. In fruit flies, distinct elements for reward learning suggest a modular structure to the insect reward processing system that broadly parallels that in the mammalian one. For example, dopamine regulates short- and long-term learning in monkeys; in fruit flies, different groups of dopamine neurons mediate reward signals for short- and long-term memories.
It had long been believed that arthropods were an exception to this with dopamine being seen as having an adverse effect. Reward was seen to be mediated instead by octopamine, a neurotransmitter closely related to norepinephrine. More recent studies, however, have shown that dopamine does play a part in reward learning in fruit flies. It has also been found that the rewarding effect of octopamine is due to its activating a set of dopaminergic neurons not previously accessed in the research.
Plants

Many plants, including a variety of food plants, synthesize dopamine to varying degrees. The highest concentrations have been observed in bananas—the fruit pulp of red and yellow bananas contains dopamine at levels of 40 to 50 parts per million by weight. Potatoes, avocados, broccoli, and Brussels sprouts may also contain dopamine at levels of 1 part per million or more; oranges, tomatoes, spinach, beans, and other plants contain measurable concentrations less than 1 part per million. The dopamine in plants is synthesized from the amino acid tyrosine, by biochemical mechanisms similar to those that animals use. It can be metabolized in a variety of ways, producing melanin and a variety of alkaloids as byproducts. The functions of plant catecholamines have not been clearly established, but there is evidence that they play a role in the response to stressors such as bacterial infection, act as growth-promoting factors in some situations, and modify the way that sugars are metabolized. The receptors that mediate these actions have not yet been identified, nor have the intracellular mechanisms that they activate.
Dopamine consumed in food cannot act on the brain, because it cannot cross the blood–brain barrier. However, there are also a variety of plants that contain L-DOPA, the metabolic precursor of dopamine. The highest concentrations are found in the leaves and bean pods of plants of the genus Mucuna, especially in Mucuna pruriens (velvet beans), which have been used as a source for L-DOPA as a drug. Another plant containing substantial amounts of L-DOPA is Vicia faba, the plant that produces fava beans (also known as "broad beans"). The level of L-DOPA in the beans, however, is much lower than in the pod shells and other parts of the plant. The seeds of Cassia and Bauhinia trees also contain substantial amounts of L-DOPA.
In a species of marine green algae Ulvaria obscura, a major component of some algal blooms, dopamine is present in very high concentrations, estimated at 4.4% of dry weight. There is evidence that this dopamine functions as an anti-herbivore defense, reducing consumption by snails and isopods.
As a precursor for melanin
Melanins are a family of dark-pigmented substances found in a wide range of organisms. Chemically they are closely related to dopamine, and there is a type of melanin, known as dopamine-melanin, that can be synthesized by oxidation of dopamine via the enzyme tyrosinase. The melanin that darkens human skin is not of this type: it is synthesized by a pathway that uses L-DOPA as a precursor but not dopamine. However, there is substantial evidence that the neuromelanin that gives a dark color to the brain's substantia nigra is at least in part dopamine-melanin.
Dopamine-derived melanin probably appears in at least some other biological systems as well. Some of the dopamine in plants is likely to be used as a precursor for dopamine-melanin. The complex patterns that appear on butterfly wings, as well as black-and-white stripes on the bodies of insect larvae, are also thought to be caused by spatially structured accumulations of dopamine-melanin.
History and development
Main article: History of catecholamine researchDopamine was first synthesized in 1910 by George Barger and James Ewens at Wellcome Laboratories in London, England and first identified in the human brain by Katharine Montagu in 1957. It was named dopamine because it is a monoamine whose precursor in the Barger-Ewens synthesis is 3,4-dihydroxyphenylalanine (levodopa or L-DOPA). Dopamine's function as a neurotransmitter was first recognized in 1958 by Arvid Carlsson and Nils-Åke Hillarp at the Laboratory for Chemical Pharmacology of the National Heart Institute of Sweden. Carlsson was awarded the 2000 Nobel Prize in Physiology or Medicine for showing that dopamine is not only a precursor of norepinephrine (noradrenaline) and epinephrine (adrenaline), but is also itself a neurotransmitter.
Polydopamine
Research motivated by adhesive polyphenolic proteins in mussels led to the discovery in 2007 that a wide variety of materials, if placed in a solution of dopamine at slightly basic pH, will become coated with a layer of polymerized dopamine, often referred to as polydopamine. This polymerized dopamine forms by a spontaneous oxidation reaction, and is formally a type of melanin. Furthermore, dopamine self-polymerization can be used to modulate the mechanical properties of peptide-based gels. Synthesis of polydopamine usually involves reaction of dopamine hydrochloride with Tris as a base in water. The structure of polydopamine is unknown.
Polydopamine coatings can form on objects ranging in size from nanoparticles to large surfaces. Polydopamine layers have chemical properties that have the potential to be extremely useful, and numerous studies have examined their possible applications. At the simplest level, they can be used for protection against damage by light, or to form capsules for drug delivery. At a more sophisticated level, their adhesive properties may make them useful as substrates for biosensors or other biologically active macromolecules.
See also
References
- Cruickshank L, Kennedy AR, Shankland N (2013). "CSD Entry TIRZAX: 5-(2-Ammonioethyl)-2-hydroxyphenolate, Dopamine". Cambridge Structural Database: Access Structures. Cambridge Crystallographic Data Centre. doi:10.5517/cc10m9nl.
- Cruickshank L, Kennedy AR, Shankland N (2013). "Tautomeric and ionisation forms of dopamine and tyramine in the solid state". J. Mol. Struct. 1051: 132–36. Bibcode:2013JMoSt1051..132C. doi:10.1016/j.molstruc.2013.08.002.
- ^ "Dopamine: Biological activity". IUPHAR/BPS guide to pharmacology. International Union of Basic and Clinical Pharmacology. Retrieved 29 January 2016.
- Berridge KC (April 2007). "The debate over dopamine's role in reward: the case for incentive salience". Psychopharmacology. 191 (3): 391–431. doi:10.1007/s00213-006-0578-x. PMID 17072591. S2CID 468204.
- ^ Wise RA, Robble MA (January 2020). "Dopamine and Addiction". Annual Review of Psychology. 71 (1): 79–106. doi:10.1146/annurev-psych-010418-103337. PMID 31905114. S2CID 210043316.
- ^ Malenka RC, Nestler EJ, Hyman SE (2009). Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 147–48, 366–67, 375–76. ISBN 978-0-07-148127-4.
- Baliki MN, Mansour A, Baria AT, Huang L, Berger SE, Fields HL, et al. (October 2013). "Parceling human accumbens into putative core and shell dissociates encoding of values for reward and pain". The Journal of Neuroscience. 33 (41): 16383–93. doi:10.1523/JNEUROSCI.1731-13.2013. PMC 3792469. PMID 24107968.
- ^ Wenzel JM, Rauscher NA, Cheer JF, Oleson EB (January 2015). "A role for phasic dopamine release within the nucleus accumbens in encoding aversion: a review of the neurochemical literature". ACS Chemical Neuroscience. 6 (1): 16–26. doi:10.1021/cn500255p. PMC 5820768. PMID 25491156.
Thus, fear-evoking stimuli are capable of differentially altering phasic dopamine transmission across NAcc subregions. The authors propose that the observed enhancement in NAcc shell dopamine likely reflects general motivational salience, perhaps due to relief from a CS-induced fear state when the US (foot shock) is not delivered. This reasoning is supported by a report from Budygin and colleagues showing that, in anesthetized rats, the termination of tail pinch results in augmented dopamine release in the shell.
- Puglisi-Allegra S, Ventura R (June 2012). "Prefrontal/accumbal catecholamine system processes high motivational salience". Front. Behav. Neurosci. 6: 31. doi:10.3389/fnbeh.2012.00031. PMC 3384081. PMID 22754514.
- Moncrieff J (2008). The myth of the chemical cure. A critique of psychiatric drug treatment. Basingstoke, UK: Palgrave MacMillan. ISBN 978-0-230-57432-8.
- Volkow ND, Wang GJ, Kollins SH, Wigal TL, Newcorn JH, Telang F, et al. (September 2009). "Evaluating dopamine reward pathway in ADHD: clinical implications". JAMA. 302 (10): 1084–91. doi:10.1001/jama.2009.1308. PMC 2958516. PMID 19738093.
- "Dopamine infusion" (PDF). Retrieved 13 October 2023.
- ^ "Shock and Hypotension in the Newborn Medication: Alpha/Beta Adrenergic Agonists, Vasodilators, Inotropic agents, Volume Expanders, Antibiotics, Other". emedicine.medscape.com. Retrieved 13 October 2023.
- "Dopamine". PubChem. Retrieved 21 September 2015.
- "Catecholamine". Britannica. Retrieved 21 September 2015.
- "Phenylethylamine". ChemicalLand21.com. Retrieved 21 September 2015.
- ^ Carter JE, Johnson JH, Baaske DM (1982). "Dopamine Hydrochloride". Analytical Profiles of Drug Substances. 11: 257–72. doi:10.1016/S0099-5428(08)60266-X. ISBN 978-0122608117.
- "Specification Sheet". www.sigmaaldrich.com. Retrieved 13 September 2019.
- ^ Broadley KJ (March 2010). "The vascular effects of trace amines and amphetamines". Pharmacology & Therapeutics. 125 (3): 363–375. doi:10.1016/j.pharmthera.2009.11.005. PMID 19948186.
- ^ Lindemann L, Hoener MC (May 2005). "A renaissance in trace amines inspired by a novel GPCR family". Trends in Pharmacological Sciences. 26 (5): 274–281. doi:10.1016/j.tips.2005.03.007. PMID 15860375.
- ^ Wang X, Li J, Dong G, Yue J (February 2014). "The endogenous substrates of brain CYP2D". European Journal of Pharmacology. 724: 211–218. doi:10.1016/j.ejphar.2013.12.025. PMID 24374199.
- ^ Seeman P (2009). "Chapter 1: Historical overview: Introduction to the dopamine receptors". In Neve K (ed.). The Dopamine Receptors. Springer. pp. 1–22. ISBN 978-1-60327-333-6.
- "EC 1.14.16.2 – Tyrosine 3-monooxygenase (Homo sapiens)". BRENDA. Technische Universität Braunschweig. July 2016. Retrieved 7 October 2016.
Substrate: L-phenylalanine + tetrahydrobiopterin + O2
Product: L-tyrosine + 3-hydroxyphenylalanine + dihydropteridine + H2O
Organism: Homo sapiens
Reaction diagram - "EC 4.1.1.28 – Aromatic-L-amino-acid decarboxylase (Homo sapiens)". BRENDA. Technische Universität Braunschweig. July 2016. Retrieved 7 October 2016.
Substrate: m-tyrosine
Product: m-tyramine + CO2
Organism: Homo sapiens
Reaction diagram - ^ Musacchio JM (2013). "Chapter 1: Enzymes involved in the biosynthesis and degradation of catecholamines". In Iverson L (ed.). Biochemistry of Biogenic Amines. Springer. pp. 1–35. ISBN 978-1-4684-3171-1.
- ^ The National Collaborating Centre for Chronic Conditions, ed. (2006). "Symptomatic pharmacological therapy in Parkinson's disease". Parkinson's Disease. London: Royal College of Physicians. pp. 59–100. ISBN 978-1-86016-283-1. Archived from the original on 24 September 2010. Retrieved 24 September 2015.
- ^ Eisenhofer G, Kopin IJ, Goldstein DS (September 2004). "Catecholamine metabolism: a contemporary view with implications for physiology and medicine". Pharmacological Reviews. 56 (3): 331–49. doi:10.1124/pr.56.3.1. PMID 15317907. S2CID 12825309.
- Zahoor I, Shafi A, Haq E (December 2018). "Pharmacological Treatment of Parkinson's Disease: Figure 1: [Metabolic pathway of dopamine synthesis...]". In Stoker TB, Greenland JC (eds.). Parkinson's Disease: Pathogenesis and Clinical Aspects . Brisbane (AU): Codon Publications.
- Amin F, Davidson M, Davis KL (1992). "Homovanillic acid measurement in clinical research: a review of methodology". Schizophrenia Bulletin. 18 (1): 123–48. doi:10.1093/schbul/18.1.123. PMID 1553492.
- Amin F, Davidson M, Kahn RS, Schmeidler J, Stern R, Knott PJ, et al. (1995). "Assessment of the central dopaminergic index of plasma HVA in schizophrenia". Schizophrenia Bulletin. 21 (1): 53–66. doi:10.1093/schbul/21.1.53. PMID 7770741.
- Sulzer D, Zecca L (February 2000). "Intraneuronal dopamine-quinone synthesis: a review". Neurotoxicity Research. 1 (3): 181–95. doi:10.1007/BF03033289. PMID 12835101. S2CID 21892355.
- Miyazaki I, Asanuma M (June 2008). "Dopaminergic neuron-specific oxidative stress caused by dopamine itself" (PDF). Acta Medica Okayama. 62 (3): 141–50. doi:10.18926/AMO/30942. PMID 18596830.
- ^ Grandy DK, Miller GM, Li JX (February 2016). ""TAARgeting Addiction" – The Alamo Bears Witness to Another Revolution: An Overview of the Plenary Symposium of the 2015 Behavior, Biology and Chemistry Conference". Drug and Alcohol Dependence. 159: 9–16. doi:10.1016/j.drugalcdep.2015.11.014. PMC 4724540. PMID 26644139.
TAAR1 is a high-affinity receptor for METH/AMPH and DA
- ^ Romanelli RJ, Williams JT, Neve KA (2009). "Chapter 6: Dopamine receptor signalling: intracellular pathways to behavior". In Neve KA (ed.). The Dopamine Receptors. Springer. pp. 137–74. ISBN 978-1-60327-333-6.
- ^ Eiden LE, Schäfer MK, Weihe E, Schütz B (February 2004). "The vesicular amine transporter family (SLC18): amine/proton antiporters required for vesicular accumulation and regulated exocytotic secretion of monoamines and acetylcholine". Pflügers Archiv. 447 (5): 636–40. doi:10.1007/s00424-003-1100-5. PMID 12827358. S2CID 20764857.
- Westerink R (1 February 2006). "Targeting Exocytosis: Ins and Outs of the Modulation of Quantal Dopamine Release". CNS & Neurological Disorders - Drug Targets. 5 (1): 57–77. doi:10.2174/187152706784111597. ISSN 1871-5273. PMID 16613554.
- ^ Miller GM (January 2011). "The emerging role of trace amine-associated receptor 1 in the functional regulation of monoamine transporters and dopaminergic activity". Journal of Neurochemistry. 116 (2): 164–76. doi:10.1111/j.1471-4159.2010.07109.x. PMC 3005101. PMID 21073468.
- ^ Beaulieu JM, Gainetdinov RR (March 2011). "The physiology, signaling, and pharmacology of dopamine receptors". Pharmacological Reviews. 63 (1): 182–217. doi:10.1124/pr.110.002642. PMID 21303898. S2CID 2545878.
- Torres GE, Gainetdinov RR, Caron MG (January 2003). "Plasma membrane monoamine transporters: structure, regulation and function". Nature Reviews. Neuroscience. 4 (1): 13–25. doi:10.1038/nrn1008. PMID 12511858. S2CID 21545649.
- ^ Rice ME, Patel JC, Cragg SJ (December 2011). "Dopamine release in the basal ganglia". Neuroscience. 198: 112–37. doi:10.1016/j.neuroscience.2011.08.066. PMC 3357127. PMID 21939738.
- Schultz W (2007). "Multiple dopamine functions at different time courses". Annual Review of Neuroscience. 30: 259–88. doi:10.1146/annurev.neuro.28.061604.135722. PMID 17600522. S2CID 13503219.
- ^ Björklund A, Dunnett SB (May 2007). "Dopamine neuron systems in the brain: an update". Trends in Neurosciences. 30 (5): 194–202. doi:10.1016/j.tins.2007.03.006. PMID 17408759. S2CID 14239716.
- ^ Dahlstroem A, Fuxe K (1964). "Evidence for the existence of monoamine-containing neurons in the central nervous system. I. Demonstration of monoamines in the cell bodies of brain stem neurons". Acta Physiologica Scandinavica. Supplementum. 232 (Suppl): 1–55. PMID 14229500.
- ^ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapter 6: Widely Projecting Systems: Monoamines, Acetylcholine, and Orexin". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 147–48, 154–57. ISBN 978-0-07-148127-4.
- Christine CW, Aminoff MJ (September 2004). "Clinical differentiation of parkinsonian syndromes: prognostic and therapeutic relevance". The American Journal of Medicine. 117 (6): 412–19. doi:10.1016/j.amjmed.2004.03.032. PMID 15380498.
- Fadok JP, Dickerson TM, Palmiter RD (September 2009). "Dopamine is necessary for cue-dependent fear conditioning". The Journal of Neuroscience. 29 (36): 11089–97. doi:10.1523/JNEUROSCI.1616-09.2009. PMC 2759996. PMID 19741115.
- Tang W, Kochubey O, Kintscher M, Schneggenburger R (April 2020). "A VTA to basal amygdala dopamine projection contributes to signal salient somatosensory events during fear learning". The Journal of Neuroscience. 40 (20): JN–RM–1796-19. doi:10.1523/JNEUROSCI.1796-19.2020. PMC 7219297. PMID 32277045.
- Jo YS, Heymann G, Zweifel LS (November 2018). "Dopamine Neurons Reflect the Uncertainty in Fear Generalization". Neuron. 100 (4): 916–925.e3. doi:10.1016/j.neuron.2018.09.028. PMC 6226002. PMID 30318411.
- ^ Paulus W, Schomburg ED (June 2006). "Dopamine and the spinal cord in restless legs syndrome: does spinal cord physiology reveal a basis for augmentation?". Sleep Medicine Reviews. 10 (3): 185–96. doi:10.1016/j.smrv.2006.01.004. PMID 16762808.
- ^ Ben-Jonathan N, Hnasko R (December 2001). "Dopamine as a prolactin (PRL) inhibitor". Endocrine Reviews. 22 (6): 724–63. doi:10.1210/er.22.6.724. PMID 11739329.
- ^ Witkovsky P (January 2004). "Dopamine and retinal function". Documenta Ophthalmologica. Advances in Ophthalmology. 108 (1): 17–40. doi:10.1023/B:DOOP.0000019487.88486.0a. PMID 15104164. S2CID 10354133.
- ^ Fix JD (2008). "Basal Ganglia and the Striatal Motor System". Neuroanatomy (Board Review Series) (4th ed.). Baltimore: Wulters Kluwer & Lippincott Williams & Wilkins. pp. 274–81. ISBN 978-0-7817-7245-7.
- ^ Chakravarthy VS, Joseph D, Bapi RS (September 2010). "What do the basal ganglia do? A modeling perspective". Biological Cybernetics. 103 (3): 237–53. doi:10.1007/s00422-010-0401-y. PMID 20644953. S2CID 853119.
- ^ Floresco SB (January 2015). "The nucleus accumbens: an interface between cognition, emotion, and action". Annual Review of Psychology. 66: 25–52. doi:10.1146/annurev-psych-010213-115159. PMID 25251489. S2CID 28268183.
- ^ Balleine BW, Dezfouli A, Ito M, Doya K (2015). "Hierarchical control of goal-directed action in the cortical–basal ganglia network". Current Opinion in Behavioral Sciences. 5: 1–7. doi:10.1016/j.cobeha.2015.06.001. S2CID 53148662.
- ^ Jankovic J (April 2008). "Parkinson's disease: clinical features and diagnosis". Journal of Neurology, Neurosurgery, and Psychiatry. 79 (4): 368–76. doi:10.1136/jnnp.2007.131045. PMID 18344392.
- Pattij T, Vanderschuren LJ (April 2008). "The neuropharmacology of impulsive behaviour". Trends in Pharmacological Sciences. 29 (4): 192–99. doi:10.1016/j.tips.2008.01.002. PMID 18304658.
- ^ Schultz W (July 2015). "Neuronal Reward and Decision Signals: From Theories to Data". Physiological Reviews. 95 (3): 853–951. doi:10.1152/physrev.00023.2014. PMC 4491543. PMID 26109341.
- ^ Robinson TE, Berridge KC (1993). "The neural basis of drug craving: an incentive-sensitization theory of addiction". Brain Research. Brain Research Reviews. 18 (3): 247–91. doi:10.1016/0165-0173(93)90013-p. hdl:2027.42/30601. PMID 8401595. S2CID 13471436.
- Wright JS, Panksepp J (2012). "An evolutionary framework to understand foraging, wanting, and desire: the neuropsychology of the SEEKING system". Neuropsychoanalysis. 14 (1): 5–39. doi:10.1080/15294145.2012.10773683. S2CID 145747459. Retrieved 24 September 2015.
- ^ Berridge KC, Robinson TE, Aldridge JW (February 2009). "Dissecting components of reward: 'liking', 'wanting', and learning". Current Opinion in Pharmacology. 9 (1): 65–73. doi:10.1016/j.coph.2008.12.014. PMC 2756052. PMID 19162544.
- Montague PR, Dayan P, Sejnowski TJ (March 1996). "A framework for mesencephalic dopamine systems based on predictive Hebbian learning". The Journal of Neuroscience. 16 (5): 1936–47. doi:10.1523/JNEUROSCI.16-05-01936.1996. PMC 6578666. PMID 8774460.
- Bromberg-Martin ES, Matsumoto M, Hikosaka O (December 2010). "Dopamine in motivational control: rewarding, aversive, and alerting". Neuron. 68 (5): 815–34. doi:10.1016/j.neuron.2010.11.022. PMC 3032992. PMID 21144997.
- Yager LM, Garcia AF, Wunsch AM, Ferguson SM (August 2015). "The ins and outs of the striatum: Role in drug addiction". Neuroscience. 301: 529–41. doi:10.1016/j.neuroscience.2015.06.033. PMC 4523218. PMID 26116518.
- ^ Saddoris MP, Cacciapaglia F, Wightman RM, Carelli RM (August 2015). "Differential Dopamine Release Dynamics in the Nucleus Accumbens Core and Shell Reveal Complementary Signals for Error Prediction and Incentive Motivation". The Journal of Neuroscience. 35 (33): 11572–82. doi:10.1523/JNEUROSCI.2344-15.2015. PMC 4540796. PMID 26290234.
- Berridge KC, Kringelbach ML (May 2015). "Pleasure systems in the brain". Neuron. 86 (3): 646–64. doi:10.1016/j.neuron.2015.02.018. PMC 4425246. PMID 25950633.
- ^ Wise RA (1996). "Addictive drugs and brain stimulation reward". Annual Review of Neuroscience. 19: 319–40. doi:10.1146/annurev.ne.19.030196.001535. PMID 8833446.
- Wise RA (October 2008). "Dopamine and reward: the anhedonia hypothesis 30 years on". Neurotoxicity Research. 14 (2–3): 169–83. doi:10.1007/BF03033808. PMC 3155128. PMID 19073424.
- Arias-Carrión O, Pöppel E (2007). "Dopamine, learning and reward-seeking behavior". Acta Neurobiol Exp. 67 (4): 481–88. doi:10.55782/ane-2007-1664. PMID 18320725.
- Ikemoto S (November 2007). "Dopamine reward circuitry: two projection systems from the ventral midbrain to the nucleus accumbens-olfactory tubercle complex". Brain Research Reviews. 56 (1): 27–78. doi:10.1016/j.brainresrev.2007.05.004. PMC 2134972. PMID 17574681.
- ^ Ferreri L, Mas-Herrero E, Zatorre RJ, Ripollés P, Gomez-Andres A, Alicart H, et al. (2019). "Dopamine modulates the reward experiences elicited by music". Proceedings of the National Academy of Sciences of the United States of America. 116 (9): 3793–98. Bibcode:2019PNAS..116.3793F. doi:10.1073/pnas.1811878116. PMC 6397525. PMID 30670642.
Listening to pleasurable music is often accompanied by measurable bodily reactions such as goose bumps or shivers down the spine, commonly called "chills" or "frissons." ... Overall, our results straightforwardly revealed that pharmacological interventions bidirectionally modulated the reward responses elicited by music. In particular, we found that risperidone impaired participants' ability to experience musical pleasure, whereas levodopa enhanced it. ... Here, in contrast, studying responses to abstract rewards in human subjects, we show that manipulation of dopaminergic transmission affects both the pleasure (i.e., amount of time reporting chills and emotional arousal measured by EDA) and the motivational components of musical reward (money willing to spend). These findings suggest that dopaminergic signaling is a sine qua non condition not only for motivational responses, as has been shown with primary and secondary rewards, but also for hedonic reactions to music. This result supports recent findings showing that dopamine also mediates the perceived pleasantness attained by other types of abstract rewards (37) and challenges previous findings in animal models on primary rewards, such as food (42, 43).
- ^ Goupil L, Aucouturier JJ (February 2019). "Musical pleasure and musical emotions". Proceedings of the National Academy of Sciences of the United States of America. 116 (9): 3364–66. Bibcode:2019PNAS..116.3364G. doi:10.1073/pnas.1900369116. PMC 6397567. PMID 30770455.
In a pharmacological study published in PNAS, Ferreri et al. (1) present evidence that enhancing or inhibiting dopamine signaling using levodopa or risperidone modulates the pleasure experienced while listening to music. ... In a final salvo to establish not only the correlational but also the causal implication of dopamine in musical pleasure, the authors have turned to directly manipulating dopaminergic signaling in the striatum, first by applying excitatory and inhibitory transcranial magnetic stimulation over their participants' left dorsolateral prefrontal cortex, a region known to modulate striatal function (5), and finally, in the current study, by administrating pharmaceutical agents able to alter dopamine synaptic availability (1), both of which influenced perceived pleasure, physiological measures of arousal, and the monetary value assigned to music in the predicted direction. ... While the question of the musical expression of emotion has a long history of investigation, including in PNAS (6), and the 1990s psychophysiological strand of research had already established that musical pleasure could activate the autonomic nervous system (7), the authors' demonstration of the implication of the reward system in musical emotions was taken as inaugural proof that these were veridical emotions whose study has full legitimacy to inform the neurobiology of our everyday cognitive, social, and affective functions (8). Incidentally, this line of work, culminating in the article by Ferreri et al. (1), has plausibly done more to attract research funding for the field of music sciences than any other in this community.
The evidence of Ferreri et al. (1) provides the latest support for a compelling neurobiological model in which musical pleasure arises from the interaction of ancient reward/valuation systems (striatal–limbic–paralimbic) with more phylogenetically advanced perception/predictions systems (temporofrontal). - Koepp MJ, Gunn RN, Lawrence AD, Cunningham VJ, Dagher A, Jones T, et al. (May 1998). "Evidence for striatal dopamine release during a video game". Nature. 393 (6682): 266–268. Bibcode:1998Natur.393..266K. doi:10.1038/30498. PMID 9607763. S2CID 205000565.
- von der Heiden JM, Braun B, Müller KW, Egloff B (2019). "The Association Between Video Gaming and Psychological Functioning". Frontiers in Psychology. 10: 1731. doi:10.3389/fpsyg.2019.01731. PMC 6676913. PMID 31402891.
- ^ Missale C, Nash SR, Robinson SW, Jaber M, Caron MG (January 1998). "Dopamine receptors: from structure to function" (PDF). Physiological Reviews. 78 (1): 189–225. doi:10.1152/physrev.1998.78.1.189. PMID 9457173. S2CID 223462. Archived from the original (PDF) on 2 March 2019.
- ^ Buttarelli FR, Fanciulli A, Pellicano C, Pontieri FE (June 2011). "The dopaminergic system in peripheral blood lymphocytes: from physiology to pharmacology and potential applications to neuropsychiatric disorders". Current Neuropharmacology. 9 (2): 278–88. doi:10.2174/157015911795596612. PMC 3131719. PMID 22131937.
- ^ Sarkar C, Basu B, Chakroborty D, Dasgupta PS, Basu S (May 2010). "The immunoregulatory role of dopamine: an update". Brain, Behavior, and Immunity. 24 (4): 525–28. doi:10.1016/j.bbi.2009.10.015. PMC 2856781. PMID 19896530.
- Hussain T, Lokhandwala MF (February 2003). "Renal dopamine receptors and hypertension". Experimental Biology and Medicine. 228 (2): 134–42. doi:10.1177/153537020322800202. PMID 12563019. S2CID 10896819.
- Choi MR, Kouyoumdzian NM, Rukavina Mikusic NL, Kravetz MC, Rosón MI, Rodríguez Fermepin M, et al. (May 2015). "Renal dopaminergic system: Pathophysiological implications and clinical perspectives". World Journal of Nephrology. 4 (2): 196–212. doi:10.5527/wjn.v4.i2.196. PMC 4419129. PMID 25949933.
- Carey RM (September 2001). "Theodore Cooper Lecture: Renal dopamine system: paracrine regulator of sodium homeostasis and blood pressure". Hypertension. 38 (3): 297–302. doi:10.1161/hy0901.096422. PMID 11566894.
- ^ Rubí B, Maechler P (December 2010). "Minireview: new roles for peripheral dopamine on metabolic control and tumor growth: let's seek the balance". Endocrinology. 151 (12): 5570–81. doi:10.1210/en.2010-0745. PMID 21047943.
- "WHO Model List of Essential Medicines" (PDF). World Health Organization. October 2013. Archived (PDF) from the original on 10 February 2014. Retrieved 24 September 2015.
- Noori S, Friedlich P, Seri I (2003). "Pharmacology Review Developmentally Regulated Cardiovascular, Renal, and Neuroendocrine Effects of Dopamine". NeoReviews. 4 (10): e283–e288. doi:10.1542/neo.4-10-e283. S2CID 71902752. Retrieved 24 September 2015.
- ^ Bhatt-Mehta V, Nahata MC (1989). "Dopamine and dobutamine in pediatric therapy". Pharmacotherapy. 9 (5): 303–14. doi:10.1002/j.1875-9114.1989.tb04142.x. PMID 2682552. S2CID 25614283.
- ^ Bronwen JB, Knights KM (2009). Pharmacology for Health Professionals (2nd ed.). Elsevier Australia. p. 192. ISBN 978-0-7295-3929-6.
- De Backer D, Biston P, Devriendt J, Madl C, Chochrad D, Aldecoa C, et al. (March 2010). "Comparison of dopamine and norepinephrine in the treatment of shock" (PDF). The New England Journal of Medicine. 362 (9): 779–89. doi:10.1056/NEJMoa0907118. PMID 20200382. S2CID 2208904. Archived from the original (PDF) on 28 February 2019.
- Karthik S, Lisbon A (2006). "Low-dose dopamine in the intensive care unit". Seminars in Dialysis. 19 (6): 465–71. doi:10.1111/j.1525-139X.2006.00208.x. PMID 17150046. S2CID 22538344.
- Moses S. "Dopamine". Family Practice Notebook. Retrieved 1 February 2016.
- Katritsis DG, Gersh BJ, Camm AJ (2013). Clinical Cardiology: Current Practice Guidelines. OUP Oxford. ISBN 978-0-19-150851-6.
Dopamine binds to beta-1, beta-2, alpha-1 and dopaminergic receptors
- Lewis RJ (2004). Sax's Dangerous Properties of Industrial Materials (11th ed.). Hoboken, NJ: Wiley & Sons. p. 1552. ISBN 978-0-471-47662-7.
- Standaert DG, Walsh RR (2011). "Pharmacology of dopaminergic neurotransmission". In Tashjian AH, Armstrong EJ, Golan DE (eds.). Principles of Pharmacology: The Pathophysiologic Basis of Drug Therapy. Lippincott Williams & Wilkins. pp. 186–206. ISBN 978-1-4511-1805-6.
- Mobbs CV, Hof PR (2009). Handbook of the neuroscience of aging. Amsterdam: Elsevier/Academic Press. ISBN 978-0-12-374898-0. OCLC 299710911.
- Ota M, Yasuno F, Ito H, Seki C, Nozaki S, Asada T, et al. (July 2006). "Age-related decline of dopamine synthesis in the living human brain measured by positron emission tomography with L-DOPA". Life Sciences. 79 (8): 730–36. doi:10.1016/j.lfs.2006.02.017. PMID 16580023.
- Kaasinen V, Vilkman H, Hietala J, Någren K, Helenius H, Olsson H, et al. (2000). "Age-related dopamine D2/D3 receptor loss in extrastriatal regions of the human brain". Neurobiology of Aging. 21 (5): 683–68. doi:10.1016/S0197-4580(00)00149-4. PMID 11016537. S2CID 40871554.
- Wang Y, Chan GL, Holden JE, Dobko T, Mak E, Schulzer M, et al. (September 1998). "Age-dependent decline of dopamine D1 receptors in human brain: a PET study". Synapse. 30 (1): 56–61. doi:10.1002/(SICI)1098-2396(199809)30:1<56::AID-SYN7>3.0.CO;2-J. PMID 9704881. S2CID 31445572.
- Wong DF, Wagner HN, Dannals RF, Links JM, Frost JJ, Ravert HT, et al. (December 1984). "Effects of age on dopamine and serotonin receptors measured by positron tomography in the living human brain". Science. 226 (4681): 1393–96. Bibcode:1984Sci...226.1393W. doi:10.1126/science.6334363. PMID 6334363. S2CID 24278577.
- ^ Wang E, Snyder SD (1998). Handbook of the aging brain. San Diego, California: Academic Press. ISBN 978-0-12-734610-6. OCLC 636693117.
- Dobryakova E, Genova HM, DeLuca J, Wylie GR (12 March 2015). "The dopamine imbalance hypothesis of fatigue in multiple sclerosis and other neurological disorders". Frontiers in Neurology. 6: 52. doi:10.3389/fneur.2015.00052. PMC 4357260. PMID 25814977.
- Marino F, Cosentino M (April 2016). "Multiple sclerosis: Repurposing dopaminergic drugs for MS—the evidence mounts". Nature Reviews. Neurology. 12 (4): 191–92. doi:10.1038/nrneurol.2016.33. PMID 27020558. S2CID 26319461.
- Dickson DV (2007). "Neuropathology of movement disorders". In Tolosa E, Jankovic JJ (eds.). Parkinson's disease and movement disorders. Hagerstown, MD: Lippincott Williams & Wilkins. pp. 271–83. ISBN 978-0-7817-7881-7.
- ^ Tuite PJ, Krawczewski K (April 2007). "Parkinsonism: a review-of-systems approach to diagnosis". Seminars in Neurology. 27 (2): 113–22. doi:10.1055/s-2007-971174. PMID 17390256. S2CID 260319916.
- ^ Olsen CM (December 2011). "Natural rewards, neuroplasticity, and non-drug addictions". Neuropharmacology. 61 (7): 1109–22. doi:10.1016/j.neuropharm.2011.03.010. PMC 3139704. PMID 21459101.
- Ceravolo R, Frosini D, Rossi C, Bonuccelli U (November 2010). "Spectrum of addictions in Parkinson's disease: from dopamine dysregulation syndrome to impulse control disorders". Journal of Neurology. 257 (Suppl 2): S276–83. doi:10.1007/s00415-010-5715-0. PMID 21080189. S2CID 19277026.
- ^ Ghodse H (2010). Ghodse's Drugs and Addictive Behaviour: A Guide to Treatment (4th ed.). Cambridge University Press. pp. 87–92. ISBN 978-1-139-48567-8.
- Siciliano CA, Jones SR (August 2017). "Cocaine Potency at the Dopamine Transporter Tracks Discrete Motivational States During Cocaine Self-Administration". Neuropsychopharmacology. 42 (9): 1893–1904. doi:10.1038/npp.2017.24. PMC 5520781. PMID 28139678.
- Heal DJ, Pierce DM (2006). "Methylphenidate and its isomers: their role in the treatment of attention-deficit hyperactivity disorder using a transdermal delivery system". CNS Drugs. 20 (9): 713–38. doi:10.2165/00023210-200620090-00002. PMID 16953648. S2CID 39535277.
- ^ Freye E (2009). Pharmacology and abuse of cocaine, amphetamines, ecstasy and related designer drugs a comprehensive review on their mode of action, treatment of abuse and intoxication. Dordrecht: Springer. ISBN 978-90-481-2448-0.
- ^ Kimko HC, Cross JT, Abernethy DR (December 1999). "Pharmacokinetics and clinical effectiveness of methylphenidate". Clinical Pharmacokinetics. 37 (6): 457–70. doi:10.2165/00003088-199937060-00002. PMID 10628897. S2CID 397390.
- Mignot EJ (October 2012). "A practical guide to the therapy of narcolepsy and hypersomnia syndromes". Neurotherapeutics. 9 (4): 739–52. doi:10.1007/s13311-012-0150-9. PMC 3480574. PMID 23065655.
- Zimmerman JL (October 2012). "Cocaine intoxication". Critical Care Clinics. 28 (4): 517–26. doi:10.1016/j.ccc.2012.07.003. PMID 22998988.
- "Quillivant XR – methylphenidate hydrochloride suspension, extended release". dailymed.nlm.nih.gov. Retrieved 11 July 2020.
- López FA, Leroux JR (September 2013). "Long-acting stimulants for treatment of attention-deficit/hyperactivity disorder: a focus on extended-release formulations and the prodrug lisdexamfetamine dimesylate to address continuing clinical challenges". Attention Deficit and Hyperactivity Disorders. 5 (3): 249–65. doi:10.1007/s12402-013-0106-x. PMC 3751218. PMID 23564273.
- Nutt DJ, Lingford-Hughes A, Erritzoe D, Stokes PR (May 2015). "The dopamine theory of addiction: 40 years of highs and lows" (PDF). Nature Reviews. Neuroscience. 16 (5): 305–12. doi:10.1038/nrn3939. PMID 25873042. S2CID 205511111.
- ^ Sinha R (August 2013). "The clinical neurobiology of drug craving". Current Opinion in Neurobiology. 23 (4): 649–54. doi:10.1016/j.conb.2013.05.001. PMC 3735834. PMID 23764204.
- Volkow ND, Baler RD (January 2014). "Addiction science: Uncovering neurobiological complexity". Neuropharmacology. 76 (Pt B): 235–49. doi:10.1016/j.neuropharm.2013.05.007. PMC 3818510. PMID 23688927.
- Nestler EJ (December 2012). "Transcriptional mechanisms of drug addiction". Clinical Psychopharmacology and Neuroscience. 10 (3): 136–43. doi:10.9758/cpn.2012.10.3.136. PMC 3569166. PMID 23430970.
- Yeo BT, Krienen FM, Sepulcre J, Sabuncu MR, Lashkari D, Hollinshead M, et al. (September 2011). "The organization of the human cerebral cortex estimated by intrinsic functional connectivity". Journal of Neurophysiology. 106 (3): 1125–65. doi:10.1152/jn.00338.2011. PMC 3174820. PMID 21653723.
- ^ Healy D (2004). The Creation of Psychopharmacology. Harvard University Press. pp. 37–73. ISBN 978-0-674-01599-9.
- ^ Brunton L. Goodman and Gilman's The Pharmacological Basis of Therapeutics (12th ed.). McGraw Hill. pp. 417–55.
- ^ Howes OD, Kapur S (May 2009). "The dopamine hypothesis of schizophrenia: version III—the final common pathway". Schizophrenia Bulletin. 35 (3): 549–62. doi:10.1093/schbul/sbp006. PMC 2669582. PMID 19325164.
- Horacek J, Bubenikova-Valesova V, Kopecek M, Palenicek T, Dockery C, Mohr P, et al. (2006). "Mechanism of action of atypical antipsychotic drugs and the neurobiology of schizophrenia". CNS Drugs. 20 (5): 389–409. doi:10.2165/00023210-200620050-00004. PMID 16696579. S2CID 18226404.
- Stahl SM (2018). "Beyond the dopamine hypothesis of schizophrenia to three neural networks of psychosis: dopamine, serotonin, and glutamate" (PDF). CNS Spectr. 23 (3): 187–91. doi:10.1017/S1092852918001013. PMID 29954475. S2CID 49599226. Archived (PDF) from the original on 29 April 2020.
- ^ Malenka RC, Nestler EJ, Hyman SE (2009). "Chapters 10 and 13". In Sydor A, Brown RY (eds.). Molecular Neuropharmacology: A Foundation for Clinical Neuroscience (2nd ed.). New York: McGraw-Hill Medical. pp. 266, 318–23. ISBN 978-0-07-148127-4.
- Wu J, Xiao H, Sun H, Zou L, Zhu LQ (June 2012). "Role of dopamine receptors in ADHD: a systematic meta-analysis". Molecular Neurobiology. 45 (3): 605–20. doi:10.1007/s12035-012-8278-5. PMID 22610946. S2CID 895006.
- ^ Berridge CW, Devilbiss DM (June 2011). "Psychostimulants as cognitive enhancers: the prefrontal cortex, catecholamines, and attention-deficit/hyperactivity disorder". Biological Psychiatry. 69 (12): e101–11. doi:10.1016/j.biopsych.2010.06.023. PMC 3012746. PMID 20875636.
- Spencer RC, Devilbiss DM, Berridge CW (June 2015). "The cognition-enhancing effects of psychostimulants involve direct action in the prefrontal cortex". Biological Psychiatry. 77 (11): 940–50. doi:10.1016/j.biopsych.2014.09.013. PMC 4377121. PMID 25499957.
- Ilieva IP, Hook CJ, Farah MJ (June 2015). "Prescription Stimulants' Effects on Healthy Inhibitory Control, Working Memory, and Episodic Memory: A Meta-analysis". Journal of Cognitive Neuroscience. 27 (6): 1069–89. doi:10.1162/jocn_a_00776. PMID 25591060. S2CID 15788121.
- ^ Wood PB (May 2008). "Role of central dopamine in pain and analgesia". Expert Review of Neurotherapeutics. 8 (5): 781–97. doi:10.1586/14737175.8.5.781. PMID 18457535. S2CID 24325199.
- ^ Flake ZA, Scalley RD, Bailey AG (March 2004). "Practical selection of antiemetics". American Family Physician. 69 (5): 1169–74. PMID 15023018.
- Connolly BS, Lang AE (2014). "Pharmacological treatment of Parkinson disease: a review". JAMA. 311 (16): 1670–83. doi:10.1001/jama.2014.3654. PMID 24756517.
- Frick A, Björkstrand J, Lubberink M, Eriksson A, Fredrikson M, Åhs F (March 2022). "Dopamine and fear memory formation in the human amygdala". Molecular Psychiatry. 27 (3): 1704–1711. doi:10.1038/s41380-021-01400-x. PMC 9095491. PMID 34862441.
- Hamati R, Ahrens J, Shvetz C, Holahan MR, Tuominen L (March 2024). "65 years of research on dopamine's role in classical fear conditioning and extinction: A systematic review". The European Journal of Neuroscience. 59 (6): 1099–1140. doi:10.1111/ejn.16157. PMID 37848184.
- Roshchina VV (2010). "Evolutionary considerations of neurotransmitters in microbial, plant, and animal cells". In Lyte M, Primrose PE (eds.). Microbial Endocrinology. New York: Springer. pp. 17–52. ISBN 978-1-4419-5576-0.
- ^ Iyer LM, Aravind L, Coon SL, Klein DC, Koonin EV (July 2004). "Evolution of cell-cell signaling in animals: did late horizontal gene transfer from bacteria have a role?". Trends in Genetics. 20 (7): 292–99. doi:10.1016/j.tig.2004.05.007. PMID 15219393.
- ^ Barron AB, Søvik E, Cornish JL (2010). "The roles of dopamine and related compounds in reward-seeking behavior across animal phyla". Frontiers in Behavioral Neuroscience. 4: 163. doi:10.3389/fnbeh.2010.00163. PMC 2967375. PMID 21048897.
- Liu H, Mishima Y, Fujiwara T, Nagai H, Kitazawa A, Mine Y, et al. (2004). "Isolation of Araguspongine M, a new stereoisomer of an Araguspongine/Xestospongin alkaloid, and dopamine from the marine sponge Neopetrosia exigua collected in Palau". Marine Drugs. 2 (4): 154–63. doi:10.3390/md204154. PMC 3783253.
- Kass-Simon G, Pierobon P (January 2007). "Cnidarian chemical neurotransmission, an updated overview". Comparative Biochemistry and Physiology. Part A, Molecular & Integrative Physiology. 146 (1): 9–25. doi:10.1016/j.cbpa.2006.09.008. PMID 17101286.
- Cottrell GA (January 1967). "Occurrence of dopamine and noradrenaline in the nervous tissue of some invertebrate species". British Journal of Pharmacology and Chemotherapy. 29 (1): 63–69. doi:10.1111/j.1476-5381.1967.tb01939.x. PMC 1557178. PMID 19108240.
- Kindt KS, Quast KB, Giles AC, De S, Hendrey D, Nicastro I, et al. (August 2007). "Dopamine mediates context-dependent modulation of sensory plasticity in C. elegans". Neuron. 55 (4): 662–76. doi:10.1016/j.neuron.2007.07.023. PMID 17698017. S2CID 2092645.
- Kalivas PW, Stewart J (1 September 1991). "Dopamine transmission in the initiation and expression of drug- and stress-induced sensitization of motor activity". Brain Research. Brain Research Reviews. 16 (3): 223–44. doi:10.1016/0165-0173(91)90007-U. PMID 1665095. S2CID 10775295.
- Perry CJ, Barron AB (2013). "Neural mechanisms of reward in insects" (PDF). Annual Review of Entomology. 58 (1): 543–62. doi:10.1146/annurev-ento-120811-153631. PMID 23020615. S2CID 19678766. Archived from the original (PDF) on 5 June 2020.
- Takikawa Y, Kawagoe R, Hikosaka O (October 2004). "A possible role of midbrain dopamine neurons in short- and long-term adaptation of saccades to position-reward mapping" (PDF). Journal of Neurophysiology. 92 (4): 2520–29. doi:10.1152/jn.00238.2004. PMID 15163669. S2CID 12534057. Archived from the original (PDF) on 2 March 2019.
- Yamagata N, Ichinose T, Aso Y, Plaçais PY, Friedrich AB, Sima RJ, et al. (January 2015). "Distinct dopamine neurons mediate reward signals for short- and long-term memories". Proceedings of the National Academy of Sciences of the United States of America. 112 (2): 578–83. Bibcode:2015PNAS..112..578Y. doi:10.1073/pnas.1421930112. PMC 4299218. PMID 25548178.
- ^ Waddell S (June 2013). "Reinforcement signalling in Drosophila; dopamine does it all after all". Current Opinion in Neurobiology. 23 (3): 324–29. doi:10.1016/j.conb.2013.01.005. PMC 3887340. PMID 23391527.
- ^ Kulma A, Szopa J (2007). "Catecholamines are active compounds in plants". Plant Science. 172 (3): 433–40. Bibcode:2007PlnSc.172..433K. doi:10.1016/j.plantsci.2006.10.013.
- ^ Ingle PK (2003). "L-DOPA bearing plants" (PDF). Natural Product Radiance. 2: 126–33. Archived (PDF) from the original on 2 March 2014. Retrieved 24 September 2015.
- Wichers HJ, Visser JF, Huizing HJ, Pras N (1993). "Occurrence of L-DOPA and dopamine in plants and cell cultures of Mucuna pruriens and effects of 2, 4-d and NaCl on these compounds". Plant Cell, Tissue and Organ Culture. 33 (3): 259–64. doi:10.1007/BF02319010. S2CID 44814336.
- Longo R, Castellani A, Sberze P, Tibolla M (1974). "Distribution of l-dopa and related amino acids in Vicia". Phytochemistry. 13 (1): 167–71. Bibcode:1974PChem..13..167L. doi:10.1016/S0031-9422(00)91287-1.
- Van Alstyne KL, Nelson AV, Vyvyan JR, Cancilla DA (June 2006). "Dopamine functions as an antiherbivore defense in the temperate green alga Ulvaria obscura". Oecologia. 148 (2): 304–11. Bibcode:2006Oecol.148..304V. doi:10.1007/s00442-006-0378-3. PMID 16489461. S2CID 5029574.
- ^ Simon JD, Peles D, Wakamatsu K, Ito S (October 2009). "Current challenges in understanding melanogenesis: bridging chemistry, biological control, morphology, and function". Pigment Cell & Melanoma Research. 22 (5): 563–79. doi:10.1111/j.1755-148X.2009.00610.x. PMID 19627559.
- Fedorow H, Tribl F, Halliday G, Gerlach M, Riederer P, Double KL (February 2005). "Neuromelanin in human dopamine neurons: comparison with peripheral melanins and relevance to Parkinson's disease". Progress in Neurobiology. 75 (2): 109–24. doi:10.1016/j.pneurobio.2005.02.001. PMID 15784302. S2CID 503902.
- Andrews RS, Pridham JB (1967). "Melanins from DOPA-containing plants". Phytochemistry. 6 (1): 13–18. Bibcode:1967PChem...6...13A. doi:10.1016/0031-9422(67)85002-7.
- Beldade P, Brakefield PM (June 2002). "The genetics and evo-devo of butterfly wing patterns". Nature Reviews. Genetics. 3 (6): 442–52. doi:10.1038/nrg818. PMID 12042771. S2CID 17417235.
- Fahn S (2008). "The history of dopamine and levodopa in the treatment of Parkinson's disease". Movement Disorders. 23 (Suppl 3): S497–508. doi:10.1002/mds.22028. PMID 18781671. S2CID 45572523.
- Benes FM (January 2001). "Carlsson and the discovery of dopamine". Trends in Pharmacological Sciences. 22 (1): 46–47. doi:10.1016/S0165-6147(00)01607-2. PMID 11165672.
- Barondes SH (2003). Better Than Prozac. New York: Oxford University Press. pp. 21–22, 39–40. ISBN 978-0-19-515130-5.
- Lee H, Dellatore SM, Miller WM, Messersmith PB (October 2007). "Mussel-inspired surface chemistry for multifunctional coatings". Science. 318 (5849): 426–30. Bibcode:2007Sci...318..426L. doi:10.1126/science.1147241. PMC 2601629. PMID 17947576.
- ^ Dreyer DR, Miller DJ, Freeman BD, Paul DR, Bielawski CW (2013). "Perspectives on poly(dopamine)". Chemical Science. 4 (10): 3796. doi:10.1039/C3SC51501J.
- ^ Lynge ME, van der Westen R, Postma A, Städler B (December 2011). "Polydopamine—a nature-inspired polymer coating for biomedical science" (PDF). Nanoscale. 3 (12): 4916–28. Bibcode:2011Nanos...3.4916L. doi:10.1039/c1nr10969c. PMID 22024699. Archived from the original on 7 March 2014.
- Fichman G, Schneider JP (March 2021). "Dopamine Self-Polymerization as a Simple and Powerful Tool to Modulate the Viscoelastic Mechanical Properties of Peptide-Based Gels". Molecules. 26 (5): 1363. doi:10.3390/molecules26051363. PMC 7961423. PMID 33806346.
Further reading (most recent first)
- Szalavitz M (13 September 2024). "A 'Dopamine Fast' Will Not Save You From Addiction". The New York Times.
- Smith DG (30 June 2023). "We Have a Dopamine Problem". The New York Times.
- Metz C (29 September 2021). One Man's Endless Hunt for a Dopamine Rush in Virtual Reality. The New York Times.
- Lembke A (2021). Dopamine Nation. Headline. ISBN 9781472294128.
External links
| Neurotransmitters | |||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Amino acid-derived |
| ||||||||||||||||||||||
| Lipid-derived |
| ||||||||||||||||||||||
| Nucleobase-derived |
| ||||||||||||||||||||||
| Vitamin-derived | |||||||||||||||||||||||
| Miscellaneous |
| ||||||||||||||||||||||
| Cardiac stimulants excluding cardiac glycosides (C01C) | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Adrenergic and dopaminergic agents |
| ||||||||||||||
| Phosphodiesterase inhibitors (PDE3I) | |||||||||||||||
| Other cardiac stimulants | |||||||||||||||
| |||||||||||||||
| Human trace amine-associated receptor ligands | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| TAAR1 |
| ||||||||||
| TAAR2 |
| ||||||||||
| TAAR5 |
| ||||||||||
| References for all endogenous human TAAR1 ligands are provided at List of trace amines
| |||||||||||
| Monoaminergic neurotoxins | |
|---|---|
| Dopaminergic |
|
| Noradrenergic | |
| Serotonergic |
|
| Unsorted | |
| See also: Receptor/signaling modulators • Adrenergics • Dopaminergics • Melatonergics • Serotonergics • Monoamine reuptake inhibitors • Monoamine releasing agents • Monoamine metabolism modulators | |
| Phenethylamines | |
|---|---|
| Phenethylamines |
|
| Amphetamines |
|
| Phentermines |
|
| Cathinones |
|
| Phenylisobutylamines | |
| Phenylalkylpyrrolidines | |
| Catecholamines (and close relatives) |
|
| Miscellaneous |
|
| Emergency medicine | |
|---|---|
| Emergency medicine | |
| Equipment |
|
| Drugs | |
| Organisations |
|
| Courses / Life support |
|
| Scoring systems | |