| Revision as of 11:57, 20 September 2006 editSadi Carnot (talk | contribs)8,673 editsm →21st century: added 2004 nanotube concept← Previous edit | Latest revision as of 14:38, 25 April 2024 edit undoQuondum (talk | contribs)Extended confirmed users36,939 edits fixes | ||
| (356 intermediate revisions by more than 100 users not shown) | |||
| Line 1: | Line 1: | ||
| {{Short description|none}} | |||
| In ], the '''history of the ]''' traces the origins of the concept or idea of the existence, in ], of a bonded structure of two or more ]s, according to which the structures of the ] are built. In a sense, the concept of united atoms, i.e. molecules, traces it origins back the 5th century BC views of the Greek philosopher ] who argued that all the universe is composed of atoms and voids. In about 450 BC, the Greek ] introduced the idea of the four "roots" of things: fire, air, water, and earth; and two "forces", attraction and repulsion, which function to join and separate the four roots. Thus, it can be argued that, from this point onward, people have speculated as to how atoms or roots might unite in combination. | |||
| ] H<sub>2</sub>O |
] of the ] molecule.]] | ||
| In ], the '''history of molecular theory''' traces the origins of the concept or idea of the existence of ] between two or more ]s. | |||
| ==Etymology== | |||
| According to ] and the ], the word "molecule" derives from the ] "]" or small unit of mass, which is akin to the ] ''molos'', meaning exertion (c.1548) possibly referring to fact that it takes a certain amount of exertion effort to lift a small mass; or specifically: | |||
| A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure ] and how individual atoms of different chemical elements such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules. | |||
| :'''Molecule''' (1794) - "extremely minute particle," from Fr. ''moléclue'' (1678), from Mod.L. ''molecula'', dim. of L. moles "mass, barrier". A vague meaning at first; the vogue for the word (used until late 18th century only in Latin form) can be traced to the philosophy of ]. | |||
| == |
== Ancient world == | ||
| Prior to the development of the concept of the "molecule" were the various essential element theories. In the early 6th century BC, the Greek scientist ] reasoned that essential element was ] and that all things derived from this element. According to legend, Thales was walking along a hillside path on the shore of ], in what is now called south-western Turkey, and he noticed some rocks which contained fossils of what were unmistakably seashells. This led Thales to believe that the hills must have once been part of the sea. On this logic, Thales reasoned that the original world must have been entirely water, and that this was the essential element. | |||
| The modern concept of molecules can be traced back towards pre-scientific and Greek philosophers such as ] and ] who argued that all the universe is composed of ]. | |||
| In the 5th century BC, the Greek ], being influenced by ], claimed that all things consisted of differing combinations of four elements: | |||
| Circa 450 BC ] imagined ] (] (]), ] (]), ] (]), and ] (])) and "forces" of attraction and repulsion allowing the elements to interact. Prior to this, ] had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties.<ref>{{cite book | last = Russell | first = Bertrand | title = A History of Western Philosophy | publisher = Simon & Schuster | date = 2007 | isbn = 978-1-4165-5477-6 | page = 41}}</ref> | |||
| ].]] | |||
| In the ], ], following ], considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass.<ref>{{cite book | last = Russell | first = Bertrand | title = A History of Western Philosophy | publisher = Simon & Schuster | date = 2007 | isbn = 978-1-4165-5477-6 | page = 145}}</ref> A fifth element, the incorruptible quintessence ], was considered to be the fundamental building block of the heavenly bodies. | |||
| Adding to the ] of the Greeks, in about 350 BC, ] both coined the term “element” and conceived of a fifth element called "]", which formed the heavens. Building on this logic, various writers over the years have speculated on possible geometric shapes, such as circles, squares, and polygons, etc., of elements and how these shapes might combine, separate, or possibly rub against each other to create new elements over evolutionary periods. | |||
| The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by ] and passed to medieval and renaissance Europe. | |||
| ==17th century== | |||
| One of the earliest molecular theories was that put forward by the famous French naturalist ] who believed that some atoms were furnished with hook-like projections, and others, with eye-like ones. He held that two atoms combined when the hook of one got caught in the eye of the other, such as shown below: | |||
| === Greek atomism === | |||
| ]' "Hook-and-Eye" bonding Model (c.1625)]] | |||
| The earliest views on the shapes and connectivity of atoms was that proposed by ], ], and ] who reasoned that the solidness of the material corresponded to the shape of the atoms involved. Thus, iron atoms are solid and strong with hooks that lock them into a solid; water atoms are smooth and slippery; salt atoms, because of their taste, are sharp and pointed; and air atoms are light and whirling, pervading all other materials.<ref>{{cite book | last = Pfeffer | first = Jeremy, I. |author2=Nir, Shlomo | title = Modern Physics: An Introduction Text | publisher = World Scientific Publishing Company | date = 2001 | isbn = 1-86094-250-4 | page = 183}}</ref> | |||
| It was Democritus that was the main proponent of this view. Using analogies based on the experiences of the ]s, he gave a picture or an image of an atom in which atoms were distinguished from each other by their shape, their size, and the arrangement of their parts. Moreover, connections were explained by material links in which single atoms were supplied with attachments: some with hooks and eyes others with balls and sockets (see diagram).<ref>See ''testimonia'' DK 68 A 80, DK 68 A 37 and DK 68 A 43. See also {{cite book | last = Cassirer | first = Ernst | title = An Essay on Man: an Introduction to the Philosophy of Human Culture | publisher = Doubleday & Co. | date = 1953 | id = ASIN B0007EK5MM | page = | isbn = 0-300-00034-0 | url = https://archive.org/details/essayonmanintrod00cass_0/page/214 }}</ref> | |||
| In a more concrete manner, however, the concept of aggregates or units of bonded atoms, i.e. "molecules", traces its origins to ]'s 1661 hypothesis, in his famous treatise ''The Sceptical Chymist'', that matter is composed of ''clusters of particles'' and that chemical change results from the rearrangement of the clusters. Boyle argued that matter's basic elements consisted of various sorts and sizes of particles, called "corpuscles", which were capable of arranging themselves into groups. | |||
| ], ], ], ] and ] adhered to such conception. Note that the composition of water was not known before ] (c. 1811).]] | |||
| ==18th century== | |||
| An early precursor to the idea of bonded "combinations of atoms", was the theory of "combination via ]". For example, in 1718, building on Boyle’s conception of combinations of clusters, the French chemist ] developed theories of ] to explain combinations of particles, reasoning that a certain alchemical “force” draws certain alchemical components together. Geoffroy's name is best known in connection with his tables of "]" (''tables des rapports''), which he presented to the ] in 1718 and 1720. | |||
| == 17th century == | |||
| <center> | |||
| With the rise of ] and the decline of the Roman Empire, the atomic theory was abandoned for many ages in favor of the various four element theories and later alchemical theories. The 17th century, however, saw a resurgence in the atomic theory primarily through the works of ], and ]. | |||
| ]’s 1718 ''Affinity Table'': at the head of the column is a substance with which all the substances below can combine.]] | |||
| </center> | |||
| Among other scientists of that time Gassendi deeply studied ancient history, wrote major works about ] natural philosophy and was a persuasive propagandist of it. He reasoned that to account for the size and shape of atoms moving in a void could account for the properties of matter. Heat was due to small, round atoms; cold, to pyramidal atoms with sharp points, which accounted for the pricking sensation of severe cold; and solids were held together by interlacing hooks.<ref>{{cite book | last = Leicester | first = Henry, M. | title = The Historical Background of Chemistry | publisher = John Wiley & Sons | date = 1956 | isbn = 0-486-61053-5 | page = 112}}</ref> | |||
| These were lists, prepared by collating observations on the actions of substances one upon another, showing the varying degrees of affinity exhibited by analogous bodies for different ]s. These tables retained their vogue for the rest of the century, until displaced by the profounder conceptions introduced by ]. In 1789, ] published views on what he called combinations of "ultimate" particles, which foreshadowed the concept of ]. If, for example, according to Higgins, the force between the ultimate particle of oxygen and the ultimate particle of nitrogen were 6, then the strength of the force would be divided accordingly, and similarly for the other combinations of ultimate particles: | |||
| Newton, though he acknowledged the various atom attachment theories in vogue at the time, i.e. "hooked atoms", "glued atoms" (bodies at rest), and the "stick together by conspiring motions" theory, rather believed, as famously stated in "Query 31" of his 1704 '']'', that particles attract one another by some force, which "in immediate contact is extremely strong, at small distances performs the chemical operations, and reaches not far from particles with any sensible effect."<ref>(a) ], (1704). ]. (p. 389). New York: Dover.{{br}}(b) {{cite book | last = Bernard | first = Pullman | author2 = Reisinger, Axel, R. | title = The Atom in the History of Human Thought | publisher = Oxford University Press | date = 2001 | isbn = 0-19-515040-6 | page = 139}}</ref> | |||
| ]' combinations of ultimate particles (1789)]] | |||
| In a more concrete manner, however, the concept of aggregates or units of bonded atoms, i.e. "]s", traces its origins to ]'s 1661 hypothesis, in his famous treatise '']'', that matter is composed of ''clusters of ]s'' and that chemical change results from the rearrangement of the clusters. Boyle argued that matter's basic elements consisted of various sorts and sizes of particles, called "]", which were capable of arranging themselves into groups. | |||
| ==19th century== | |||
| Similar to these views, in 1803 ] took the atomic weight of hydrogen, the lightest element, as unity, and determined, for example, that the ratio for nitrous anhydride was 2 to 3 which gives the formula N<sub>2</sub>O<sub>3</sub>. Interestingly, Dalton incorrectly imagined that atoms “hooked” together to form molecules. Later, in 1808, Dalton published his famous diagram of combined "atoms": | |||
| In 1680, using the ] as a basis, French chemist ] stipulated that the ] of any substance consisted in its pointed particles, while ] were endowed with pores of various sizes.<ref>Lemery, Nicolas. (1680). An Appendix to a Course of Chymistry. London, pgs 14-15.</ref> A molecule, according to this view, consisted of corpuscles united through a geometric locking of points and pores. | |||
| ]'s union of atoms combined in ratios (1808)]] | |||
| == 18th century == | |||
| In ]'s famous "Essay on Determining the Relative Masses of the Elementary Molecules of Bodies", he essentially states, i.e. according to ]'s ''A Short History of Chemistry'', that: | |||
| ]’s 1718 ''Affinity Table'': at the head of the column is a substance with which all the substances below can combine.]] | |||
| An early precursor to the idea of bonded "combinations of atoms", was the theory of "combination via ]". For example, in 1718, building on Boyle's conception of combinations of clusters, the French chemist ] developed theories of ] to explain combinations of particles, reasoning that a certain alchemical "force" draws certain alchemical components together. Geoffroy's name is best known in connection with his tables of "]" (''tables des rapports''), which he presented to the ] in 1718 and 1720. | |||
| These were lists, prepared by collating observations on the actions of substances one upon another, showing the varying degrees of affinity exhibited by analogous bodies for different ]s. These tables retained their vogue for the rest of the century, until displaced by the profounder conceptions introduced by ]. | |||
| <div style="font-size:125%"> | |||
| {{cquote|The smallest particles of gases are not necessarily simple atoms, but are made up of a certain number of these atoms united by attraction to form a single '''molecule'''.}}</div> | |||
| In 1738, Swiss physicist and mathematician ] published '']'', which laid the basis for the ] of gases. In this work, Bernoulli positioned the argument, still used to this day, that ]es consist of great numbers of molecules moving in all directions, that their impact on a surface causes the gas ] that we feel, and that what we experience as ] is simply the kinetic energy of their motion. The theory was not immediately accepted, in part because ] had not yet been established, and it was not obvious to physicists how the collisions between molecules could be perfectly elastic. | |||
| It must be noted here that this quote is a literal translation. Avogadro uses the name "molecule" for both atoms and molecules. Specifically, he uses the name "elementary molecule" when referring to atoms and to complicate the matter also speaks of "compound molecules" and "composite molecules". | |||
| In 1789, ] published views on what he called combinations of "ultimate" particles, which foreshadowed the concept of ]. If, for example, according to Higgins, the force between the ultimate particle of oxygen and the ultimate particle of nitrogen were 6, then the strength of the force would be divided accordingly, and similarly for the other combinations of ultimate particles: | |||
| During his stay in Vercelli Avogadro wrote a concise note (''memoria'') in which he declared the hypothesis of what we now call ]: ''equal volumes of gases, at the same temperature and pressure, contain the same number of molecules''. This law implies that the relationship occurring between the weights of same volumes of different gases, at the same temperature and pressure, corresponds to the relationship between respective ]s. Hence, relative molecular masses could now be calculated from the masses of gas samples. | |||
| ]' combinations of ultimate particles (1789)]] | |||
| Avogadro developed this hypothesis in order to reconcile ]'s 1808 ] with Dalton's 1803 ]. The greatest difficulty Avogadro had to resolve was the huge confusion at that time regarding atoms and molecules – one of the most important contributions of Avogadro's work was clearly distinguishing one from the other, admitting that simple particles too could be composed of molecules, and that these are composed of atoms. Dalton, by contrast, did not consider this possibility. Curiously, Avogadro considers only molecules containing even numbers of atoms; he does not say why odd numbers are left out? | |||
| == 19th century == | |||
| ]'s union of atoms combined in ratios (1808)]] | |||
| Similar to these views, in 1803 ] took the atomic weight of hydrogen, the lightest element, as unity, and determined, for example, that the ratio for ] was 2 to 3 which gives the formula N<sub>2</sub>O<sub>3</sub>. Dalton incorrectly imagined that atoms "hooked" together to form molecules. Later, in 1808, Dalton published his famous diagram of combined "atoms": | |||
| ] created the word "molecule".<ref name="ley196606"> | |||
| {{Cite magazine | |||
| |last=Ley | |||
| |first=Willy | |||
| |date=June 1966 | |||
| |title=The Re-Designed Solar System | |||
| |department=For Your Information | |||
| |url=https://archive.org/stream/Galaxy_v24n05_1966-06#page/n93/mode/2up | |||
| |magazine=Galaxy Science Fiction | |||
| |pages=94–106 | |||
| }}</ref> His 1811 paper "Essay on Determining the Relative Masses of the Elementary Molecules of Bodies", he essentially states, i.e. according to ]'s ''A Short History of Chemistry'', that:<ref>{{cite journal | last1 = Avogadro | first1 = Amedeo | date = 1811 | title = Masses of the Elementary Molecules of Bodies | url = http://web.lemoyne.edu/~giunta/AVOGADRO.HTML | journal = Journal de Physique | volume = 73 | pages = 58–76 }}</ref> | |||
| {{quote|The smallest particles of gases are not necessarily simple atoms, but are made up of a certain number of these atoms united by attraction to form a single '''molecule'''.}} | |||
| Note that this quote is not a literal translation. Avogadro uses the name "molecule" for both atoms and molecules. Specifically, he uses the name "elementary molecule" when referring to atoms and to complicate the matter also speaks of "compound molecules" and "composite molecules". | |||
| During his stay in Vercelli, Avogadro wrote a concise note (''memoria'') in which he declared the hypothesis of what we now call ]: ''equal volumes of gases, at the same temperature and pressure, contain the same number of molecules''. This law implies that the relationship occurring between the weights of same volumes of different gases, at the same temperature and pressure, corresponds to the relationship between respective ]s. Hence, relative molecular masses could now be calculated from the masses of gas samples. | |||
| Avogadro developed this hypothesis to reconcile ]'s 1808 ] with Dalton's 1803 ]. The greatest difficulty Avogadro had to resolve was the huge confusion at that time regarding atoms and molecules—one of the most important contributions of Avogadro's work was clearly distinguishing one from the other, admitting that simple particles too could be composed of molecules and that these are composed of atoms. Dalton, by contrast, did not consider this possibility. Curiously, Avogadro considers only molecules containing even numbers of atoms; he does not say why odd numbers are left out. | |||
| In 1826, building on the work of Avogadro, the French chemist ] states: | In 1826, building on the work of Avogadro, the French chemist ] states: | ||
| <div style="font-size:125%"> | |||
| {{cquote|Gases in similar circumstances are composed of '''molecules''' or atoms placed at the same distance, which is the same as saying that they contain the same number in the same volume.}}</div> | |||
| {{quote|Gases in similar circumstances are composed of '''molecules''' or atoms placed at the same distance, which is the same as saying that they contain the same number in the same volume.}} | |||
| In coordination with these concepts, in 1833 the french chemist ] presented a clear account of Avogadro's hypothesis, regarding atomic weights, by making use of “volume diagrams”, which clearly show both semi-correct molecular geometries, such as a linear water molecule, and correct molecular formulas, such as H<sub>2</sub>O: | |||
| In coordination with these concepts, in 1833 the French chemist ] presented a clear account of Avogadro's hypothesis,<ref>{{cite journal | |||
| | title = The Atomic Structural Theories of Ampère and Gaudin: Molecular Speculation and Avogadro's Hypothesis| author = Seymour H. Mauskopf| journal = Isis| date = 1969| volume = 60| issue = 1| pages = 61–74| doi = 10.1086/350449| jstor=229022| s2cid = 143759556}}</ref> regarding atomic weights, by making use of "volume diagrams", which clearly show both semi-correct molecular geometries, such as a linear water molecule, and correct molecular formulas, such as H<sub>2</sub>O: | |||
| ]'s volume diagrams of molecules in the gas phase (1833)]] | |||
| In 1863, an unknown Vienna high-school teacher named ] published, at his own expense, a booklet entitled ''Chemische Studine I'' (see: ), containing pioneering molecular images which showed both "ringed" structures as well as double-bonded structures, such as: | |||
| In two papers outlining his "theory of atomicity of the elements" (1857–58), ] was the first to offer a theory of how every atom in an organic molecule was bonded to every other atom. He proposed that carbon atoms were tetravalent, and could bond to themselves to form the carbon skeletons of organic molecules. | |||
| ]'s molecule drawings of ] H<sub>2</sub>C=CH<sub>2</sub> and ] HC=CH (1861)]] | |||
| In 1856, Scottish chemist ] began research on the ] of benzene at the laboratory of ] in Paris.<ref>{{Cite web |title=Chemical Bonding Concept/Skills Development |url=http://intro.chem.okstate.edu/ChemSource/Bond/bondpage37.html |access-date=2023-01-01 |website=intro.chem.okstate.edu}}</ref> One month after Kekulé's second paper appeared, Couper's independent and largely identical theory of molecular structure was published. He offered a very concrete idea of molecular structure, proposing that atoms joined to each other like modern-day ] in specific three-dimensional structures. Couper was the first to use lines between atoms, in conjunction with the older method of using brackets, to represent bonds, and also postulated straight chains of atoms as the structures of some molecules, ring-shaped molecules of others, such as in ] and ].<ref>{{cite book|last1=Bowden|first1=Mary Ellen|title=Chemical achievers : the human face of the chemical sciences|url=https://archive.org/details/chemicalachiever0000bowd|url-access=registration|date=1997|publisher=Chemical Heritage Foundation|location=Philadelphia, PA|isbn=9780941901123|pages=}}</ref> In later publications, Couper's bonds were represented using straight dotted lines (although it is not known if this is the typesetter's preference) such as with ] and ] below: | |||
| It is argued that Loschmidt's ringed molecular images formed the seed of thought for ]'s story of how he "dreamed up" the ] ring structure. An often-repeated story claims that after years of studying carbon bonding, benzene and related molecules, he dreamt one night of the ], a ] eating its own tail, and that upon waking he was inspired to deduce the ring structure of benzene. The story, however, first appeared in the ''Berichte der Durstigen Chemischen Gesellschaft'' (Journal of the Thirsty Chemical Society), a ] of the ''Berichte der Deutschen Chemischen Gesellschaft'', which appeared annually in the late-19th century on the occasion of the congress of German chemists; as such, it is probably to be treated with circumspection. | |||
| ]'s molecular structures, for ] and ], using elemental symbols for atoms and lines for bonds (1858)]] | |||
| While his (more formal) claims were well-publicized and accepted, biographers came to the conclusion that Kekulé's understanding of the tetravalent nature of carbon bonding depended on the previous research of ] and ]. The cyclic nature of benzene was finally confirmed by the eminent crystallographer ]. Benzene presents a special problem in that, to account for all the bonds, there must be alternating ] carbon bonds: | |||
| In 1861, an unknown Vienna high-school teacher named ] published, at his own expense, a booklet entitled ''Chemische Studien I'', containing pioneering molecular images which showed both "ringed" structures as well as double-bonded structures, such as:<ref>Bader, A. & Parker, L. (2001). "", ''Physics Today'', Mar.</ref> | |||
| ] molecule with alternating ]]] | |||
| ]'s molecule drawings of ] H<sub>2</sub>C=CH<sub>2</sub> and ] HC≡CH (1861)]] | |||
| In 1894, ] postulated the famous "Lock and Key" molecule bonding theory. According to this theory molecules fit togethter according to complementary geometric shape. Enzymes, for example, are very specific, and Fischer suggested that this was because both the enzyme and the substrate possess specific complementary geometric shapes that fit exactly into one another.<ref>{{cite journal |author= Fischer E.|year= 1894|title= Einfluss der Configuration auf die Wirkung der Enzyme| journal=Ber. Dt. | |||
| Chem. Ges.|volume=27|pages=2985-2993}}</ref> This is often referred to as "the lock and key" model. An enzyme combines with its substrate(s) to form a short-lived enzyme-substrate complex. However, while this model explains enzyme specificity, it fails to explain the stabilization of the transition state which occurs. | |||
| Loschmidt also suggested a possible formula for benzene, but left the issue open. The first proposal of the modern structure for benzene was due to Kekulé, in 1865. The cyclic nature of benzene was finally confirmed by the crystallographer ]. Benzene presents a special problem in that, to account for all the bonds, there must be alternating ] carbon bonds: | |||
| ] | |||
| ] molecule with alternating ]s]] | |||
| Later, Fisher developed the ] technique for viewing 3-D molecules on a 2-D sheet of paper: | |||
| In 1865, German chemist ] was the first to make stick-and-ball molecular models, which he used in lecture at the ], such as methane shown below: | |||
| <center>],]</center> | |||
| ]'s 1865 ] of ] ''CH<sub>4</sub>''.]] | |||
| ==20th century== | |||
| In the early 1900s, the American chemist ] began to use dots in lecture, while teaching undergraduates at ], to represent the electrons around atoms. His students favored these drawings, which stimulated him in this direction. From these lectures, Lewis noted that elements with a certain number of electrons seemed to have a special stability. To Lewis it appeared that once a core of eight electrons has formed around a nucleus, the layer is filled, and a new layer is started. Lewis also noted that various ]s with eight electrons also seemed to have a special stability. On these views, he proposed the rule of eight or ]: ''Ions or atoms with a filled layer of eight electrons have a special stability''.<ref>{{cite book | last = Cobb | first = Cathy | title = Creations of Fire - Chemistry's Lively History From Alchemy to the Atomic Age | publisher = Perseus Publishing | year = 1995 | id = ISBN 073820594X}}</ref> | |||
| The basis of this model followed the earlier 1855 suggestion by his colleague ] that ] is ]. Hofmann's color scheme, to note, is ]: ] = black, ] = blue, ] = red, ] = green, ] = yellow, ] = white.<ref>{{cite journal | last1 = Ollis | first1 = W. D. | date = 1972 | title = Models and molecules | journal = Proceedings of the Royal Institution of Great Britain | volume = 45 | pages = 1–31 }}</ref> The deficiencies in Hofmann's model were essentially geometric: ] bonding was shown as ], rather than tetrahedral, and the atoms were out of proportion, e.g. carbon was smaller in size than the hydrogen. | |||
| Moreover, noting that a cube has eight corners Lewis envisioned an atom as having eight sides available for electrons, like the corner of a cube. Subsequently, in 1902 he devised a conception in which ] can bond on their sides to form cubic-structured molecules: | |||
| In 1864, Scottish organic chemist ] began to draw pictures of molecules, in which he enclosed the symbols for atoms in circles, and used broken lines to connect the atoms together in a way that satisfied each atom's valence. | |||
| ]' notes showing the possible "cubic" arrangement of electrons about an atom. (1902)]] | |||
| The year 1873, by many accounts, was a seminal point in the history of the development of the concept of the "molecule". In this year, the renowned Scottish physicist ] published his famous thirteen page article 'Molecules' in the September issue of ''Nature''.<ref>Maxwell, James Clerk, " {{webarchive|url=https://web.archive.org/web/20070209001003/http://www.thecore.nus.edu.sg/landow/victorian/science/science_texts/molecules.html |date=2007-02-09 }}". ''Nature'', September, 1873.</ref> In the opening section to this article, Maxwell clearly states: | |||
| In other words, electron-pair bonds are formed when two atoms share an edge, as in structure '''C''' below. This results in the sharing of two electrons. Similarly, charged ionic-bonds are formed by the transfer of an electron from one cube to another, without sharing an edge '''A'''. An intermediate state '''B''' where only one corner is shared was also postulated by Lewis. | |||
| {{quote|An atom is a body which cannot be cut in two; a '''molecule''' is the smallest possible portion of a particular substance.}} | |||
| ] | |||
| After speaking about the ] of ], Maxwell goes on to tell us that the word 'molecule' is a modern word. He states, "it does not occur in '']''. The ideas it embodies are those belonging to modern chemistry." We are told that an 'atom' is a material point, invested and surrounded by 'potential forces' and that when 'flying molecules' strike against a solid body in constant succession it causes what is called ] of air and other gases. At this point, however, Maxwell notes that no one has ever seen or handled a molecule. | |||
| Double bonds are formed by sharing a face between two cubic atoms. This results in sharing four electrons: | |||
| In 1874, ] and ] independently proposed that the phenomenon of ] could be explained by assuming that the chemical bonds between carbon atoms and their neighbors were directed towards the corners of a regular tetrahedron. This led to a better understanding of the three-dimensional nature of molecules. | |||
| ] | |||
| ] developed the ] technique for viewing 3-D molecules on a 2-D sheet of paper: | |||
| About ten years later, while working as the chair of the department of chemistry at the ], Lewis read a paper by an English graduate student, ], who was visiting Berkeley for a year. In this paper, Parson suggested that a ] results from two electrons being shared between two atoms. This, according to Lewis, meant that bonding occurred when two electrons formed a shared edge between two complete cubes. | |||
| ] | |||
| On these views, in his famous 1916 article ''The Molecule and the Atom'', Lewis introduced the “Lewis structure” to represent atoms and molecules, where dots represent ]s and lines represent ]s. Moreover, he proposed that an atom tended to form an ion by gaining or losing the number of electrons needed to complete a cube. Thus, Lewis structures show each atom in the structure of the molecule using its chemical symbol. Lines are drawn between atoms that are bonded to one another; occasionally, pairs of dots are used instead of lines. Excess electrons that form lone pairs are represented as pair of dots, and are placed next to the atoms on which they reside: | |||
| In 1898, ], in his ''Lectures on Gas Theory'', used the theory of ] to explain the phenomenon of gas phase molecular dissociation, and in doing so drew one of the first rudimentary yet detailed atomic orbital overlap drawings. Noting first the known fact that molecular ] vapor dissociates into atoms at higher temperatures, Boltzmann states that we must explain the existence of molecules composed of two atoms, the "double atom" as Boltzmann calls it, by an attractive force acting between the two atoms. Boltzmann states that this chemical attraction, owing to certain facts of chemical valence, must be associated with a relatively small region on the surface of the atom called the ''sensitive region''. | |||
| ] | |||
| Boltzmann states that this "sensitive region" will lie on the surface of the atom, or may partially lie inside the atom, and will firmly be connected to it. Specifically, he states "only when two atoms are situated so that their sensitive regions are in contact, or partly overlap, will there be a chemical attraction between them. We then say that they are chemically bound to each other." This picture is detailed below, showing the ''α-sensitive region'' of atom-A overlapping with the ''β-sensitive region'' of atom-B:<ref>{{cite book | last = Boltzmann | first = Ludwig | title = Lectures on Gas Theory | publisher = Dover |edition=Reprint | date = 1898 | isbn = 0-486-68455-5}}</ref> | |||
| To summarize his views on his new bonding model, Lewis states: <ref>"Valence and The Structure of Atoms and Molecules", G. N. Lewis, American Chemical Society Monograph Series, page 79 and 81.</ref> | |||
| ]’s 1898 I<sub>2</sub> molecule diagram showing atomic "sensitive region" (α, β) overlap.]] | |||
| <div style="font-size:115%"> | |||
| {{cquote|Two atoms may conform to the rule of eight, or the octet rule, not only by the transfer of electrons from one atom to another, but also by sharing one or more pairs of electrons...Two electrons thus coupled together, when lying between two atomic centers, and held jointly in the shells of the two atoms, I have considered to be the chemical bond. We thus have a concrete picture of that physical entity, that "hook and eye" which is part of the creed of the organic chemist.}}</div> | |||
| == 20th century == | |||
| The following year, in 1917, an unknown American undergraduate chemical engineer named ] was learning the Dalton hook-and-eye bonding method at the ], which was the vogue description of bonds between atoms at the time. Each atom had a certain number of hooks that allowed it to attach to other atoms, and a certain number of eyes that allowed other atoms to attach to it. A chemical bond resulted when a hook and eye connected. Pauling, however, wasn't satisfied with this archaic method and looked to the newly-emerging field of ] for a new method. | |||
| In the early 20th century, the American chemist ] began to use dots in lecture, while teaching undergraduates at ], to represent the electrons around atoms. His students favored these drawings, which stimulated him in this direction. From these lectures, Lewis noted that elements with a certain number of electrons seemed to have a special stability. This phenomenon was pointed out by the German chemist ] in 1904, to which Lewis referred to as "Abegg's law of valence" (now generally known as ]). To Lewis it appeared that once a core of eight electrons has formed around a nucleus, the layer is filled, and a new layer is started. Lewis also noted that various ]s with eight electrons also seemed to have a special stability. On these views, he proposed the rule of eight or ]: ''Ions or atoms with a filled layer of eight electrons have a special stability''.<ref>{{cite book | last = Cobb | first = Cathy | title = Creations of Fire - Chemistry's Lively History From Alchemy to the Atomic Age | publisher = Perseus Publishing | date = 1995 | isbn = 0-7382-0594-X}}</ref> | |||
| Moreover, noting that a cube has eight corners Lewis envisioned an atom as having eight sides available for electrons, like the corner of a cube. Subsequently, in 1902 he devised a conception in which ] can bond on their sides to form cubic-structured molecules. | |||
| Subsequently, in 1931, building on theories found in Lewis' famous article, Pauling published his ground-breaking article "The Nature of the Chemical Bond" (see: ) in which he used ] to calculate properties and structures of molecules, such as angles between bonds and rotation about bonds. On these concepts, Lewis developed ] to account for bonds in molecules such as CH<sub>4</sub>, in which four sp³ hybridised orbitals are overlapped by ]'s ''1s'' orbital, yielding four ]. The four bonds are of the same length and strength, which yields a molecular structure as shown below: | |||
| In other words, electron-pair bonds are formed when two atoms share an edge, as in structure '''C''' below. This results in the sharing of two electrons. Similarly, charged ionic-bonds are formed by the transfer of an electron from one cube to another, without sharing an edge '''A'''. An intermediate state '''B''' where only one corner is shared was also postulated by Lewis. | |||
| <center>] translates into ]</center> | |||
| ] | |||
| Owing to these exceptional theories, Pauling has been the only person two ever win two unshared ]s | |||
| Hence, ]s are formed by sharing a face between two cubic atoms. This results in the sharing of four electrons. | |||
| In 1937, chemist K.L Wolf introduced the concept of ] (''Übermoleküle'') to describe ] in ] ]s. Supermolecules are organized polymolecular systems, held together by non-covalent interactions. Examples of a supermolecules are the ]s, which have a cavity in which a guest molecule can fit: | |||
| In 1913, while working as the chair of the department of chemistry at the ], Lewis read a preliminary outline of paper by an English graduate student, ], who was visiting Berkeley for a year. In this paper, Parson suggested that the ] is not merely an electric charge but is also a small magnet (or "]" as he called it) and furthermore that a ] results from two electrons being shared between two atoms.<ref>Parson, A.L. (1915). "A Magneton Theory of the Structure of the Atom". ''Smithsonian Publication'' 2371, Washington.</ref> This, according to Lewis, meant that bonding occurred when two electrons formed a shared edge between two complete cubes. | |||
| ] - chemical structure of the three main types of ].]] | |||
| On these views, in his famous 1916 article '''', Lewis introduced the "Lewis structure" to represent atoms and molecules, where dots represent ]s and lines represent ]s. In this article, he developed the concept of the ], in which two atoms may share one to six electrons, thus forming the ], a ], a ], or a ]. | |||
| In the mid-twentieth century, neurochemists began to discover molecules that have function in human brain activity. In 1948, for example, ] isolated and named the now-famous structure “]”. In the ], serotonin is believed to play an important role in the regulation of ], ], ], ], and ]. By many accounts, serotonin is considered the “confidence chemical”, in that, for example, cerebral levels of this neurochemical are higher in alpha-males and alpha-females, such as captains, group leaders, and high-ranking chimpanzees in troop hierarchies. Moreover, during the first six months of being in love, paradoxically, serotonin levels in couples drop to 40 percent below those in normal subjects: | |||
| ] | |||
| <center> | |||
| <gallery> | |||
| Image:Serotonin-skeletal.png|Molecular-structure of ] (1948) | |||
| Image:Serotonin-3D-vdW.png|3D-structure of serotonin C<sub>10</sub>H<sub>12</sub>N<sub>2</sub>O | |||
| </gallery> | |||
| </center> | |||
| In Lewis' own words: | |||
| In 1953, American scientists ] and ] determined the molecular structure of ] or “deoxyribonucleic acid”, which consists of a pair of ]s, organized as strands running start-to-end and joined by ]s along their lengths.<ref name=Butler>Butler, John M. (2001) ''Forensic DNA Typing'' "Elsevier". pp. 14-15. ISBN 012147951X.</ref> Each strand is a chain of chemical "building blocks", called ]s, of which there are four types: ] (abbreviated A), ] (C), ] (G) and ] (T).<ref name=Butler /> These molcular strands links together in the form a spiral structure, as shown below: | |||
| {{quote|An electron may form a part of the shell of two different atoms and cannot be said to belong to either one exclusively.}} | |||
| Moreover, he proposed that an atom tended to form an ion by gaining or losing the number of electrons needed to complete a cube. Thus, Lewis structures show each atom in the structure of the molecule using its chemical symbol. Lines are drawn between atoms that are bonded to one another; occasionally, pairs of dots are used instead of lines. Excess electrons that form lone pairs are represented as pair of dots, and are placed next to the atoms on which they reside: | |||
| ] molecule''' rotating (1953).]] | |||
| ] | |||
| In 1958, the American biochemist ] and his co-workers discovered that a photosensitive molecule in the rod cells of the eye, called ] C<sub>20</sub>H<sub>28</sub>O, straightens its configuration in response to light, i.e. ]s. This straightened configuration results to trigger a nerve impulse; statistically it takes five ]s to trigger such a nerve impulse. This is the core mechanism of the vision process: | |||
| To summarize his views on his new bonding model, Lewis states:<ref>"Valence and The Structure of Atoms and Molecules", G. N. Lewis, American Chemical Society Monograph Series, page 79 and 81.</ref> | |||
| ] γ (light), of the correct wavelength]] | |||
| {{quote|Two atoms may conform to the rule of eight, or the octet rule, not only by the transfer of electrons from one atom to another, but also by sharing one or more pairs of electrons...Two electrons thus coupled together, when lying between two atomic centers, and held jointly in the shells of the two atoms, I have considered to be the chemical bond. We thus have a concrete picture of that physical entity, that "hook and eye" which is part of the creed of the organic chemist.}} | |||
| For his work, Wald won a share of the ] ] with ] and ]. | |||
| The following year, in 1917, an unknown American undergraduate chemical engineer named ] was learning the Dalton hook-and-eye bonding method at the ], which was the vogue description of bonds between atoms at the time. Each atom had a certain number of hooks that allowed it to attach to other atoms, and a certain number of eyes that allowed other atoms to attach to it. A chemical bond resulted when a hook and eye connected. Pauling, however, wasn't satisfied with this archaic method and looked to the newly emerging field of ] for a new method. | |||
| During the later half of the twentieth century, computer technologies began to increase; this led to the determination of larger molecular structures such as ]s. In 1958, for example, the structure of the ] molecule was determined using ] by ] and ], as shown below, where the colored segments are ]: <ref>Kendrew JC, Bodo G, Dintzis HM, Parrish RG, Wyckoff H, Phillips DC. (1958). A three-dimensional model of the myoglobin molecule obtained by x-ray analysis. ''Nature'' 181(4610):662-6.</ref> | |||
| In 1927, the physicists ] and ] applied the new quantum mechanics to the deal with the saturable, nondynamic forces of attraction and repulsion, i.e., exchange forces, of the hydrogen molecule. Their valence bond treatment of this problem, in their joint paper,<ref>{{cite journal | doi = 10.1007/BF01397394 | last1 = Heitler | first1 = Walter | last2 = London | first2 = Fritz | date = 1927 | title = Wechselwirkung neutraler Atome und homöopolare Bindung nach der Quantenmechanik | journal = Zeitschrift für Physik | volume = 44 | issue = 6–7 | pages = 455–472 |bibcode = 1927ZPhy...44..455H | s2cid = 119739102 }}</ref> was a landmark in that it brought chemistry under quantum mechanics. Their work was an influence on Pauling, who had just received his doctorate and visited Heitler and London in Zürich on a ]. | |||
| ] molecule''' (1958)]] | |||
| Subsequently, in 1931, building on the work of Heitler and London and on theories found in Lewis' famous article, Pauling published his ground-breaking article "The Nature of the Chemical Bond"<ref>{{cite journal | doi = 10.1021/ja01355a027 | last1 = Pauling | first1 = Linus | date = 1931 | title = The nature of the chemical bond. Application of results obtained from the quantum mechanics and from a theory of paramagnetic susceptibility to the structure of molecules | journal = J. Am. Chem. Soc. | volume = 53 | issue = 4 | pages = 1367–1400 }}</ref> (see: ) in which he used ] to calculate properties and structures of molecules, such as angles between bonds and rotation about bonds. On these concepts, Pauling developed ] to account for bonds in molecules such as CH<sub>4</sub>, in which four sp³ hybridised orbitals are overlapped by ]'s ''1s'' orbital, yielding four ]. The four bonds are of the same length and strength, which yields a molecular structure as shown below: | |||
| Perutz and Kendrew received the ] for determining the structure of this molecule. | |||
| ] | |||
| In 1985, ] of the ], and ], ], ] and ], from ], discovered C<sub>60</sub> or what is famously known as a "buckyball": | |||
| Owing to these exceptional theories, Pauling won the 1954 ]. Notably he has been the only person to ever win two unshared ]s, winning the ] in 1963. | |||
| ] | |||
| In 1926, French physicist ] received the Nobel Prize in physics for proving, conclusively, the existence of molecules. He did this by calculating the ] using three different methods, all involving liquid phase systems. First, he used a ] soap-like emulsion, second by doing experimental work on ], and third by confirming Einstein's theory of particle rotation in the liquid phase.<ref>Perrin, Jean, B. (1926). , Nobel Lecture, December 11.</ref> | |||
| In ], discrete peaks were observed corresponding to molecules with the exact mass of sixty or seventy or more carbon atoms. Shortly thereafter came the discover the ]s. Kroto, Curl, and Smalley were awarded the 1996 ] for their roles in the discovery of this class of compounds. C<sub>60</sub>. Other fullerenes were later noticed occurring outside of a laboratory environment, e.g., such as in normal ] ]. | |||
| In 1937, chemist ] introduced the concept of ] (''Übermoleküle'') to describe ] in ] ]s. This would eventually lead to the area of ], which is the study of non-covalent bonding. | |||
| In ], researchers from the ] of ] demonstrated that the ] applied to macro-molecules such as fullerene.<ref>{{cite journal| title=Wave-particle duality of C60| first=M.| last=Arndt| coauthors=O. Nairz, J. Voss-Andreae, C. Keller, G. van der Zouw, ]| journal=Nature| volume=401| pages=680-682| month=14 October| year=1999}}</ref> | |||
| In 1951, physicist ] invents the ] and is the first to see ]s, e.g. bonded atomic arrangements at the tip of a metal point.<ref>Mitch Jacoby, , ''Chemical & engineering News'', '''83''':48, pp. 13–16, 28 November 2005</ref> | |||
| ==21st century== | |||
| On the cutting edge is ], which is the science and engineering of matter at the atomic scale. In other words, recent areas of interest in the molecular world have been to build functionable molecules atom-by-atom, such as, for example, a molecular gear: | |||
| ] computer simulation.]] | |||
| In 1968-1970 Leroy Cooper, PhD of the University of California at Davis completed his thesis which showed what molecules looked like. He used x-ray deflection off crystals and a complex computer program written by Bill Pentz of the UC Davis Computer Center. This program took the mapped deflections and used them to calculate the basic shapes of crystal molecules. His work showed that actual molecular shapes in quartz crystals and other tested crystals looked similar to the long envisioned merged various sized soap bubbles theorized, except instead of being merged spheres of different sizes, actual shapes were rigid mergers of more tear dropped shapes that stayed fixed in orientation. This work verified for the first time that crystal molecules are actually linked or stacked merged tear drop constructions. {{citation needed|date=December 2020}} | |||
| In June of 2004, scientists from China's ] and ] demonstrated the use of nanotubes in ]s, replacing a ] ] in a ] with a carbon nanotube one: | |||
| In 1999, researchers from the ] of ] reported results from experiments on ] for ] molecules.<ref>{{cite journal| title=Wave-particle duality of C60 molecules| first=M.| last=Arndt| author2=O. Nairz |author3=J. Voss-Andreae |author3-link=Julian Voss-Andreae |author4=C. Keller |author5=G. van der Zouw |author6=A. Zeilinger |author6-link=Anton Zeilinger | journal=Nature| volume=401| issue=6754| pages=680–682|date=14 October 1999| pmid=18494170|doi=10.1038/44348| bibcode=1999Natur.401..680A| s2cid=4424892}}</ref> The data published by ] et al. were consistent with ]'s ]. This experiment was noted for extending the applicability of wave–particle duality by about one order of magnitude in the macroscopic direction.<ref>{{cite journal| title=Quantum physics: Waves, particles and fullerenes| first=A. I. M.| last=Rae| journal=Nature| volume=401| pages=651–653|date=14 October 1999|doi=10.1038/44294| issue=6754|bibcode = 1999Natur.401..651R | s2cid=5416065}}</ref> | |||
| ] | |||
| In 2009, researchers from ] managed to take the first picture of a real molecule.<ref>.</ref> Using an ] every single atom and bond of a ] molecule could be imaged. | |||
| In September of 2005, a research team, led by Ludwig Bartel at the ] designed a molecule that walks over a surface like a human, i.e. a '''walking molecule'''<ref>Pittalwala, M. (2005). , Sept., Source: UCR Newsroom, Sept. 26</ref> The molecule, 9,10-dithioanthracene or “DTA”, has two linkers that act as feet. With a source of ], the molecule moves such that only one of the linkers is lifted from the surface while the other stabilizes the molecule and guides its motion. By alternating its two feet, the nano-walker is able to move over atomic surfaces without the assistance of nano-rails or atomic-groves. | |||
| == See also == | |||
| Similarly, in October of 2005, researchers at ] designed the world's first '''molecular car'''.<ref>Lamba, R. (2005) , Oct. Source: physOrg.com</ref> With a wheelbase of less than 5 nm, the tiny atom-sized car was driven on a gold-plated microscopic highway. The wheels were buckyballs, spheres of pure carbon containing 60 atoms a piece. According to these researchers, this "nanocar" represents the first step towards molecular manufacturing. | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| == References == | |||
| The modern theory of molecules makes great use of the many numerical techniques offered by ]. Dozens of molecules have now been identified in ] by ]. | |||
| {{reflist|30em}} | |||
| == |
== Further reading == | ||
| {{refbegin}} | |||
| *] | |||
| * {{cite book | last = Partington | first = J.R. | title = A Short History of Chemistry | url = https://archive.org/details/shorthistoryofch0000part_q6h4 | url-access = registration | publisher = Dover Publications, Inc | date = 1989 | isbn = 0-486-65977-1}} | |||
| *] | |||
| * {{cite book | last = Atkins | first = Peter | title = Atkins' Molecules, 2nd Ed | publisher = Cambridge University Press | date = 2003 | isbn = 0-521-53536-0}} | |||
| *] | |||
| * {{cite book | last = Sargent | first = Ted | title = The Dance of Molecules - How Nanotechnology is Changing our Lives | publisher = Thunder's Mouth Press | date = 2006 | isbn = 1-56025-809-8 | url = https://archive.org/details/danceofmolecules00sarg }} | |||
| *] | |||
| * {{cite book | last = Scerri | first = Eric R. | title = The Periodic Table, Its Story and Its Significance | url = https://archive.org/details/periodictableits0000scer | url-access = registration | publisher =Oxford University Press| date = 2007 | isbn = 978-0-19-530573-9}} | |||
| {{refend}} | |||
| == External links == | |||
| ==References== | |||
| * - Middlebury College | |||
| <references /> | |||
| * - McMaster University | |||
| * - The Wileys Family | |||
| * - School of Chemistry, University of Bristol | |||
| * - Eric Scerri's history & philosophy of chemistry website | |||
| == |
=== Types === | ||
| * - The National Health Museum | |||
| *{{cite book | last = Partington | first = J.R. | title = A Short History of Chemistry | publisher = Dover Publications, Inc. | year = 1989 | id = ISBN 0486659771}}</ref> | |||
| * - IUPAC Definitions | |||
| *{{cite book | last = Atkins | first = Peter | title = Atkins' Molecules, 2nd Ed. | publisher = Cambridge University Press | year = 2003 | id = ISBN 0521535360}}</ref> | |||
| *{{cite book | last = Sargent | first = Ted | title = The Dance of Molecules - How Nanotechnology is Changing our Lives | publisher = Thunder's Mouth Press | year = 2006 | id = ISBN 1560258098}}</ref> | |||
| === Definitions === | |||
| ==External links== | |||
| * - ] (Department of Chemistry) | |||
| * - Middlebury College | |||
| * - IUPAC | |||
| * - McMaster University | |||
| *, , and - Institute of Human Thermodynamics | |||
| * - Institute of Human Thermodynamics | |||
| ===Articles=== | === Articles === | ||
| * - TRN Newswire | * - TRN Newswire | ||
| * - HP Labs | * - HP Labs | ||
| {{History of chemistry}} | |||
| {{History of physics}} | |||
| {{DEFAULTSORT:History Of Molecular Theory}} | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 14:38, 25 April 2024

In chemistry, the history of molecular theory traces the origins of the concept or idea of the existence of strong chemical bonds between two or more atoms.
A modern conceptualization of molecules began to develop in the 19th century along with experimental evidence for pure chemical elements and how individual atoms of different chemical elements such as hydrogen and oxygen can combine to form chemically stable molecules such as water molecules.
Ancient world
The modern concept of molecules can be traced back towards pre-scientific and Greek philosophers such as Leucippus and Democritus who argued that all the universe is composed of atoms and voids.
Circa 450 BC Empedocles imagined fundamental elements (fire (![]() ), earth (
), earth (![]() ), air (
), air (![]() ), and water (
), and water (![]() )) and "forces" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties.
)) and "forces" of attraction and repulsion allowing the elements to interact. Prior to this, Heraclitus had claimed that fire or change was fundamental to our existence, created through the combination of opposite properties.
In the Timaeus, Plato, following Pythagoras, considered mathematical entities such as number, point, line and triangle as the fundamental building blocks or elements of this ephemeral world, and considered the four elements of fire, air, water and earth as states of substances through which the true mathematical principles or elements would pass. A fifth element, the incorruptible quintessence aether, was considered to be the fundamental building block of the heavenly bodies.
The viewpoint of Leucippus and Empedocles, along with the aether, was accepted by Aristotle and passed to medieval and renaissance Europe.
Greek atomism
The earliest views on the shapes and connectivity of atoms was that proposed by Leucippus, Democritus, and Epicurus who reasoned that the solidness of the material corresponded to the shape of the atoms involved. Thus, iron atoms are solid and strong with hooks that lock them into a solid; water atoms are smooth and slippery; salt atoms, because of their taste, are sharp and pointed; and air atoms are light and whirling, pervading all other materials.
It was Democritus that was the main proponent of this view. Using analogies based on the experiences of the senses, he gave a picture or an image of an atom in which atoms were distinguished from each other by their shape, their size, and the arrangement of their parts. Moreover, connections were explained by material links in which single atoms were supplied with attachments: some with hooks and eyes others with balls and sockets (see diagram).
17th century
With the rise of scholasticism and the decline of the Roman Empire, the atomic theory was abandoned for many ages in favor of the various four element theories and later alchemical theories. The 17th century, however, saw a resurgence in the atomic theory primarily through the works of Gassendi, and Newton.
Among other scientists of that time Gassendi deeply studied ancient history, wrote major works about Epicurus natural philosophy and was a persuasive propagandist of it. He reasoned that to account for the size and shape of atoms moving in a void could account for the properties of matter. Heat was due to small, round atoms; cold, to pyramidal atoms with sharp points, which accounted for the pricking sensation of severe cold; and solids were held together by interlacing hooks.
Newton, though he acknowledged the various atom attachment theories in vogue at the time, i.e. "hooked atoms", "glued atoms" (bodies at rest), and the "stick together by conspiring motions" theory, rather believed, as famously stated in "Query 31" of his 1704 Opticks, that particles attract one another by some force, which "in immediate contact is extremely strong, at small distances performs the chemical operations, and reaches not far from particles with any sensible effect."
In a more concrete manner, however, the concept of aggregates or units of bonded atoms, i.e. "molecules", traces its origins to Robert Boyle's 1661 hypothesis, in his famous treatise The Sceptical Chymist, that matter is composed of clusters of particles and that chemical change results from the rearrangement of the clusters. Boyle argued that matter's basic elements consisted of various sorts and sizes of particles, called "corpuscles", which were capable of arranging themselves into groups.
In 1680, using the corpuscular theory as a basis, French chemist Nicolas Lemery stipulated that the acidity of any substance consisted in its pointed particles, while alkalis were endowed with pores of various sizes. A molecule, according to this view, consisted of corpuscles united through a geometric locking of points and pores.
18th century

An early precursor to the idea of bonded "combinations of atoms", was the theory of "combination via chemical affinity". For example, in 1718, building on Boyle's conception of combinations of clusters, the French chemist Étienne François Geoffroy developed theories of chemical affinity to explain combinations of particles, reasoning that a certain alchemical "force" draws certain alchemical components together. Geoffroy's name is best known in connection with his tables of "affinities" (tables des rapports), which he presented to the French Academy in 1718 and 1720.
These were lists, prepared by collating observations on the actions of substances one upon another, showing the varying degrees of affinity exhibited by analogous bodies for different reagents. These tables retained their vogue for the rest of the century, until displaced by the profounder conceptions introduced by CL Berthollet.
In 1738, Swiss physicist and mathematician Daniel Bernoulli published Hydrodynamica, which laid the basis for the kinetic theory of gases. In this work, Bernoulli positioned the argument, still used to this day, that gases consist of great numbers of molecules moving in all directions, that their impact on a surface causes the gas pressure that we feel, and that what we experience as heat is simply the kinetic energy of their motion. The theory was not immediately accepted, in part because conservation of energy had not yet been established, and it was not obvious to physicists how the collisions between molecules could be perfectly elastic.
In 1789, William Higgins published views on what he called combinations of "ultimate" particles, which foreshadowed the concept of valency bonds. If, for example, according to Higgins, the force between the ultimate particle of oxygen and the ultimate particle of nitrogen were 6, then the strength of the force would be divided accordingly, and similarly for the other combinations of ultimate particles:

19th century

Similar to these views, in 1803 John Dalton took the atomic weight of hydrogen, the lightest element, as unity, and determined, for example, that the ratio for nitrous anhydride was 2 to 3 which gives the formula N2O3. Dalton incorrectly imagined that atoms "hooked" together to form molecules. Later, in 1808, Dalton published his famous diagram of combined "atoms":
Amedeo Avogadro created the word "molecule". His 1811 paper "Essay on Determining the Relative Masses of the Elementary Molecules of Bodies", he essentially states, i.e. according to Partington's A Short History of Chemistry, that:
The smallest particles of gases are not necessarily simple atoms, but are made up of a certain number of these atoms united by attraction to form a single molecule.
Note that this quote is not a literal translation. Avogadro uses the name "molecule" for both atoms and molecules. Specifically, he uses the name "elementary molecule" when referring to atoms and to complicate the matter also speaks of "compound molecules" and "composite molecules".
During his stay in Vercelli, Avogadro wrote a concise note (memoria) in which he declared the hypothesis of what we now call Avogadro's law: equal volumes of gases, at the same temperature and pressure, contain the same number of molecules. This law implies that the relationship occurring between the weights of same volumes of different gases, at the same temperature and pressure, corresponds to the relationship between respective molecular weights. Hence, relative molecular masses could now be calculated from the masses of gas samples.
Avogadro developed this hypothesis to reconcile Joseph Louis Gay-Lussac's 1808 law on volumes and combining gases with Dalton's 1803 atomic theory. The greatest difficulty Avogadro had to resolve was the huge confusion at that time regarding atoms and molecules—one of the most important contributions of Avogadro's work was clearly distinguishing one from the other, admitting that simple particles too could be composed of molecules and that these are composed of atoms. Dalton, by contrast, did not consider this possibility. Curiously, Avogadro considers only molecules containing even numbers of atoms; he does not say why odd numbers are left out.
In 1826, building on the work of Avogadro, the French chemist Jean-Baptiste Dumas states:
Gases in similar circumstances are composed of molecules or atoms placed at the same distance, which is the same as saying that they contain the same number in the same volume.
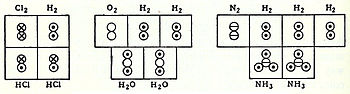
In coordination with these concepts, in 1833 the French chemist Marc Antoine Auguste Gaudin presented a clear account of Avogadro's hypothesis, regarding atomic weights, by making use of "volume diagrams", which clearly show both semi-correct molecular geometries, such as a linear water molecule, and correct molecular formulas, such as H2O:
In two papers outlining his "theory of atomicity of the elements" (1857–58), Friedrich August Kekulé was the first to offer a theory of how every atom in an organic molecule was bonded to every other atom. He proposed that carbon atoms were tetravalent, and could bond to themselves to form the carbon skeletons of organic molecules.
In 1856, Scottish chemist Archibald Couper began research on the bromination of benzene at the laboratory of Charles Wurtz in Paris. One month after Kekulé's second paper appeared, Couper's independent and largely identical theory of molecular structure was published. He offered a very concrete idea of molecular structure, proposing that atoms joined to each other like modern-day Tinkertoys in specific three-dimensional structures. Couper was the first to use lines between atoms, in conjunction with the older method of using brackets, to represent bonds, and also postulated straight chains of atoms as the structures of some molecules, ring-shaped molecules of others, such as in tartaric acid and cyanuric acid. In later publications, Couper's bonds were represented using straight dotted lines (although it is not known if this is the typesetter's preference) such as with alcohol and oxalic acid below:

In 1861, an unknown Vienna high-school teacher named Joseph Loschmidt published, at his own expense, a booklet entitled Chemische Studien I, containing pioneering molecular images which showed both "ringed" structures as well as double-bonded structures, such as:
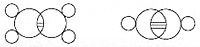
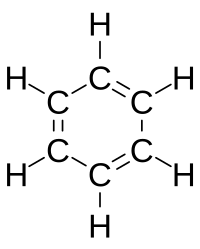
Loschmidt also suggested a possible formula for benzene, but left the issue open. The first proposal of the modern structure for benzene was due to Kekulé, in 1865. The cyclic nature of benzene was finally confirmed by the crystallographer Kathleen Lonsdale. Benzene presents a special problem in that, to account for all the bonds, there must be alternating double carbon bonds:
In 1865, German chemist August Wilhelm von Hofmann was the first to make stick-and-ball molecular models, which he used in lecture at the Royal Institution of Great Britain, such as methane shown below:

The basis of this model followed the earlier 1855 suggestion by his colleague William Odling that carbon is tetravalent. Hofmann's color scheme, to note, is still used to this day: carbon = black, nitrogen = blue, oxygen = red, chlorine = green, sulfur = yellow, hydrogen = white. The deficiencies in Hofmann's model were essentially geometric: carbon bonding was shown as planar, rather than tetrahedral, and the atoms were out of proportion, e.g. carbon was smaller in size than the hydrogen.
In 1864, Scottish organic chemist Alexander Crum Brown began to draw pictures of molecules, in which he enclosed the symbols for atoms in circles, and used broken lines to connect the atoms together in a way that satisfied each atom's valence.
The year 1873, by many accounts, was a seminal point in the history of the development of the concept of the "molecule". In this year, the renowned Scottish physicist James Clerk Maxwell published his famous thirteen page article 'Molecules' in the September issue of Nature. In the opening section to this article, Maxwell clearly states:
An atom is a body which cannot be cut in two; a molecule is the smallest possible portion of a particular substance.
After speaking about the atomic theory of Democritus, Maxwell goes on to tell us that the word 'molecule' is a modern word. He states, "it does not occur in Johnson's Dictionary. The ideas it embodies are those belonging to modern chemistry." We are told that an 'atom' is a material point, invested and surrounded by 'potential forces' and that when 'flying molecules' strike against a solid body in constant succession it causes what is called pressure of air and other gases. At this point, however, Maxwell notes that no one has ever seen or handled a molecule.
In 1874, Jacobus Henricus van 't Hoff and Joseph Achille Le Bel independently proposed that the phenomenon of optical activity could be explained by assuming that the chemical bonds between carbon atoms and their neighbors were directed towards the corners of a regular tetrahedron. This led to a better understanding of the three-dimensional nature of molecules.
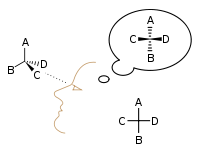
Emil Fischer developed the Fischer projection technique for viewing 3-D molecules on a 2-D sheet of paper:
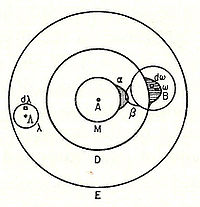
In 1898, Ludwig Boltzmann, in his Lectures on Gas Theory, used the theory of valence to explain the phenomenon of gas phase molecular dissociation, and in doing so drew one of the first rudimentary yet detailed atomic orbital overlap drawings. Noting first the known fact that molecular iodine vapor dissociates into atoms at higher temperatures, Boltzmann states that we must explain the existence of molecules composed of two atoms, the "double atom" as Boltzmann calls it, by an attractive force acting between the two atoms. Boltzmann states that this chemical attraction, owing to certain facts of chemical valence, must be associated with a relatively small region on the surface of the atom called the sensitive region.
Boltzmann states that this "sensitive region" will lie on the surface of the atom, or may partially lie inside the atom, and will firmly be connected to it. Specifically, he states "only when two atoms are situated so that their sensitive regions are in contact, or partly overlap, will there be a chemical attraction between them. We then say that they are chemically bound to each other." This picture is detailed below, showing the α-sensitive region of atom-A overlapping with the β-sensitive region of atom-B:
20th century
In the early 20th century, the American chemist Gilbert N. Lewis began to use dots in lecture, while teaching undergraduates at Harvard, to represent the electrons around atoms. His students favored these drawings, which stimulated him in this direction. From these lectures, Lewis noted that elements with a certain number of electrons seemed to have a special stability. This phenomenon was pointed out by the German chemist Richard Abegg in 1904, to which Lewis referred to as "Abegg's law of valence" (now generally known as Abegg's rule). To Lewis it appeared that once a core of eight electrons has formed around a nucleus, the layer is filled, and a new layer is started. Lewis also noted that various ions with eight electrons also seemed to have a special stability. On these views, he proposed the rule of eight or octet rule: Ions or atoms with a filled layer of eight electrons have a special stability.
Moreover, noting that a cube has eight corners Lewis envisioned an atom as having eight sides available for electrons, like the corner of a cube. Subsequently, in 1902 he devised a conception in which cubic atoms can bond on their sides to form cubic-structured molecules.
In other words, electron-pair bonds are formed when two atoms share an edge, as in structure C below. This results in the sharing of two electrons. Similarly, charged ionic-bonds are formed by the transfer of an electron from one cube to another, without sharing an edge A. An intermediate state B where only one corner is shared was also postulated by Lewis.

Hence, double bonds are formed by sharing a face between two cubic atoms. This results in the sharing of four electrons.
In 1913, while working as the chair of the department of chemistry at the University of California, Berkeley, Lewis read a preliminary outline of paper by an English graduate student, Alfred Lauck Parson, who was visiting Berkeley for a year. In this paper, Parson suggested that the electron is not merely an electric charge but is also a small magnet (or "magneton" as he called it) and furthermore that a chemical bond results from two electrons being shared between two atoms. This, according to Lewis, meant that bonding occurred when two electrons formed a shared edge between two complete cubes.
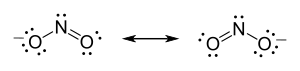
On these views, in his famous 1916 article The Atom and the Molecule, Lewis introduced the "Lewis structure" to represent atoms and molecules, where dots represent electrons and lines represent covalent bonds. In this article, he developed the concept of the electron-pair bond, in which two atoms may share one to six electrons, thus forming the single electron bond, a single bond, a double bond, or a triple bond.

In Lewis' own words:
An electron may form a part of the shell of two different atoms and cannot be said to belong to either one exclusively.
Moreover, he proposed that an atom tended to form an ion by gaining or losing the number of electrons needed to complete a cube. Thus, Lewis structures show each atom in the structure of the molecule using its chemical symbol. Lines are drawn between atoms that are bonded to one another; occasionally, pairs of dots are used instead of lines. Excess electrons that form lone pairs are represented as pair of dots, and are placed next to the atoms on which they reside:
To summarize his views on his new bonding model, Lewis states:
Two atoms may conform to the rule of eight, or the octet rule, not only by the transfer of electrons from one atom to another, but also by sharing one or more pairs of electrons...Two electrons thus coupled together, when lying between two atomic centers, and held jointly in the shells of the two atoms, I have considered to be the chemical bond. We thus have a concrete picture of that physical entity, that "hook and eye" which is part of the creed of the organic chemist.
The following year, in 1917, an unknown American undergraduate chemical engineer named Linus Pauling was learning the Dalton hook-and-eye bonding method at the Oregon Agricultural College, which was the vogue description of bonds between atoms at the time. Each atom had a certain number of hooks that allowed it to attach to other atoms, and a certain number of eyes that allowed other atoms to attach to it. A chemical bond resulted when a hook and eye connected. Pauling, however, wasn't satisfied with this archaic method and looked to the newly emerging field of quantum physics for a new method.
In 1927, the physicists Fritz London and Walter Heitler applied the new quantum mechanics to the deal with the saturable, nondynamic forces of attraction and repulsion, i.e., exchange forces, of the hydrogen molecule. Their valence bond treatment of this problem, in their joint paper, was a landmark in that it brought chemistry under quantum mechanics. Their work was an influence on Pauling, who had just received his doctorate and visited Heitler and London in Zürich on a Guggenheim Fellowship.
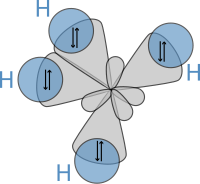
Subsequently, in 1931, building on the work of Heitler and London and on theories found in Lewis' famous article, Pauling published his ground-breaking article "The Nature of the Chemical Bond" (see: manuscript) in which he used quantum mechanics to calculate properties and structures of molecules, such as angles between bonds and rotation about bonds. On these concepts, Pauling developed hybridization theory to account for bonds in molecules such as CH4, in which four sp³ hybridised orbitals are overlapped by hydrogen's 1s orbital, yielding four sigma (σ) bonds. The four bonds are of the same length and strength, which yields a molecular structure as shown below:
Owing to these exceptional theories, Pauling won the 1954 Nobel Prize in Chemistry. Notably he has been the only person to ever win two unshared Nobel prizes, winning the Nobel Peace Prize in 1963.
In 1926, French physicist Jean Perrin received the Nobel Prize in physics for proving, conclusively, the existence of molecules. He did this by calculating the Avogadro number using three different methods, all involving liquid phase systems. First, he used a gamboge soap-like emulsion, second by doing experimental work on Brownian motion, and third by confirming Einstein's theory of particle rotation in the liquid phase.
In 1937, chemist K.L. Wolf introduced the concept of supermolecules (Übermoleküle) to describe hydrogen bonding in acetic acid dimers. This would eventually lead to the area of supermolecular chemistry, which is the study of non-covalent bonding.
In 1951, physicist Erwin Wilhelm Müller invents the field ion microscope and is the first to see atoms, e.g. bonded atomic arrangements at the tip of a metal point.
In 1968-1970 Leroy Cooper, PhD of the University of California at Davis completed his thesis which showed what molecules looked like. He used x-ray deflection off crystals and a complex computer program written by Bill Pentz of the UC Davis Computer Center. This program took the mapped deflections and used them to calculate the basic shapes of crystal molecules. His work showed that actual molecular shapes in quartz crystals and other tested crystals looked similar to the long envisioned merged various sized soap bubbles theorized, except instead of being merged spheres of different sizes, actual shapes were rigid mergers of more tear dropped shapes that stayed fixed in orientation. This work verified for the first time that crystal molecules are actually linked or stacked merged tear drop constructions.
In 1999, researchers from the University of Vienna reported results from experiments on wave-particle duality for C60 molecules. The data published by Anton Zeilinger et al. were consistent with Louis de Broglie's matter waves. This experiment was noted for extending the applicability of wave–particle duality by about one order of magnitude in the macroscopic direction.
In 2009, researchers from IBM managed to take the first picture of a real molecule. Using an atomic force microscope every single atom and bond of a pentacene molecule could be imaged.
See also
- History of chemistry
- History of quantum mechanics
- History of thermodynamics
- History of molecular biology
- Kinetic theory of gases
- Atomic theory
References
- Russell, Bertrand (2007). A History of Western Philosophy. Simon & Schuster. p. 41. ISBN 978-1-4165-5477-6.
- Russell, Bertrand (2007). A History of Western Philosophy. Simon & Schuster. p. 145. ISBN 978-1-4165-5477-6.
- Pfeffer, Jeremy, I.; Nir, Shlomo (2001). Modern Physics: An Introduction Text. World Scientific Publishing Company. p. 183. ISBN 1-86094-250-4.
{{cite book}}: CS1 maint: multiple names: authors list (link) - See testimonia DK 68 A 80, DK 68 A 37 and DK 68 A 43. See also Cassirer, Ernst (1953). An Essay on Man: an Introduction to the Philosophy of Human Culture. Doubleday & Co. p. 214. ISBN 0-300-00034-0. ASIN B0007EK5MM.
- Leicester, Henry, M. (1956). The Historical Background of Chemistry. John Wiley & Sons. p. 112. ISBN 0-486-61053-5.
{{cite book}}: CS1 maint: multiple names: authors list (link) - (a) Isaac Newton, (1704). Opticks. (p. 389). New York: Dover.
(b) Bernard, Pullman; Reisinger, Axel, R. (2001). The Atom in the History of Human Thought. Oxford University Press. p. 139. ISBN 0-19-515040-6.{{cite book}}: CS1 maint: multiple names: authors list (link) - Lemery, Nicolas. (1680). An Appendix to a Course of Chymistry. London, pgs 14-15.
- Ley, Willy (June 1966). "The Re-Designed Solar System". For Your Information. Galaxy Science Fiction. pp. 94–106.
- Avogadro, Amedeo (1811). "Masses of the Elementary Molecules of Bodies". Journal de Physique. 73: 58–76.
- Seymour H. Mauskopf (1969). "The Atomic Structural Theories of Ampère and Gaudin: Molecular Speculation and Avogadro's Hypothesis". Isis. 60 (1): 61–74. doi:10.1086/350449. JSTOR 229022. S2CID 143759556.
- "Chemical Bonding Concept/Skills Development". intro.chem.okstate.edu. Retrieved 2023-01-01.
- Bowden, Mary Ellen (1997). Chemical achievers : the human face of the chemical sciences. Philadelphia, PA: Chemical Heritage Foundation. pp. 90–93. ISBN 9780941901123.
- Bader, A. & Parker, L. (2001). "Joseph Loschmidt", Physics Today, Mar.
- Ollis, W. D. (1972). "Models and molecules". Proceedings of the Royal Institution of Great Britain. 45: 1–31.
- Maxwell, James Clerk, "Molecules Archived 2007-02-09 at the Wayback Machine". Nature, September, 1873.
- Boltzmann, Ludwig (1898). Lectures on Gas Theory (Reprint ed.). Dover. ISBN 0-486-68455-5.
- Cobb, Cathy (1995). Creations of Fire - Chemistry's Lively History From Alchemy to the Atomic Age. Perseus Publishing. ISBN 0-7382-0594-X.
- Parson, A.L. (1915). "A Magneton Theory of the Structure of the Atom". Smithsonian Publication 2371, Washington.
- "Valence and The Structure of Atoms and Molecules", G. N. Lewis, American Chemical Society Monograph Series, page 79 and 81.
- Heitler, Walter; London, Fritz (1927). "Wechselwirkung neutraler Atome und homöopolare Bindung nach der Quantenmechanik". Zeitschrift für Physik. 44 (6–7): 455–472. Bibcode:1927ZPhy...44..455H. doi:10.1007/BF01397394. S2CID 119739102.
- Pauling, Linus (1931). "The nature of the chemical bond. Application of results obtained from the quantum mechanics and from a theory of paramagnetic susceptibility to the structure of molecules". J. Am. Chem. Soc. 53 (4): 1367–1400. doi:10.1021/ja01355a027.
- Perrin, Jean, B. (1926). Discontinuous Structure of Matter, Nobel Lecture, December 11.
- Mitch Jacoby, "Atomic Imaging Turns 50", Chemical & engineering News, 83:48, pp. 13–16, 28 November 2005
- Arndt, M.; O. Nairz; J. Voss-Andreae; C. Keller; G. van der Zouw; A. Zeilinger (14 October 1999). "Wave-particle duality of C60 molecules". Nature. 401 (6754): 680–682. Bibcode:1999Natur.401..680A. doi:10.1038/44348. PMID 18494170. S2CID 4424892.
- Rae, A. I. M. (14 October 1999). "Quantum physics: Waves, particles and fullerenes". Nature. 401 (6754): 651–653. Bibcode:1999Natur.401..651R. doi:10.1038/44294. S2CID 5416065.
- Single molecule's stunning image.
Further reading
- Partington, J.R. (1989). A Short History of Chemistry. Dover Publications, Inc. ISBN 0-486-65977-1.
- Atkins, Peter (2003). Atkins' Molecules, 2nd Ed. Cambridge University Press. ISBN 0-521-53536-0.
- Sargent, Ted (2006). The Dance of Molecules - How Nanotechnology is Changing our Lives. Thunder's Mouth Press. ISBN 1-56025-809-8.
- Scerri, Eric R. (2007). The Periodic Table, Its Story and Its Significance. Oxford University Press. ISBN 978-0-19-530573-9.
External links
- Geometric Structures of Molecules - Middlebury College
- Atoms and Molecules - McMaster University
- 3D Molecule Viewer - The Wileys Family
- Molecule of the Month - School of Chemistry, University of Bristol
- - Eric Scerri's history & philosophy of chemistry website
Types
- Antibody Molecule - The National Health Museum
- 15 Types of Molecules - IUPAC Definitions
Definitions
- Molecule Definition - Frostburg State University (Department of Chemistry)
- Definition of Molecule - IUPAC
Articles
- Molecules Used to Make Nano-sized Containers - TRN Newswire
- Molecular Computer Processors - HP Labs
| History of chemistry (timeline) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| By branch |
| ||||||||
| Ancient history | |||||||||
| Periodic table (timeline) |
| ||||||||
| On specific discoveries |
| ||||||||
| Scientific disputes | |||||||||
| Other | |||||||||
| History of physics (timeline) | |
|---|---|
| Classical physics | |
| Modern physics |
|
| Recent developments |
|
| On specific discoveries |
|
| By periods | |
| By groups | |
| Scientific disputes | |