| Revision as of 12:35, 18 September 2014 editJytdog (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers187,951 edits →Heart failure: clarify that lack of date makes independent interpretation impossible; this is very different from an experiment that is designed badly so that its results are impossible to interpret← Previous edit | Latest revision as of 05:13, 12 November 2024 edit undoCitation bot (talk | contribs)Bots5,414,846 edits Altered volume. | Use this bot. Report bugs. | Suggested by Whywhenwhohow | #UCB_webform 496/617 | ||
| (346 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|Combination medication}} | |||
| {{Use dmy dates|date=May 2020}} | |||
| {{cs1 config|name-list-style=vanc|display-authors=6}} | |||
| {{Drugbox | {{Drugbox | ||
| | |
| type = combo | ||
| | image = Valsartan.svg | | image = Valsartan and sacubitril.svg | ||
| | width = | |||
| | alt = Valsartan | |||
| | |
| alt = Sacubitril/valsartan | ||
| | caption = | |||
| | alt2 = AHU-377 | |||
| | type = combo | |||
| | component1 = Valsartan | |||
| | class1 = ] | |||
| | component2 = Sacubitril | |||
| | class2 = ] inhibitor | |||
| <!-- |
<!-- Combo data --> | ||
| | component1 = Sacubitril | |||
| | tradename = | |||
| | class1 = ] | |||
| | pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| | component2 = Valsartan | |||
| | pregnancy_US = <!-- A / B / C / D / X --> | |||
| | class2 = ] | |||
| | pregnancy_category = | |||
| | legal_AU = <!-- S2, S3, S4, S5, S6, S7, S8, S9 or Unscheduled--> | |||
| | legal_CA = <!-- Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| | legal_UK = <!-- GSL, P, POM, CD, or Class A, B, C --> | |||
| | legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> | |||
| | legal_status = Investigational | |||
| | routes_of_administration = Oral | |||
| <!-- |
<!-- Clinical data --> | ||
| | pronounce = | |||
| | bioavailability = | |||
| | tradename = Entresto, Azmarda, Neparvis, others | |||
| | protein_bound = | |||
| | Drugs.com = {{Drugs.com|monograph|sacubitril-and-valsartan}} | |||
| | metabolism = | |||
| | MedlinePlus = a615039 | |||
| | DailyMedID = Sacubitril and valsartan | |||
| | pregnancy_AU = D | |||
| | pregnancy_AU_comment = <ref name="Entresto AU PI" /> | |||
| | pregnancy_category= | |||
| | routes_of_administration = ] | |||
| | ATC_prefix = C09 | |||
| | ATC_suffix = DX04 | |||
| | ATC_supplemental = | |||
| <!-- Legal status --> | |||
| | legal_AU = S4 | |||
| | legal_AU_comment = <ref name="Entresto AU PI" /><ref>{{cite web | title=Prescription medicines: registration of new chemical entities in Australia, 2016 | website=Therapeutic Goods Administration (TGA) | date=21 June 2022 | url=https://www.tga.gov.au/prescription-medicines-registration-new-chemical-entities-australia-2016 | access-date=10 April 2023 | archive-date=10 April 2023 | archive-url=https://web.archive.org/web/20230410065503/https://www.tga.gov.au/prescription-medicines-registration-new-chemical-entities-australia-2016 | url-status=live }}</ref><ref>{{cite report | title= AusPAR for sacubitril / valsartan salt complex | url=https://www.tga.gov.au/sites/default/files/161013_auspar-sacubitril-valsartan-salt-complex-160923.pdf | publisher=] (TGA) | date=September 2016 }}</ref> | |||
| | legal_BR = <!-- OTC, A1, A2, A3, B1, B2, C1, C2, C3, C4, C5, D1, D2, E, F--> | |||
| | legal_BR_comment = | |||
| | legal_CA = Rx-only | |||
| | legal_CA_comment = <ref>{{cite web | title=Health Canada New Drug Authorizations: 2015 Highlights | website=] | date=4 May 2016 | url=https://www.canada.ca/en/health-canada/services/publications/drugs-health-products/health-canada-new-drug-authorizations-2015-highlights.html | access-date=7 April 2024}}</ref> | |||
| | legal_DE = <!-- Anlage I, II, III or Unscheduled--> | |||
| | legal_DE_comment = | |||
| | legal_NZ = <!-- Class A, B, C --> | |||
| | legal_NZ_comment = | |||
| | legal_UK = POM | |||
| | legal_UK_comment = <ref>{{cite web | title=Entresto 24 mg/26 mg film-coated tablets - Summary of Product Characteristics (SmPC) | website=(emc) | url=https://www.medicines.org.uk/emc/product/7751/smpc | access-date=21 September 2020 | archive-date=24 January 2021 | archive-url=https://web.archive.org/web/20210124224526/https://www.medicines.org.uk/emc/product/7751/smpc | url-status=live }}</ref> | |||
| | legal_US = Rx-only | |||
| | legal_US_comment = <ref name="Entresto FDA label" /> | |||
| | legal_EU = Rx-only | |||
| | legal_EU_comment = <ref name="Entresto EPAR" /><ref name="Neparvis EPAR">{{cite web | title=Neparvis EPAR | website=] (EMA) | date=17 September 2018 | url=https://www.ema.europa.eu/en/medicines/human/EPAR/neparvis | access-date=23 September 2020 | archive-date=28 December 2020 | archive-url=https://web.archive.org/web/20201228000243/https://www.ema.europa.eu/en/medicines/human/EPAR/neparvis | url-status=live }}</ref> | |||
| | legal_UN = <!-- N I, II, III, IV / P I, II, III, IV--> | |||
| | legal_UN_comment = | |||
| | legal_status = Rx-only | |||
| <!-- Pharmacokinetic data --> | |||
| | bioavailability = | |||
| | protein_bound = | |||
| | metabolism = | |||
| | metabolites = | |||
| | onset = | |||
| | elimination_half-life = | | elimination_half-life = | ||
| | |
| duration_of_action = | ||
| | excretion = | |||
| <!--Identifiers--> | <!-- Identifiers --> | ||
| | CAS_number = | | CAS_number = 936623-90-4 | ||
| | CAS_supplemental = | |||
| | ATC_prefix = none | |||
| | |
| PubChem = 71449007 | ||
| | |
| PubChemSubstance = | ||
| | IUPHAR_ligand = | |||
| | PubChem = 24755604 | |||
| | DrugBank = | | DrugBank = | ||
| | |
| ChemSpiderID = | ||
| | |
| UNII = WB8FT61183 | ||
| | KEGG = D10226 | |||
| | ChEBI = | |||
| | ChEMBL = | |||
| | NIAID_ChemDB = | |||
| | PDB_ligand = | |||
| | synonyms = LCZ696 | |||
| <!-- Chemical and physical data --> | |||
| | IUPAC_name = | |||
| | C = 96 | H = 120 | N = 12 | Na = 6 | O = 21 | |||
| | SMILES = CCCCC(=O)N(CC1=CC=C(C=C1)C2=CC=CC=C2C3=NN=N3)C(C(C)C)C(=O).CCCCC(=O)N(CC1=CC=C(C=C1)C2=CC=CC=C2C3=NN=N3)C(C(C)C)C(=O).CCOC(=O)C(C)CC(CC1=CC=C(C=C1)C2=CC=CC=C2)NC(=O)CCC(=O).CCOC(=O)C(C)CC(CC1=CC=C(C=C1)C2=CC=CC=C2)NC(=O)CCC(=O).O.O.O.O.O...... | |||
| | StdInChI = 1S/2C24H29N5O3.2C24H29NO5.6Na.5H2O/c2*1-4-5-10-21(30)29(22(16(2)3)24(31)32)15-17-11-13-18(14-12-17)19-8-6-7-9-20(19)23-25-27-28-26-23;2*1-3-30-24(29)17(2)15-21(25-22(26)13-14-23(27)28)16-18-9-11-20(12-10-18)19-7-5-4-6-8-19;;;;;;;;;;;/h2*6-9,11-14,16,22H,4-5,10,15H2,1-3H3,(H2,25,26,27,28,31,32);2*4-12,17,21H,3,13-16H2,1-2H3,(H,25,26)(H,27,28);;;;;;;5*1H2/q;;;;6*+1;;;;;/p-6/t2*22-;2*17-,21+;;;;;;;;;;;/m0011.........../s1 | |||
| | StdInChI_comment = | |||
| | StdInChIKey = ZASXKEGREHRXDL-CAWNUZPDSA-H | |||
| | density = | |||
| | density_notes = | |||
| | melting_point = | |||
| | melting_high = | |||
| | melting_notes = | |||
| | boiling_point = | |||
| | boiling_notes = | |||
| | solubility = | |||
| | sol_units = | |||
| | specific_rotation = | |||
| }} | }} | ||
| <!-- Definition and medical uses --> | |||
| '''Valsartan/sacubitril''' (codenamed '''LCZ696''') is an investigational ] consisting of two ]s (blood pressure lowering drugs), ] and ], in a 1:1 mixture. As of 2014 it is being developed by ]. The combination is often described as a dual-acting angiotensin receptor-neprilysin inhibitor (ARNi)<ref name="Gu" /><ref name="Ruilope" /> although the two effects are achieved by two different molecules. | |||
| '''Sacubitril/valsartan''', sold under the brand name '''Entresto''' among others, is a fixed-dose ] for use in ]. It consists of the ] ] and the ] ]. The combination is sometimes described as an "angiotensin receptor-neprilysin inhibitor" (ARNi).<ref name=pmid26976916>{{cite journal | vauthors = Hubers SA, Brown NJ | title = Combined Angiotensin Receptor Antagonism and Neprilysin Inhibition | journal = Circulation | volume = 133 | issue = 11 | pages = 1115–1124 | date = March 2016 | pmid = 26976916 | pmc = 4800749 | doi = 10.1161/CIRCULATIONAHA.115.018622 }}</ref> | |||
| In 2016, the American College of Cardiology/American Heart Association Task Force recommended it as a replacement for an ] or an ] in people with heart failure with reduced ].<ref name=Van2016>{{cite journal | vauthors = Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, Drazner MH, Filippatos G, Fonarow GC, Givertz MM, Hollenberg SM, Lindenfeld J, Masoudi FA, McBride PE, Peterson PN, Stevenson LW, Westlake C | title = 2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America | journal = Circulation | volume = 134 | issue = 13 | pages = e282–e293 | date = September 2016 | pmid = 27208050 | doi = 10.1161/CIR.0000000000000435 | doi-access = free }}</ref> | |||
| <!-- Side effects and mechanism --> | |||
| Potential side effects include ], ], and ].<ref name=Van2016/> | |||
| <!-- History and culture --> | |||
| It was approved for medical use in the United States and in the European Union in 2015,<ref name=FDApr2015>{{cite press release|publisher=U.S. ] (FDA) |date=7 July 2015|url=https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm453845.htm|title=FDA approves new drug to treat heart failure | archive-url=https://wayback.archive-it.org/7993/20180126023455/https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm453845.htm | archive-date=26 January 2018 | url-status=dead | df=dmy-all }}</ref><ref>{{cite web | title=Entresto (sacubitril/valsartan) Tablets | website=U.S. ] (FDA) | date=14 August 2015 | url=https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207620Orig1s000TOC.cfm | access-date=22 September 2020 | archive-date=31 March 2021 | archive-url=https://web.archive.org/web/20210331081554/https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207620Orig1s000TOC.cfm | url-status=live }}</ref><ref>{{Cite report|url=https://www.accessdata.fda.gov/drugsatfda_docs/nda/2015/207620Orig1s000SumR.pdf|title=Summary review of LCZ696, a fixed-dose combination of valsartan and sacubitril|publisher=Center for Drug Evaluation and Research|id=207620Orig1s000|date=12 June 2015| vauthors = Thompson A }} Partially redacted.</ref><ref name="Entresto EPAR">{{cite web | title=Entresto EPAR | website=] (EMA) | date=17 September 2018 | url=https://www.ema.europa.eu/en/medicines/human/EPAR/entresto | access-date=21 September 2020 | archive-date=27 February 2021 | archive-url=https://web.archive.org/web/20210227125734/https://www.ema.europa.eu/en/medicines/human/EPAR/entresto | url-status=live }}</ref> and in Australia in 2016.<ref name="Entresto AU PI">{{cite web | title=Entresto 24/26 tablets, Entresto 49/51 tablets, Entresto 97/103 tablets (sacubitril/valsartan) Product Information | website=] (TGA) | publisher=Novartis | url=https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2016-PI-01107-1 | access-date=21 September 2020 | archive-date=11 November 2020 | archive-url=https://web.archive.org/web/20201111173322/https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent | url-status=live }}</ref> In 2022, it was the 165th most commonly prescribed medication in the United States, with more than 3{{nbsp}}million prescriptions.<ref>{{cite web | title=The Top 300 of 2022 | url=https://clincalc.com/DrugStats/Top300Drugs.aspx | website=ClinCalc | access-date=30 August 2024 | archive-date=30 August 2024 | archive-url=https://web.archive.org/web/20240830202410/https://clincalc.com/DrugStats/Top300Drugs.aspx | url-status=live }}</ref><ref>{{cite web | title = Sacubitril; Valsartan Drug Usage Statistics, United States, 2013 - 2022 | website = ClinCalc | url = https://clincalc.com/DrugStats/Drugs/SacubitrilValsartan | access-date = 30 August 2024 }}</ref> It is available as a ].<ref name="FDA PR 20240531">{{cite press release | title=FDA Roundup: May 31, 2024 | website=U.S. ] (FDA) | date=31 May 2024 | url=https://www.fda.gov/news-events/press-announcements/fda-roundup-may-31-2024 | access-date=31 May 2024}} {{PD-notice}}</ref> | |||
| ==Medical uses== | ==Medical uses== | ||
| Sacubitril/valsartan can be used instead of an ] or an ] in people with ] and a reduced ] (LVEF),<ref>{{cite journal | vauthors = Chang HY, Chen KC, Fong MC, Feng AN, Fu HN, Huang KC, Chong E, Yin WH | title = Recovery of left ventricular dysfunction after sacubitril/valsartan: predictors and management | journal = Journal of Cardiology | volume = 75 | issue = 3 | pages = 233–241 | date = March 2020 | pmid = 31563433 | doi = 10.1016/j.jjcc.2019.08.005 | doi-access = free }}</ref><ref name=pmid26976916 /> alongside other standard therapies (e.g. ]s) for heart failure.<ref name=Van2016/><ref name=FDApr2015/><ref name="McMurray" /> To investigate its use for heart failure in those with a preserved LVEF (HFpEF), Novartis funded the PARAGON-HF trial which was designed to investigate the use of sacubitril/valsartan in the treatment of HFpEF patients with a LVEF of 45% or more. Concluding in 2019, it failed to show significance for reducing hospitalisation related to heart failure or reducing death from cardiovascular causes, and therefore appearing to show limited benefit to those with HFpEF.<ref name="Solomon_2019">{{cite journal | vauthors = Solomon SD, McMurray JJ, Anand IS, Ge J, Lam CS, Maggioni AP, Martinez F, Packer M, Pfeffer MA, Pieske B, Redfield MM, Rouleau JL, van Veldhuisen DJ, Zannad F, Zile MR, Desai AS, Claggett B, Jhund PS, Boytsov SA, Comin-Colet J, Cleland J, Düngen HD, Goncalvesova E, Katova T, Kerr Saraiva JF, Lelonek M, Merkely B, Senni M, Shah SJ, Zhou J, Rizkala AR, Gong J, Shi VC, Lefkowitz MP | title = Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction | journal = The New England Journal of Medicine | volume = 381 | issue = 17 | pages = 1609–1620 | date = October 2019 | pmid = 31475794 | doi = 10.1056/NEJMoa1908655 | doi-access = free | hdl = 2445/175935 | hdl-access = free }}</ref> | |||
| Valsartan/sacubitril is not currently used as a medicine; it is in clinical trials as a treatment for ] and ].<ref name=Monge>Monge M et al. New drug therapies interfering with the renin-angiotensin-aldosterone system for resistant hypertension. J Renin Angiotensin Aldosterone Syst. 2013 Dec;14(4):285-9. doi: 10.1177/1470320313513408. Epub 2013 Nov 12. PMID 24222656</ref><ref>Zouein FA1 et al. Heart failure with preserved ejection fraction: emerging drug strategies. J Cardiovasc Pharmacol. 2013 Jul;62(1):13-21. doi: 10.1097/FJC.0b013e31829a4e61. PMID 23714774 PMC 3724214</ref><ref name="PARADIGM-HF">{{ClinicalTrialsGov|NCT01035255|This Study Will Evaluate the Efficacy and Safety of LCZ696 Compared to Enalapril on Morbidity and Mortality of Patients With Chronic Heart Failure (PARADIGM-HF)}}</ref> | |||
| There is moderate evidence to suggest that treating people with HFpEF using MRA and ARNI may reduce the rate of people being hospitalized due to heart failure, however, there is no evidence that this type of treatment improves the person's quality of life or rate of survival due to cardiovascular disease.<ref>{{cite journal | vauthors = Martin N, Manoharan K, Davies C, Lumbers RT | title = Beta-blockers and inhibitors of the renin-angiotensin aldosterone system for chronic heart failure with preserved ejection fraction | journal = The Cochrane Database of Systematic Reviews | volume = 2021 | issue = 5 | pages = CD012721 | date = May 2021 | pmid = 34022072 | pmc = 8140651 | doi = 10.1002/14651858.CD012721.pub3 }}</ref> Evidence is lacking to support the use of ACE Inhibitors, ARBs or ARNIs in people with HFpEF at this time, and that the mainstay ] for HFpEF still remains the treatment of co-morbidities such as hypertension or other triggers for decompensation. Patients who exhibit symptoms of ] II or III heart failure and are still symptomatic despite maximally tolerated dose of an ACE inhibitor or ARB alone, may be considered for sacubitril/valsartan dual therapy to decrease the risk of cardiovascular-related and all-cause mortality.<ref name="McMurray_2014" /> Mortality benefits have only been observed to date in those with LVEF less than 35%.<ref name="Solomon_2019" /><ref name="McMurray_2014" /> | |||
| ==Mechanism of action== | |||
| ] blocks the ] (AT<sub>1</sub>) and thereby causes ] and increases excretion of ] and water via the kidneys (by reducing ] production). The latter mechanism leads to a reduction in blood volume.<ref name="Mutschler" /> | |||
| ==Adverse effects== | |||
| ] is a ] that is activated to ] by de-] via ]s.<ref name="Solomon" /> LBQ657 inhibits the enzyme ],<ref name="Gu" /> which is responsible for the degradation of ] and ], two blood pressure lowering ]s that work mainly by reducing blood volume.<ref name="Schubert" /> | |||
| Common adverse effects include hyperkalaemia , hypotension , a persistent dry cough and renal impairment .<ref name="McMurray_2014">{{cite journal | vauthors = McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR | title = Angiotensin-neprilysin inhibition versus enalapril in heart failure | journal = The New England Journal of Medicine | volume = 371 | issue = 11 | pages = 993–1004 | date = September 2014 | pmid = 25176015 | doi = 10.1056/NEJMoa1409077 | hdl-access = free | hdl = 2336/552372 | s2cid = 11383 }}</ref><ref name="Solomon_2019" /><ref name="Velazquez_2019">{{cite journal | vauthors = Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, Rocha R, Braunwald E | title = Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure | journal = The New England Journal of Medicine | volume = 380 | issue = 6 | pages = 539–548 | date = February 2019 | pmid = 30415601 | doi = 10.1056/NEJMoa1812851 | s2cid = 53280900 | doi-access =free }}</ref> | |||
| Angioedema, a rare but more serious reaction, can occur in some patients and involves swelling of the face and lips.<ref name="McMurray_2014" /><ref name="Solomon_2019" /><ref name="Velazquez_2019" /> Angioedema is more common in black (African American) patients.<ref name="McMurray_2014" /> Sacubitril/Valsartan should not be taken within 36 hours of an Angiotensin Converting Enzyme Inhibitor to reduce the risk of developing Angioedema.<ref name="McMurray_2014" /> | |||
| ==Physical and chemical properties== | |||
| LCZ696 is synthesized by co-crystallisation of ] and ], in a one-to-one ratio; it is not simply a ] of the two drugs together.<ref name=Monge/> | |||
| The side effect profile in trials of sacubitril/valsartan compared to valsartan alone or ] is very similar, with the incidence of hypotension slightly higher in sacubitril/valsartan, the risk comparable for angioedema, and the chance of hyperkalaemia, renal impairment and cough slightly lower.<ref name="McMurray_2014" /><ref name="Solomon_2019" /><ref name="Velazquez_2019" /> | |||
| ==Research== | |||
| Sacubitril/valsartan is ] because it contains valsartan, a known risk for birth defects.<ref name="Entresto FDA label">{{cite web | title=Entresto- sacubitril and valsartan tablet, film coated | website=DailyMed | date=14 June 2020 | url=https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=000dc81d-ab91-450c-8eae-8eb74e72296f | access-date=21 September 2020 | archive-date=19 April 2020 | archive-url=https://web.archive.org/web/20200419215543/https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=000dc81d-ab91-450c-8eae-8eb74e72296f | url-status=live }}</ref> | |||
| ===Hypertension=== | |||
| A randomized, double-blinded clinical study published in 2010 found that LCZ696 decreased blood pressure more than placebo, or either of ] and ] given alone, in people with stage 1–2 hypertension.<ref name=Monge/> | |||
| == |
==Pharmacology== | ||
| Valsartan blocks the ] (AT<sub>1</sub>). This receptor is found on both vascular smooth muscle cells, and on the ] cells of the ] which are responsible for ] secretion. In the absence of AT<sub>1</sub> blockade, angiotensin causes both direct ] and adrenal aldosterone secretion, the aldosterone then acting on the distal tubular cells of the kidney to promote sodium reabsorption which expands ] (ECF) volume. Blockade of (AT<sub>1</sub>) thus causes blood vessel dilation and reduction of ECF volume.<ref name="Mutschler">{{cite book| vauthors = Mutschler E, Schäfer-Korting M |title=Arzneimittelwirkungen|language=de|location=Stuttgart|publisher=Wissenschaftliche Verlagsgesellschaft|year=2001|edition=8|page=579|isbn=978-3-8047-1763-3 }}</ref><ref name="Zouein">{{cite journal | vauthors = Zouein FA, de Castro Brás LE, da Costa DV, Lindsey ML, Kurdi M, Booz GW | title = Heart failure with preserved ejection fraction: emerging drug strategies | journal = Journal of Cardiovascular Pharmacology | volume = 62 | issue = 1 | pages = 13–21 | date = July 2013 | pmid = 23714774 | pmc = 3724214 | doi = 10.1097/FJC.0b013e31829a4e61 }}</ref> | |||
| On 30 August 2014, the PARADIGM-HF investigators and committees reported in the ] (NEJM) that LCZ696 significantly reduced the risks of overall death, death from heart failure, and hospitalization for heart failure, compared to ], in a Phase III trial.<ref name="McMurray">{{cite journal | |||
| | title =Angiotensin–Neprilysin Inhibition versus Enalapril in Heart Failure | |||
| | journal =N Eng J Med | |||
| | date =August 30, 2014 | |||
| | authors =John J.V. McMurray, Milton Packer, Akshay S. Desai, et al. for the PARADIGM-HF Investigators and Committees | |||
| | volume =371 | |||
| | issue = | |||
| | pages = | |||
| | url =http://www.nejm.org/doi/full/10.1056/NEJMoa1409077 | |||
| | doi =10.1056/NEJMoa1409077 | |||
| | pmid = | |||
| | pmc = | |||
| }}</ref> 8,442 people with with moderate to severe heart failure and an ejection fraction of 40% or less were enrolled in the trial, which randomized and double-blinded. The primary endpoint of the trial was the number of patients in each arm who experienced death from cardiovascular disease or hospitalization. At the time the study was stopped, 21.8% of those in the LCZ696 arm and 26.5% of those in the enalapril arm had died of cardiovascular causes or been hospitalized (hazard ratio 0.80); 17.0% of those receiving LCZ696 and 19.8% of those receiving enalapril had died (hazard ratio 0.84); and 13.3% of those receiving LCZ696 and 16.5% of those receiving enalapril had died of cardiovascular causes. | |||
| Sacubitril is a ] that is activated to ] (LBQ657) by de-] via ]s.<ref name="Solomon">{{cite web|url=http://spo.escardio.org/eSlides/view.aspx?eevtid=46&fp=13|title=HFpEF in the Future: New Diagnostic Techniques and Treatments in the Pipeline|vauthors=Solomon SD|location=Boston|page=48|access-date=26 January 2012|archive-date=12 September 2014|archive-url=https://web.archive.org/web/20140912012635/http://spo.escardio.org/eSlides/view.aspx?eevtid=46&fp=13|url-status=live}}</ref> Sacubitrilat inhibits the enzyme ],<ref name="Gu">{{cite journal | vauthors = Gu J, Noe A, Chandra P, Al-Fayoumi S, Ligueros-Saylan M, Sarangapani R, Maahs S, Ksander G, Rigel DF, Jeng AY, Lin TH, Zheng W, Dole WP | title = Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi) | journal = Journal of Clinical Pharmacology | volume = 50 | issue = 4 | pages = 401–414 | date = April 2010 | pmid = 19934029 | doi = 10.1177/0091270009343932 | s2cid = 24853279 }}</ref> a ] that degrades ] peptides, including ], ], and ]. Thus, sacubitril increases the levels of these peptides, causing blood vessel dilation and reduction of ECF volume via sodium excretion.<ref name="Schubert">{{cite news| vauthors = Schubert-Zsilavecz M, Wurglics M |title=Neue Arzneimittel 2010/2011|language=de }}</ref> | |||
| Expert commentary on publication of the PARADIGM-HF clinical trial varied widely, with some declaring a "new paradigm" in the treatment of heart failure,<ref>{{cite web |url=http://www.medscape.com/viewarticle/830711#2 |title=PARADIGM-HF: New Drug Class Outclasses ACE-I in Chronic HF |format= |work= |accessdate=}}</ref><ref>{{cite web |url=http://www.forbes.com/sites/larryhusten/2014/08/30/paradigm-hf-establishes-a-new-paradigm-for-heart-failure-treatment/ |title=PARADIGM-HF Establishes a New Paradigm for Heart Failure Treatment - Forbes |format= |work= |accessdate=}}</ref> and others arguing that flaws in the trial design and lack of availability of patient level data makes independent interpretation of the results impossible.<ref name="Pollack">, By Andrew Pollack, New York Times, AUG. 30, 2014</ref><ref> Vol 371. The BMJ, 8 September 2014.</ref> | |||
| Despite these actions, neprilysin inhibitors have been found to have limited efficacy in the treatment of hypertension and heart failure when taken on their own.<ref>{{cite journal | vauthors = Bevan EG, Connell JM, Doyle J, Carmichael HA, Davies DL, Lorimer AR, McInnes GT | title = Candoxatril, a neutral endopeptidase inhibitor: efficacy and tolerability in essential hypertension | journal = Journal of Hypertension | volume = 10 | issue = 7 | pages = 607–613 | date = July 1992 | pmid = 1321186 | doi = 10.1097/00004872-199207000-00002 | s2cid = 23507064 }}</ref><ref name="Richards_1993">{{cite journal | vauthors = Richards AM, Wittert GA, Crozier IG, Espiner EA, Yandle TG, Ikram H, Frampton C | title = Chronic inhibition of endopeptidase 24.11 in essential hypertension: evidence for enhanced atrial natriuretic peptide and angiotensin II | journal = Journal of Hypertension | volume = 11 | issue = 4 | pages = 407–416 | date = April 1993 | pmid = 8390508 | doi = 10.1097/00004872-199304000-00011 | s2cid = 25333484 }}</ref> This is attributed to a reduction in enzymatic breakdown of angiotensin II by the reduction of neprilysin activity, which results in an increase in systemic angiotensin II levels and the negation of the positive effects of this drug family in cardiovascular disease treatment.<ref name="Richards_1993" /> Combined treatment with a neprilysin inhibitor and an angiotensin converting enzyme (ACE) inhibitor has been shown to be effective in reducing angiotensin II levels, and demonstrated superiority in lowering blood pressure compared to ACE inhibition alone.<ref name="Kostis_2004">{{cite journal | vauthors = Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E | title = Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial | journal = American Journal of Hypertension | volume = 17 | issue = 2 | pages = 103–111 | date = February 2004 | pmid = 14751650 | doi = 10.1016/j.amjhyper.2003.09.014 | doi-access = free }}</ref> However, due to an increase in bradykinins from the inhibition of both ACE and neprilysin, there was a threefold increase in relative risk of angioedema compared with ACE inhibition alone following this combination treatment.<ref name="Kostis_2004" /> The combination of a neprilysin inhibitor with an angiotensin receptor blocker instead of the ACE inhibitor has been shown to have a comparable risk of angioedema, whilst also demonstrating superiority in treating moderate-severe heart failure to ACE inhibitor treatment.<ref name="McMurray" /><ref name="Velazquez_2019"/> | |||
| ==References== | |||
| {{reflist|2|refs= | |||
| Neprilysin also has a role in clearing the protein ] from the ], and its inhibition by sacubitril has shown increased levels of AB<sub>1-38</sub> in healthy subjects (Entresto 194/206 for two weeks). Amyloid beta is considered to contribute to the development of Alzheimer's disease, and there exist concerns that sacubitril may promote the development of Alzheimer's disease.<ref name="Entresto FDA label" /><ref>{{cite journal | vauthors = Patel N, Gluck J | title = Is Entresto good for the brain? | journal = World Journal of Cardiology | volume = 9 | issue = 7 | pages = 594–599 | date = July 2017 | pmid = 28824789 | pmc = 5545143 | doi = 10.4330/wjc.v9.i7.594 | doi-access = free }}</ref> | |||
| <ref name="Gu">{{cite pmid|19934029}}</ref> | |||
| <ref name="Schubert">{{Cite document|last1=Schubert-Zsilavecz|first1=M|last2=Wurglics|first2=M|title=Neue Arzneimittel 2010/2011.|language=German|postscript=<!-- Bot inserted parameter. Either remove it; or change its value to "." for the cite to end in a ".", as necessary. -->{{inconsistent citations}}}}</ref> | |||
| == Structure activity relationship == | |||
| <ref name="Ruilope">{{cite doi|10.1016/S0140-6736(09)61966-8}}</ref> | |||
| Sacubitril is the molecule that is metabolically activated by de-ethylation by esterases. The active form of the molecule, sacubitrilat, is responsible for the molecule's drug lowering effects.<ref name="pmid35921043">{{cite journal |vauthors=Abdin A, Schulz M, Riemer U, Hadëri B, Wachter R, Laufs U, Bauersachs J, Kindermann I, Vukadinović D, Böhm M |title=Sacubitril/valsartan in heart failure: efficacy and safety in and outside clinical trials |journal=ESC Heart Fail |volume= 9|issue= 6|pages= 3737–3750|date=August 2022 |pmid=35921043 |doi=10.1002/ehf2.14097 |pmc=9773772 |url=}}</ref> | |||
| <ref name="Mutschler">{{Cite book|last1=Mutschler|first1=Ernst|last2=Schäfer-Korting|first2=Monika|title=Arzneimittelwirkungen|language=German|location=Stuttgart|publisher=Wissenschaftliche Verlagsgesellschaft|year=2001|edition=8|page=579|isbn=3-8047-1763-2}}</ref> | |||
| <ref name="Solomon">{{cite web|url=http://spo.escardio.org/eSlides/view.aspx?eevtid=46&fp=13|title=HFpEF in the Future: New Diagnostic Techniques and Treatments in the Pipeline|last=Solomon|first=SD|location=Boston|page=48|accessdate=2012-01-26}}</ref> | |||
| ==Chemistry== | |||
| }} | |||
| Sacubitril/valsartan is co-crystallized sacubitril and valsartan, in a one-to-one ] ratio. One sacubitril/valsartan ] consists of six sacubitril ]s, six valsartan dianions, 18 sodium cations, and 15 molecules of water, resulting in the molecular formula C<sub>288</sub>H<sub>330</sub>N<sub>36</sub>Na<sub>18</sub>O<sub>48</sub>·15H<sub>2</sub>O and a ] of 5748.03 g/mol.<ref name=Monge>{{cite journal | vauthors = Monge M, Lorthioir A, Bobrie G, Azizi M | title = New drug therapies interfering with the renin-angiotensin-aldosterone system for resistant hypertension | journal = Journal of the Renin-Angiotensin-Aldosterone System | volume = 14 | issue = 4 | pages = 285–289 | date = December 2013 | pmid = 24222656 | doi = 10.1177/1470320313513408 | doi-access = free }}</ref><ref name="Feng" /> | |||
| The substance is a white powder consisting of thin hexagonal plates. It is stable in solid form as well as in aqueous (water) solution with a ] of 5 to 7, and has a melting point of about {{convert|138|C|F}}.<ref name="Feng">{{Cite journal| vauthors = Feng L, Karpinski PH, Sutton P, Liu Y, Hook DF, Hu B, Blacklock TJ, Fanwick PE, Prashad M, Godtfredsen S, Ziltener C |title=LCZ696: a dual-acting sodium supramolecular complex| journal=Tetrahedron Letters| volume=53|issue=3| year=2012| pages=275–276 | doi= 10.1016/j.tetlet.2011.11.029}}</ref> | |||
| ==History== | |||
| During its development by Novartis, Entresto was known as LCZ696.<ref name=pmid26976916 /> It was approved under the FDA's ] process on 7 July 2015.<ref name=FDApr2015/> It was also approved in Europe in 2015.<ref name="Entresto EPAR" /> In 2022, Novartis sold its India marketing rights of Sacubitril Valsartan to JB Pharma, under the brand name Azmarda.<ref>{{cite news|url=https://medicaldialogues.in/partner/jbcpl/azmarda-sacubitril-valsartan|title=Azmarda (Sacubitril Valsartan): Overview, Indications, Clinical Evidence & Dosage|date=30 June 2022|work=Medical Dialogues|access-date=30 June 2022|archive-date=5 July 2022|archive-url=https://web.archive.org/web/20220705000846/https://medicaldialogues.in/partner/jbcpl/azmarda-sacubitril-valsartan|url-status=live}}</ref> | |||
| ==Society and culture== | |||
| ===Trial design=== | |||
| There was controversy over the PARADIGM-HF trial—the Phase III trial on the basis of which the drug was approved by the FDA. For example, both Richard Lehman, a physician who writes a weekly review of key medical articles for the BMJ Blog and a December 2015, report from the ] (ICER) found that the ] was not adequately determined because the design of the clinical trial was too artificial and did not reflect people with heart failure that doctors usually encounter.<ref name=ICERfinal>{{cite news | url=http://resource.nlm.nih.gov/101672986 | vauthors=Ollendorf DA, Tarlochan Sandhu A, Chapman R, Heidenreich PA, Russo E, Shore KK, Synnott P, Travers K, Weissberg J, Pearson SD | title=CardioMEMS HF System (St. Jude Medical, Inc.) and Sacubitril/Valsartan (Entresto, Novartis AG) for management of congestive heart failure: effectiveness, value, and value-based price benchmarks : final report | date=1 December 2015 | id=101672986 | access-date=4 October 2019 | archive-date=29 August 2021 | archive-url=https://web.archive.org/web/20210829043221/https://collections.nlm.nih.gov/catalog/nlm:nlmuid-101672986-pdf | url-status=live }}</ref>{{rp|28}}<ref> {{Webarchive|url=https://web.archive.org/web/20210516220729/https://blogs.bmj.com/bmj/2014/09/08/richard-lehmans-journal-review-8-september-2014/#more-32277 |date=16 May 2021 }} Vol 371. The BMJ, 8 September 2014.</ref> In 2019, the PIONEER-HF and PARAGON-HF trials studied the effect of sacubitril/valsartan in 800 patients recently hospitalised with severe heart failure and 4800 patients with less severe symptoms of heart failure respectively.<ref name="Velazquez_2019" /><ref name="Solomon_2019" /> The medication consistently demonstrated similar levels of safety, with higher rates of very low blood pressure, compared to current treatments across all three trials in a variety of patients, however it has only shown effectiveness in those with more advanced heart failure.<ref name="McMurray_2014" /><ref name="Solomon_2019" /><ref name="Velazquez_2019" /> In December 2015, ] and other thought leaders in cardiology said that the approval of sacubitril/valsartan had the greatest impact on clinical practice in cardiology in 2015, and Nissen called the drug "truly a breakthrough approach."<ref>Roger Sergel for Medpage Today. {{Webarchive|url=https://web.archive.org/web/20210627214226/https://www.medpagetoday.com/Cardiology/CHF/55415 |date=27 June 2021 }}</ref> | |||
| One 2015 review stated that sacubitril/valsartan represents "an advancement in the chronic treatment of heart failure with reduced ejection fraction" but that widespread clinical success with the drug will require taking care to use it in appropriate patients, specifically those with characteristics similar to those in the clinical trial population.<ref>{{cite journal | vauthors = Lillyblad MP | title = Dual Angiotensin Receptor and Neprilysin Inhibition with Sacubitril/Valsartan in Chronic Systolic Heart Failure: Understanding the New PARADIGM | journal = The Annals of Pharmacotherapy | volume = 49 | issue = 11 | pages = 1237–1251 | date = November 2015 | pmid = 26175499 | doi = 10.1177/1060028015593093 | s2cid = 28918702 }}</ref> Another 2015 review called the reductions in mortality and hospitalization conferred by sacubitril/valsartan "striking", but noted that its effects in heart failure people with hypertension, diabetes, ], and the elderly needed to be evaluated further.<ref>{{cite journal | vauthors = Bavishi C, Messerli FH, Kadosh B, Ruilope LM, Kario K | title = Role of neprilysin inhibitor combinations in hypertension: insights from hypertension and heart failure trials | journal = European Heart Journal | volume = 36 | issue = 30 | pages = 1967–1973 | date = August 2015 | pmid = 25898846 | doi = 10.1093/eurheartj/ehv142 | doi-access = free }}</ref> | |||
| ===Economics=== | |||
| The wholesale cost to the ] (NHS) in the UK is approximately {{GBP|1,200}} per person per year as of 2017.<ref name=MIMS>{{cite web|title=Entresto|url=http://www.mims.co.uk/drugs/cardiovascular-system/heart-failure/entresto|website=MIMS|access-date=25 July 2017|archive-date=5 August 2017|archive-url=https://web.archive.org/web/20170805222559/http://www.mims.co.uk/drugs/cardiovascular-system/heart-failure/entresto|url-status=live}}</ref> | |||
| The wholesale cost in the United States is {{US$|4,560}} per year {{as of|2015|lc=yes}}.<ref name=ICERfinal/> | |||
| One industry-funded analysis found a cost of {{US$|45,017}} per ] (QALY).<ref name=Gaziano>{{cite journal | vauthors = Gaziano TA, Fonarow GC, Claggett B, Chan WW, Deschaseaux-Voinet C, Turner SJ, Rouleau JL, Zile MR, McMurray JJ, Solomon SD | title = Cost-effectiveness Analysis of Sacubitril/Valsartan vs Enalapril in Patients With Heart Failure and Reduced Ejection Fraction | journal = JAMA Cardiology | volume = 1 | issue = 6 | pages = 666–672 | date = September 2016 | pmid = 27438344 | doi = 10.1001/jamacardio.2016.1747 | doi-access = free }}</ref> | |||
| Similar class generic drugs without sacubitril, such as valsartan alone, cost approximately {{US$|48}} a year.<ref name="Pollack">{{cite news |url=https://www.nytimes.com/2014/08/31/business/new-novartis-drug-shows-striking-efficacy-in-treating-heart-failure.html |title=New Novartis Drug Effective in Treating Heart Failure |vauthors=Pollack A |newspaper=] |date=30 August 2014 |access-date=3 March 2017 |archive-date=5 May 2021 |archive-url=https://web.archive.org/web/20210505133259/https://www.nytimes.com/2014/08/31/business/new-novartis-drug-shows-striking-efficacy-in-treating-heart-failure.html |url-status=live }}</ref> | |||
| ==Research== | |||
| The PARADIGM-HF trial (in which ] was one of the principal investigators) compared treatment with sacubitril/valsartan to treatment with ].<ref>{{cite news|vauthors=Husten L|title=Novartis Trial Was Stopped Early Because Of A Significant Drop In Cardiovascular Mortality|url=https://www.forbes.com/sites/larryhusten/2014/03/31/novartis-trial-was-stopped-early-because-of-a-significant-drop-in-cardiovascular-mortality/#2226cab84010|work=Forbes|date=31 March 2014|access-date=6 August 2017|archive-date=24 June 2021|archive-url=https://web.archive.org/web/20210624200934/https://www.forbes.com/sites/larryhusten/2014/03/31/novartis-trial-was-stopped-early-because-of-a-significant-drop-in-cardiovascular-mortality/#2226cab84010|url-status=live}}</ref> People with heart failure and reduced LVEF (10,513) were sequentially treated on a short-term basis with enalapril and then with sacubitril/valsartan. Those that were able to tolerate both regimens (8442, 80%) were randomly assigned to long-term treatment with either enalapril or sacubitril/valsartan. Participants were mainly white (66%), male (78%), middle aged (median 63.8 +/- 11 years) with NYHA stage II (71.6%) or stage III (23.1%) heart failure.<ref name="ReferenceA">{{cite journal | vauthors = King JB, Bress AP, Reese AD, Munger MA | title = Neprilysin Inhibition in Heart Failure with Reduced Ejection Fraction: A Clinical Review | journal = Pharmacotherapy | volume = 35 | issue = 9 | pages = 823–837 | date = September 2015 | pmid = 26406774 | doi = 10.1002/phar.1629 | s2cid = 6363036 }}</ref> | |||
| The trial was stopped early after a prespecified interim analysis revealed a reduction in the primary endpoint of cardiovascular death or heart failure in the sacubitril/valsartan group relative to those treated with enalapril. Taken individually, the reductions in cardiovascular death and heart failure hospitalizations retained statistical significance.<ref name="McMurray">{{cite journal | vauthors = McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, Rouleau JL, Shi VC, Solomon SD, Swedberg K, Zile MR | title = Angiotensin-neprilysin inhibition versus enalapril in heart failure | journal = The New England Journal of Medicine | volume = 371 | issue = 11 | pages = 993–1004 | date = September 2014 | pmid = 25176015 | doi = 10.1056/NEJMoa1409077 | hdl-access = free | hdl = 2336/552372 | s2cid = 11383 }}</ref> Relative to enalapril, sacubitril/valsartan provided reductions<ref name="ReferenceA"/><ref>{{cite web | title=Sacubitril/Valsartan Combination Drug: 2 Years Later | date=3 March 2017 | vauthors=Drescher CS, Desai AS | publisher=American College of Cardiology | url=https://www.acc.org/latest-in-cardiology/articles/2017/03/03/09/30/sacubitril-valsartan-combination-drug | access-date=20 June 2019 | archive-date=24 June 2021 | archive-url=https://web.archive.org/web/20210624200924/https://www.acc.org/latest-in-cardiology/articles/2017/03/03/09/30/sacubitril-valsartan-combination-drug | url-status=live }}</ref> in: | |||
| * the composite endpoint of cardiovascular death or hospitalization for heart failure (incidence 21.8% vs 26.5%) | |||
| * cardiovascular death (incidence 13.3% vs 16.5%) | |||
| * first hospitalization for worsening heart failure (incidence 12.8% vs 15.6%), and | |||
| * all-cause mortality (incidence 17.0% vs 19.8%) | |||
| Limitations of the trial include scarce experience with initiation of therapy in hospitalized patients and in those with NYHA heart failure class IV symptoms.<ref>{{cite journal | vauthors = Havakuk O, Elkayam U | title = Angiotensin Receptor-Neprilysin Inhibition | journal = Journal of Cardiovascular Pharmacology and Therapeutics | volume = 22 | issue = 4 | pages = 356–364 | date = July 2017 | pmid = 28587583 | doi = 10.1177/1074248416683049 | s2cid = 4066142 }}</ref><ref>{{cite journal | vauthors = Perez AL, Kittipibul V, Tang WH, Starling RC | title = Patients Not Meeting PARADIGM-HF Enrollment Criteria Are Eligible for Sacubitril/Valsartan on the Basis of FDA Approval: The Need to Close the Gap | journal = JACC. Heart Failure | volume = 5 | issue = 6 | pages = 460–463 | date = June 2017 | pmid = 28571599 | doi = 10.1016/j.jchf.2017.03.007 | doi-access = free }}</ref> Additionally the trial compared a maximal dose of valsartan (plus sacubitril) with a sub-maximal dose of enalapril, and was thus not directly comparable with current gold-standard use of ACE inhibitors in heart failure, diminishing the validity of the trial results.<ref>{{Cite web|url=https://blogs.bmj.com/bmj/2014/09/08/richard-lehmans-journal-review-8-september-2014/|title=Richard Lehman's journal review—8 September 2014|date=8 September 2014|website=The BMJ|access-date=20 May 2019|archive-date=11 February 2018|archive-url=https://web.archive.org/web/20180211144219/http://blogs.bmj.com/bmj/2014/09/08/richard-lehmans-journal-review-8-september-2014/|url-status=live}}</ref><ref>{{cite journal | vauthors = Ahn R, Prasad V | title = Do Limitations in the Design of PARADIGM-HF Justify the Slow Real World Uptake of Sacubitril/Valsartan (Entresto)? | journal = Cardiovascular Drugs and Therapy | volume = 32 | issue = 6 | pages = 633–635 | date = December 2018 | pmid = 30232657 | doi = 10.1007/s10557-018-6830-x | s2cid = 52298581 }}</ref> | |||
| == References == | |||
| ] | |||
| {{reflist}} | |||
| ] | |||
| {{ACE inhibitors}} | |||
| {{Portal bar | Medicine}} | |||
| {{Authority control}} | |||
| {{DEFAULTSORT:Sacubitril Valsartan}} | |||
| {{antihypertensive-stub}} | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 05:13, 12 November 2024
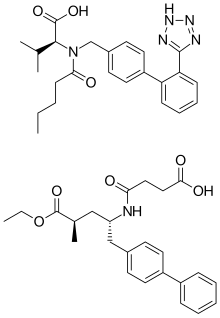
Combination medicationPharmaceutical compound
 | |
| Combination of | |
|---|---|
| Sacubitril | Neprilysin inhibitor |
| Valsartan | Angiotensin II receptor antagonist |
| Clinical data | |
| Trade names | Entresto, Azmarda, Neparvis, others |
| Other names | LCZ696 |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a615039 |
| License data | |
| Pregnancy category |
|
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Identifiers | |
| CAS Number | |
| PubChem CID | |
| UNII | |
| KEGG | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| Formula | C96H120N12Na6O21 |
| Molar mass | 1916.018 g·mol |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
Sacubitril/valsartan, sold under the brand name Entresto among others, is a fixed-dose combination medication for use in heart failure. It consists of the neprilysin inhibitor sacubitril and the angiotensin receptor blocker valsartan. The combination is sometimes described as an "angiotensin receptor-neprilysin inhibitor" (ARNi). In 2016, the American College of Cardiology/American Heart Association Task Force recommended it as a replacement for an ACE inhibitor or an angiotensin receptor blocker in people with heart failure with reduced ejection fraction.
Potential side effects include angioedema, nephrotoxicity, and low blood pressure.
It was approved for medical use in the United States and in the European Union in 2015, and in Australia in 2016. In 2022, it was the 165th most commonly prescribed medication in the United States, with more than 3 million prescriptions. It is available as a generic medication.
Medical uses
Sacubitril/valsartan can be used instead of an ACE inhibitor or an angiotensin receptor blocker in people with heart failure and a reduced left ventricular ejection fraction (LVEF), alongside other standard therapies (e.g. beta-blockers) for heart failure. To investigate its use for heart failure in those with a preserved LVEF (HFpEF), Novartis funded the PARAGON-HF trial which was designed to investigate the use of sacubitril/valsartan in the treatment of HFpEF patients with a LVEF of 45% or more. Concluding in 2019, it failed to show significance for reducing hospitalisation related to heart failure or reducing death from cardiovascular causes, and therefore appearing to show limited benefit to those with HFpEF.
There is moderate evidence to suggest that treating people with HFpEF using MRA and ARNI may reduce the rate of people being hospitalized due to heart failure, however, there is no evidence that this type of treatment improves the person's quality of life or rate of survival due to cardiovascular disease. Evidence is lacking to support the use of ACE Inhibitors, ARBs or ARNIs in people with HFpEF at this time, and that the mainstay pharmacological therapy for HFpEF still remains the treatment of co-morbidities such as hypertension or other triggers for decompensation. Patients who exhibit symptoms of NYHA Class II or III heart failure and are still symptomatic despite maximally tolerated dose of an ACE inhibitor or ARB alone, may be considered for sacubitril/valsartan dual therapy to decrease the risk of cardiovascular-related and all-cause mortality. Mortality benefits have only been observed to date in those with LVEF less than 35%.
Adverse effects
Common adverse effects include hyperkalaemia , hypotension , a persistent dry cough and renal impairment .
Angioedema, a rare but more serious reaction, can occur in some patients and involves swelling of the face and lips. Angioedema is more common in black (African American) patients. Sacubitril/Valsartan should not be taken within 36 hours of an Angiotensin Converting Enzyme Inhibitor to reduce the risk of developing Angioedema.
The side effect profile in trials of sacubitril/valsartan compared to valsartan alone or enalapril is very similar, with the incidence of hypotension slightly higher in sacubitril/valsartan, the risk comparable for angioedema, and the chance of hyperkalaemia, renal impairment and cough slightly lower.
Sacubitril/valsartan is contraindicated in pregnancy because it contains valsartan, a known risk for birth defects.
Pharmacology
Valsartan blocks the angiotensin II receptor type 1 (AT1). This receptor is found on both vascular smooth muscle cells, and on the zona glomerulosa cells of the adrenal gland which are responsible for aldosterone secretion. In the absence of AT1 blockade, angiotensin causes both direct vasoconstriction and adrenal aldosterone secretion, the aldosterone then acting on the distal tubular cells of the kidney to promote sodium reabsorption which expands extracellular fluid (ECF) volume. Blockade of (AT1) thus causes blood vessel dilation and reduction of ECF volume.
Sacubitril is a prodrug that is activated to sacubitrilat (LBQ657) by de-ethylation via esterases. Sacubitrilat inhibits the enzyme neprilysin, a neutral endopeptidase that degrades vasoactive peptides, including natriuretic peptides, bradykinin, and adrenomedullin. Thus, sacubitril increases the levels of these peptides, causing blood vessel dilation and reduction of ECF volume via sodium excretion.
Despite these actions, neprilysin inhibitors have been found to have limited efficacy in the treatment of hypertension and heart failure when taken on their own. This is attributed to a reduction in enzymatic breakdown of angiotensin II by the reduction of neprilysin activity, which results in an increase in systemic angiotensin II levels and the negation of the positive effects of this drug family in cardiovascular disease treatment. Combined treatment with a neprilysin inhibitor and an angiotensin converting enzyme (ACE) inhibitor has been shown to be effective in reducing angiotensin II levels, and demonstrated superiority in lowering blood pressure compared to ACE inhibition alone. However, due to an increase in bradykinins from the inhibition of both ACE and neprilysin, there was a threefold increase in relative risk of angioedema compared with ACE inhibition alone following this combination treatment. The combination of a neprilysin inhibitor with an angiotensin receptor blocker instead of the ACE inhibitor has been shown to have a comparable risk of angioedema, whilst also demonstrating superiority in treating moderate-severe heart failure to ACE inhibitor treatment.
Neprilysin also has a role in clearing the protein amyloid beta from the cerebrospinal fluid, and its inhibition by sacubitril has shown increased levels of AB1-38 in healthy subjects (Entresto 194/206 for two weeks). Amyloid beta is considered to contribute to the development of Alzheimer's disease, and there exist concerns that sacubitril may promote the development of Alzheimer's disease.
Structure activity relationship
Sacubitril is the molecule that is metabolically activated by de-ethylation by esterases. The active form of the molecule, sacubitrilat, is responsible for the molecule's drug lowering effects.
Chemistry
Sacubitril/valsartan is co-crystallized sacubitril and valsartan, in a one-to-one molar ratio. One sacubitril/valsartan complex consists of six sacubitril anions, six valsartan dianions, 18 sodium cations, and 15 molecules of water, resulting in the molecular formula C288H330N36Na18O48·15H2O and a molecular mass of 5748.03 g/mol.
The substance is a white powder consisting of thin hexagonal plates. It is stable in solid form as well as in aqueous (water) solution with a pH of 5 to 7, and has a melting point of about 138 °C (280 °F).
History
During its development by Novartis, Entresto was known as LCZ696. It was approved under the FDA's priority review process on 7 July 2015. It was also approved in Europe in 2015. In 2022, Novartis sold its India marketing rights of Sacubitril Valsartan to JB Pharma, under the brand name Azmarda.
Society and culture
Trial design
There was controversy over the PARADIGM-HF trial—the Phase III trial on the basis of which the drug was approved by the FDA. For example, both Richard Lehman, a physician who writes a weekly review of key medical articles for the BMJ Blog and a December 2015, report from the Institute for Clinical and Economic Review (ICER) found that the risk–benefit ratio was not adequately determined because the design of the clinical trial was too artificial and did not reflect people with heart failure that doctors usually encounter. In 2019, the PIONEER-HF and PARAGON-HF trials studied the effect of sacubitril/valsartan in 800 patients recently hospitalised with severe heart failure and 4800 patients with less severe symptoms of heart failure respectively. The medication consistently demonstrated similar levels of safety, with higher rates of very low blood pressure, compared to current treatments across all three trials in a variety of patients, however it has only shown effectiveness in those with more advanced heart failure. In December 2015, Steven Nissen and other thought leaders in cardiology said that the approval of sacubitril/valsartan had the greatest impact on clinical practice in cardiology in 2015, and Nissen called the drug "truly a breakthrough approach."
One 2015 review stated that sacubitril/valsartan represents "an advancement in the chronic treatment of heart failure with reduced ejection fraction" but that widespread clinical success with the drug will require taking care to use it in appropriate patients, specifically those with characteristics similar to those in the clinical trial population. Another 2015 review called the reductions in mortality and hospitalization conferred by sacubitril/valsartan "striking", but noted that its effects in heart failure people with hypertension, diabetes, chronic kidney disease, and the elderly needed to be evaluated further.
Economics
The wholesale cost to the National Health Service (NHS) in the UK is approximately £1,200 per person per year as of 2017.
The wholesale cost in the United States is US$4,560 per year as of 2015. One industry-funded analysis found a cost of US$45,017 per quality-adjusted life year (QALY).
Similar class generic drugs without sacubitril, such as valsartan alone, cost approximately US$48 a year.
Research
The PARADIGM-HF trial (in which Milton Packer was one of the principal investigators) compared treatment with sacubitril/valsartan to treatment with enalapril. People with heart failure and reduced LVEF (10,513) were sequentially treated on a short-term basis with enalapril and then with sacubitril/valsartan. Those that were able to tolerate both regimens (8442, 80%) were randomly assigned to long-term treatment with either enalapril or sacubitril/valsartan. Participants were mainly white (66%), male (78%), middle aged (median 63.8 +/- 11 years) with NYHA stage II (71.6%) or stage III (23.1%) heart failure.
The trial was stopped early after a prespecified interim analysis revealed a reduction in the primary endpoint of cardiovascular death or heart failure in the sacubitril/valsartan group relative to those treated with enalapril. Taken individually, the reductions in cardiovascular death and heart failure hospitalizations retained statistical significance. Relative to enalapril, sacubitril/valsartan provided reductions in:
- the composite endpoint of cardiovascular death or hospitalization for heart failure (incidence 21.8% vs 26.5%)
- cardiovascular death (incidence 13.3% vs 16.5%)
- first hospitalization for worsening heart failure (incidence 12.8% vs 15.6%), and
- all-cause mortality (incidence 17.0% vs 19.8%)
Limitations of the trial include scarce experience with initiation of therapy in hospitalized patients and in those with NYHA heart failure class IV symptoms. Additionally the trial compared a maximal dose of valsartan (plus sacubitril) with a sub-maximal dose of enalapril, and was thus not directly comparable with current gold-standard use of ACE inhibitors in heart failure, diminishing the validity of the trial results.
References
- ^ "Entresto 24/26 tablets, Entresto 49/51 tablets, Entresto 97/103 tablets (sacubitril/valsartan) Product Information". Therapeutic Goods Administration (TGA). Novartis. Archived from the original on 11 November 2020. Retrieved 21 September 2020.
- "Prescription medicines: registration of new chemical entities in Australia, 2016". Therapeutic Goods Administration (TGA). 21 June 2022. Archived from the original on 10 April 2023. Retrieved 10 April 2023.
- AusPAR for sacubitril / valsartan salt complex (PDF) (Report). Therapeutic Goods Administration (TGA). September 2016.
- "Health Canada New Drug Authorizations: 2015 Highlights". Health Canada. 4 May 2016. Retrieved 7 April 2024.
- "Entresto 24 mg/26 mg film-coated tablets - Summary of Product Characteristics (SmPC)". (emc). Archived from the original on 24 January 2021. Retrieved 21 September 2020.
- ^ "Entresto- sacubitril and valsartan tablet, film coated". DailyMed. 14 June 2020. Archived from the original on 19 April 2020. Retrieved 21 September 2020.
- ^ "Entresto EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 27 February 2021. Retrieved 21 September 2020.
- "Neparvis EPAR". European Medicines Agency (EMA). 17 September 2018. Archived from the original on 28 December 2020. Retrieved 23 September 2020.
- ^ Hubers SA, Brown NJ (March 2016). "Combined Angiotensin Receptor Antagonism and Neprilysin Inhibition". Circulation. 133 (11): 1115–1124. doi:10.1161/CIRCULATIONAHA.115.018622. PMC 4800749. PMID 26976916.
- ^ Yancy CW, Jessup M, Bozkurt B, Butler J, Casey DE, Colvin MM, et al. (September 2016). "2016 ACC/AHA/HFSA Focused Update on New Pharmacological Therapy for Heart Failure: An Update of the 2013 ACCF/AHA Guideline for the Management of Heart Failure: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines and the Heart Failure Society of America". Circulation. 134 (13): e282 – e293. doi:10.1161/CIR.0000000000000435. PMID 27208050.
- ^ "FDA approves new drug to treat heart failure" (Press release). U.S. Food and Drug Administration (FDA). 7 July 2015. Archived from the original on 26 January 2018.
- "Entresto (sacubitril/valsartan) Tablets". U.S. Food and Drug Administration (FDA). 14 August 2015. Archived from the original on 31 March 2021. Retrieved 22 September 2020.
- Thompson A (12 June 2015). Summary review of LCZ696, a fixed-dose combination of valsartan and sacubitril (PDF) (Report). Center for Drug Evaluation and Research. 207620Orig1s000. Partially redacted.
- "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- "Sacubitril; Valsartan Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- "FDA Roundup: May 31, 2024". U.S. Food and Drug Administration (FDA) (Press release). 31 May 2024. Retrieved 31 May 2024.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- Chang HY, Chen KC, Fong MC, Feng AN, Fu HN, Huang KC, et al. (March 2020). "Recovery of left ventricular dysfunction after sacubitril/valsartan: predictors and management". Journal of Cardiology. 75 (3): 233–241. doi:10.1016/j.jjcc.2019.08.005. PMID 31563433.
- ^ McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. (September 2014). "Angiotensin-neprilysin inhibition versus enalapril in heart failure". The New England Journal of Medicine. 371 (11): 993–1004. doi:10.1056/NEJMoa1409077. hdl:2336/552372. PMID 25176015. S2CID 11383.
- ^ Solomon SD, McMurray JJ, Anand IS, Ge J, Lam CS, Maggioni AP, et al. (October 2019). "Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction". The New England Journal of Medicine. 381 (17): 1609–1620. doi:10.1056/NEJMoa1908655. hdl:2445/175935. PMID 31475794.
- Martin N, Manoharan K, Davies C, Lumbers RT (May 2021). "Beta-blockers and inhibitors of the renin-angiotensin aldosterone system for chronic heart failure with preserved ejection fraction". The Cochrane Database of Systematic Reviews. 2021 (5): CD012721. doi:10.1002/14651858.CD012721.pub3. PMC 8140651. PMID 34022072.
- ^ McMurray JJ, Packer M, Desai AS, Gong J, Lefkowitz MP, Rizkala AR, et al. (September 2014). "Angiotensin-neprilysin inhibition versus enalapril in heart failure". The New England Journal of Medicine. 371 (11): 993–1004. doi:10.1056/NEJMoa1409077. hdl:2336/552372. PMID 25176015. S2CID 11383.
- ^ Velazquez EJ, Morrow DA, DeVore AD, Duffy CI, Ambrosy AP, McCague K, et al. (February 2019). "Angiotensin-Neprilysin Inhibition in Acute Decompensated Heart Failure". The New England Journal of Medicine. 380 (6): 539–548. doi:10.1056/NEJMoa1812851. PMID 30415601. S2CID 53280900.
- Mutschler E, Schäfer-Korting M (2001). Arzneimittelwirkungen (in German) (8 ed.). Stuttgart: Wissenschaftliche Verlagsgesellschaft. p. 579. ISBN 978-3-8047-1763-3.
- Zouein FA, de Castro Brás LE, da Costa DV, Lindsey ML, Kurdi M, Booz GW (July 2013). "Heart failure with preserved ejection fraction: emerging drug strategies". Journal of Cardiovascular Pharmacology. 62 (1): 13–21. doi:10.1097/FJC.0b013e31829a4e61. PMC 3724214. PMID 23714774.
- Solomon SD. "HFpEF in the Future: New Diagnostic Techniques and Treatments in the Pipeline". Boston. p. 48. Archived from the original on 12 September 2014. Retrieved 26 January 2012.
- Gu J, Noe A, Chandra P, Al-Fayoumi S, Ligueros-Saylan M, Sarangapani R, et al. (April 2010). "Pharmacokinetics and pharmacodynamics of LCZ696, a novel dual-acting angiotensin receptor-neprilysin inhibitor (ARNi)". Journal of Clinical Pharmacology. 50 (4): 401–414. doi:10.1177/0091270009343932. PMID 19934029. S2CID 24853279.
- Schubert-Zsilavecz M, Wurglics M. "Neue Arzneimittel 2010/2011" (in German).
- Bevan EG, Connell JM, Doyle J, Carmichael HA, Davies DL, Lorimer AR, et al. (July 1992). "Candoxatril, a neutral endopeptidase inhibitor: efficacy and tolerability in essential hypertension". Journal of Hypertension. 10 (7): 607–613. doi:10.1097/00004872-199207000-00002. PMID 1321186. S2CID 23507064.
- ^ Richards AM, Wittert GA, Crozier IG, Espiner EA, Yandle TG, Ikram H, et al. (April 1993). "Chronic inhibition of endopeptidase 24.11 in essential hypertension: evidence for enhanced atrial natriuretic peptide and angiotensin II". Journal of Hypertension. 11 (4): 407–416. doi:10.1097/00004872-199304000-00011. PMID 8390508. S2CID 25333484.
- ^ Kostis JB, Packer M, Black HR, Schmieder R, Henry D, Levy E (February 2004). "Omapatrilat and enalapril in patients with hypertension: the Omapatrilat Cardiovascular Treatment vs. Enalapril (OCTAVE) trial". American Journal of Hypertension. 17 (2): 103–111. doi:10.1016/j.amjhyper.2003.09.014. PMID 14751650.
- Patel N, Gluck J (July 2017). "Is Entresto good for the brain?". World Journal of Cardiology. 9 (7): 594–599. doi:10.4330/wjc.v9.i7.594. PMC 5545143. PMID 28824789.
- Abdin A, Schulz M, Riemer U, Hadëri B, Wachter R, Laufs U, et al. (August 2022). "Sacubitril/valsartan in heart failure: efficacy and safety in and outside clinical trials". ESC Heart Fail. 9 (6): 3737–3750. doi:10.1002/ehf2.14097. PMC 9773772. PMID 35921043.
- Monge M, Lorthioir A, Bobrie G, Azizi M (December 2013). "New drug therapies interfering with the renin-angiotensin-aldosterone system for resistant hypertension". Journal of the Renin-Angiotensin-Aldosterone System. 14 (4): 285–289. doi:10.1177/1470320313513408. PMID 24222656.
- ^ Feng L, Karpinski PH, Sutton P, Liu Y, Hook DF, Hu B, et al. (2012). "LCZ696: a dual-acting sodium supramolecular complex". Tetrahedron Letters. 53 (3): 275–276. doi:10.1016/j.tetlet.2011.11.029.
- "Azmarda (Sacubitril Valsartan): Overview, Indications, Clinical Evidence & Dosage". Medical Dialogues. 30 June 2022. Archived from the original on 5 July 2022. Retrieved 30 June 2022.
- ^ Ollendorf DA, Tarlochan Sandhu A, Chapman R, Heidenreich PA, Russo E, Shore KK, et al. (1 December 2015). "CardioMEMS HF System (St. Jude Medical, Inc.) and Sacubitril/Valsartan (Entresto, Novartis AG) for management of congestive heart failure: effectiveness, value, and value-based price benchmarks : final report". 101672986. Archived from the original on 29 August 2021. Retrieved 4 October 2019.
- Richard Lehman's journal review—8 September 2014. NEJM 4 Sep 2014. Archived 16 May 2021 at the Wayback Machine Vol 371. The BMJ, 8 September 2014.
- Roger Sergel for Medpage Today. 5 Game-Changers in Cardiology in 2015: Entresto Archived 27 June 2021 at the Wayback Machine
- Lillyblad MP (November 2015). "Dual Angiotensin Receptor and Neprilysin Inhibition with Sacubitril/Valsartan in Chronic Systolic Heart Failure: Understanding the New PARADIGM". The Annals of Pharmacotherapy. 49 (11): 1237–1251. doi:10.1177/1060028015593093. PMID 26175499. S2CID 28918702.
- Bavishi C, Messerli FH, Kadosh B, Ruilope LM, Kario K (August 2015). "Role of neprilysin inhibitor combinations in hypertension: insights from hypertension and heart failure trials". European Heart Journal. 36 (30): 1967–1973. doi:10.1093/eurheartj/ehv142. PMID 25898846.
- "Entresto". MIMS. Archived from the original on 5 August 2017. Retrieved 25 July 2017.
- Gaziano TA, Fonarow GC, Claggett B, Chan WW, Deschaseaux-Voinet C, Turner SJ, et al. (September 2016). "Cost-effectiveness Analysis of Sacubitril/Valsartan vs Enalapril in Patients With Heart Failure and Reduced Ejection Fraction". JAMA Cardiology. 1 (6): 666–672. doi:10.1001/jamacardio.2016.1747. PMID 27438344.
- Pollack A (30 August 2014). "New Novartis Drug Effective in Treating Heart Failure". The New York Times. Archived from the original on 5 May 2021. Retrieved 3 March 2017.
- Husten L (31 March 2014). "Novartis Trial Was Stopped Early Because Of A Significant Drop In Cardiovascular Mortality". Forbes. Archived from the original on 24 June 2021. Retrieved 6 August 2017.
- ^ King JB, Bress AP, Reese AD, Munger MA (September 2015). "Neprilysin Inhibition in Heart Failure with Reduced Ejection Fraction: A Clinical Review". Pharmacotherapy. 35 (9): 823–837. doi:10.1002/phar.1629. PMID 26406774. S2CID 6363036.
- Drescher CS, Desai AS (3 March 2017). "Sacubitril/Valsartan Combination Drug: 2 Years Later". American College of Cardiology. Archived from the original on 24 June 2021. Retrieved 20 June 2019.
- Havakuk O, Elkayam U (July 2017). "Angiotensin Receptor-Neprilysin Inhibition". Journal of Cardiovascular Pharmacology and Therapeutics. 22 (4): 356–364. doi:10.1177/1074248416683049. PMID 28587583. S2CID 4066142.
- Perez AL, Kittipibul V, Tang WH, Starling RC (June 2017). "Patients Not Meeting PARADIGM-HF Enrollment Criteria Are Eligible for Sacubitril/Valsartan on the Basis of FDA Approval: The Need to Close the Gap". JACC. Heart Failure. 5 (6): 460–463. doi:10.1016/j.jchf.2017.03.007. PMID 28571599.
- "Richard Lehman's journal review—8 September 2014". The BMJ. 8 September 2014. Archived from the original on 11 February 2018. Retrieved 20 May 2019.
- Ahn R, Prasad V (December 2018). "Do Limitations in the Design of PARADIGM-HF Justify the Slow Real World Uptake of Sacubitril/Valsartan (Entresto)?". Cardiovascular Drugs and Therapy. 32 (6): 633–635. doi:10.1007/s10557-018-6830-x. PMID 30232657. S2CID 52298581.