| Revision as of 19:08, 5 October 2006 editCollegehealth-e (talk | contribs)21 editsNo edit summary← Previous edit | Latest revision as of 01:01, 25 December 2024 edit undoDMacks (talk | contribs)Edit filter managers, Autopatrolled, Administrators186,225 edits ref? Undid revision 1265067145 by NW49ERSFAN24 (talk)Tag: Undo | ||
| Line 1: | Line 1: | ||
| {{Short description|Cough suppressant, antidepressant, and dissociative drug}} | |||
| {{drugbox | | |||
| {{Distinguish|Dextrorphan|Dexamethasone|Dextroamphetamine}} | |||
| | IUPAC_name = <small>D</small>-(+)-3-methoxy-17-methyl-<br/>(9α,13α,14α)-morphinan | |||
| {{cs1 config |name-list-style=vanc |display-authors=6}} | |||
| | image = Dextromethorphan.png | |||
| {{Infobox drug | |||
| | CAS_number = 125-71-3 | |||
| | Verifiedfields = changed | |||
| | Watchedfields = changed | |||
| | verifiedrevid = 470624518 | |||
| | image = Dextromethorphan.svg | |||
| | alt = | |||
| | image2 = Dextromethorphan-from-xtal-3D-balls-A.png | |||
| | alt2 = | |||
| <!-- Clinical data --> | |||
| | pronounce = {{IPAc-en|ˌ|d|ɛ|k|.|s|t|r|oʊ|.|m|ə|ˈ|θ|ɔː|ɹ|ˌ|f|æ|n}}<br />{{respell|DEK|stroh|məth|OR|fan}} | |||
| | tradename = Robitussin, Delsym, others | |||
| | Drugs.com = {{drugs.com|monograph|dextromethorphan}} | |||
| | MedlinePlus = a682492 | |||
| | DailyMedID = Dextromethorphan | |||
| | pregnancy_AU = A | |||
| | addiction_liability = Low–moderate | |||
| | routes_of_administration = ] | |||
| | class = ];<ref>{{cite web|url=https://www.drugs.com/monograph/dextromethorphan.html|title=Dextromethorphan Monograph for Professionals|website=Drugs.com}}</ref><ref>{{cite journal | vauthors = Windhab LG, Gastberger S, Hulka LM, Baumgartner MR, Soyka M, Müller TJ, Seifritz E, Mutschler J | title = Dextromethorphan Abuse Among Opioid-Dependent Patients | journal = Clinical Neuropharmacology | volume = 43 | issue = 5 | pages = 127–133 | year = 2020 | pmid = 32947422 | doi = 10.1097/WNF.0000000000000403 | s2cid = 221798401 | url = https://boris.unibe.ch/150461/ }}</ref><br>];<ref>{{Cite journal |last=McCarthy |first=Brian|date=2023-11-30 |title=Dextromethorphan-bupropion (Auvelity) for the Treatment of Major Depressive Disorder |journal=Clinical Psychopharmacology and Neuroscience|volume=21 |issue=4 |pages=609–616 |doi=10.9758/cpn.23.1081 |pmid=37859435|pmc=10591164 }}</ref> ]; ] | |||
| | ATC_prefix = R05 | | ATC_prefix = R05 | ||
| | ATC_suffix = DA09 | | ATC_suffix = DA09 | ||
| | PubChem = 5360696 | |||
| <!-- Legal status --> | |||
| | DrugBank = APRD00655 | |||
| | chemical_formula = {{Carbon}}<sub>18</sub>{{Hydrogen}}<sub>25</sub>{{Nitrogen}}{{Oxygen}} | |||
| | molecular_weight = 271.4 g/mol | |||
| | bioavailability = ? | |||
| | metabolism = Hepatic (], ] and ]-mediated) | |||
| | elimination_half-life = 1.4–3.9 hours | |||
| | excretion = ] | |||
| | pregnancy_AU = A | |||
| | pregnancy_US = C | |||
| | legal_AU = S2 | | legal_AU = S2 | ||
| | legal_UK = P | |||
| | routes_of_administration = Oral | |||
| | legal_US = OTC | |||
| | legal_CA = OTC | |||
| | legal_UN = Unscheduled | |||
| | legal_status = SE: Förteckning V | |||
| <!-- Pharmacokinetic data --> | |||
| | bioavailability = 11%<ref name="pmid15500572">{{cite journal | vauthors = Kukanich B, Papich MG | title = Plasma profile and pharmacokinetics of dextromethorphan after intravenous and oral administration in healthy dogs | journal = Journal of Veterinary Pharmacology and Therapeutics | volume = 27 | issue = 5 | pages = 337–341 | date = October 2004 | pmid = 15500572 | doi = 10.1111/j.1365-2885.2004.00608.x }}</ref> | |||
| | metabolism = ] enzymes: major ], minor ], and minor ] | |||
| | metabolites = * ] | |||
| * ] | |||
| | elimination_half-life = 2–4 hours (]s); 24 hours (]s)<ref name = MSR>{{cite web|title=Balminil DM, Benylin DM (dextromethorphan) dosing, indications, interactions, adverse effects, and more|work=Medscape Reference|publisher=WebMD|access-date=15 April 2014|url=http://reference.medscape.com/drug/balminil-dm-benylin-dm-dextromethorphan-343401#showall}}</ref> | |||
| | duration_of_action = 3–8 hours | |||
| | excretion = ] | |||
| <!-- Identifiers --> | |||
| | CAS_number_Ref = {{cascite|correct|??}} | |||
| | CAS_number = 125-71-3 | |||
| | PubChem = 5360696 | |||
| | IUPHAR_ligand = 6953 | |||
| | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| | DrugBank = DB00514 | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| | ChemSpiderID = 13109865 | |||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| | UNII = 7355X3ROTS | |||
| | KEGG_Ref = {{keggcite|correct|kegg}} | |||
| | KEGG = D03742 | |||
| | ChEBI_Ref = {{ebicite|changed|EBI}} | |||
| | ChEBI = 4470 | |||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| | ChEMBL = 52440 | |||
| | synonyms = DXM, 3-methoxy-N-methylmorphinan | |||
| <!-- Chemical data --> | |||
| | IUPAC_name = (4b''S'',8a''R'',9''S'')-3-Methoxy-11-methyl-6,7,8,8a,9,10-hexahydro-5''H''-9,4b-(epiminoethano)phenanthrene | |||
| | C = 18 | |||
| | H = 25 | |||
| | N = 1 | |||
| | O = 1 | |||
| | chirality = ] ] | |||
| | SMILES = CN1CC23CCCC21Cc4c3cc(cc4)OC | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChI = 1S/C18H25NO/c1-19-10-9-18-8-4-3-5-15(18)17(19)11-13-6-7-14(20-2)12-16(13)18/h6-7,12,15,17H,3-5,8-11H2,1-2H3/t15-,17+,18+/m1/s1 | |||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | StdInChIKey = MKXZASYAUGDDCJ-NJAFHUGGSA-N | |||
| | melting_point = 111 | |||
| }} | }} | ||
| '''Dextromethorphan''' ('''DM''' or '''DXM''') is an ] drug that is found in many ] ] and ] preparations, usually in the form of dextromethorphan ]. It is also used as a ]. | |||
| '''Dextromethorphan''', sold under the brand name '''Robitussin''' among others, is a ] used in many ] and ] medicines.<ref>{{cite journal |last=Dicpinigaitis |first=P |date=2022-09-12 |title=The Current and Emerging Treatment Landscape for Chronic Cough |url=https://www.ajmc.com/view/the-current-and-emerging-treatment-landscape-for-chronic-cough |journal=The American Journal of Managed Care |series=Uncovering the Economic Burden of Chronic Cough and the Promising Role of Emerging Targeted Therapies |language=en |volume=28 |issue=9 |pages=S159–S165 |doi=10.37765/ajmc.2022.89244 |pmid=36198074 |s2cid=252736111 |quote=By sales, dextromethorphan is the most widely used OTC antitussive drug in the United States, and approximately 85% to 90% of OTC cough medicines contain dextromethorphan}}</ref> In 2022, the US ] (FDA) approved the combination ] to serve as a rapid-acting ] in people with ].<ref>{{cite journal | vauthors = Majeed A, Xiong J, Teopiz KM, Ng J, Ho R, Rosenblat JD, Phan L, Cao B, McIntyre RS | title = Efficacy of dextromethorphan for the treatment of depression: a systematic review of preclinical and clinical trials | journal = Expert Opinion on Emerging Drugs | volume = 26 | issue = 1 | pages = 63–74 | date = March 2021 | pmid = 33682569 | doi = 10.1080/14728214.2021.1898588 | s2cid = 232141396 }}</ref> | |||
| It is in the ] class of medications with ], ], and ] properties (at lower doses). Dextromethorphan does not have a significant ] for the ] activity typical of morphinan compounds and exerts its therapeutic effects through several other receptors.<ref name="pmid27139517" /> In its pure form, dextromethorphan occurs as a white powder.<ref name="ref tables">{{cite web|url=http://www.pharmacopeia.cn/v29240/usp29nf24s0_alpha-2-13.html|title=Reference Tables: Description and Solubility{{dash}}D|archive-url=https://web.archive.org/web/20170704164745/http://www.pharmacopeia.cn/v29240/usp29nf24s0_alpha-2-13.html|archive-date=2017-07-04|url-status=dead|access-date=2011-05-06 }}</ref> | |||
| When exceeding approved dosages, dextromethorphan acts as a ]. It has multiple ], including actions as a nonselective ]<ref name="Schwartz, Pizon, Brooks 2008">{{cite journal | vauthors = Schwartz AR, Pizon AF, Brooks DE | title = Dextromethorphan-induced serotonin syndrome | journal = Clinical Toxicology | volume = 46 | issue = 8 | pages = 771–773 | date = September 2008 | pmid = 19238739 | doi = 10.1080/15563650701668625 | s2cid = 37817922 | doi-access = free | title-link = doi }}</ref> and a ] agonist.<ref name="pmid17386960">{{cite journal | vauthors = Shin EJ, Nah SY, Chae JS, Bing G, Shin SW, Yen TP, Baek IH, Kim WK, Maurice T, Nabeshima T, Kim HC | title = Dextromethorphan attenuates trimethyltin-induced neurotoxicity via sigma1 receptor activation in rats | journal = Neurochemistry International | volume = 50 | issue = 6 | pages = 791–799 | date = May 2007 | pmid = 17386960 | doi = 10.1016/j.neuint.2007.01.008 | s2cid = 43230896 }}</ref><ref name="pmid15723099">{{cite journal | vauthors = Shin EJ, Nah SY, Kim WK, Ko KH, Jhoo WK, Lim YK, Cha JY, Chen CF, Kim HC | title = The dextromethorphan analog dimemorfan attenuates kainate-induced seizures via sigma1 receptor activation: comparison with the effects of dextromethorphan | journal = British Journal of Pharmacology | volume = 144 | issue = 7 | pages = 908–918 | date = April 2005 | pmid = 15723099 | pmc = 1576070 | doi = 10.1038/sj.bjp.0705998 }}</ref> Dextromethorphan and its major ], ], also block the ] at high doses, which produces effects similar to other ] such as ], ], and ]. | |||
| <!-- Society and culture --> | |||
| It was patented in 1949 and approved for medical use in 1953.<ref name=Fis2006>{{cite book | vauthors = Fischer J, Ganellin CR |title= Analogue-based Drug Discovery |date=2006 |publisher=John Wiley & Sons |isbn=9783527607495 |page=527 |url=https://books.google.com/books?id=FjKfqkaKkAAC&pg=PA527 }}</ref> In 2022, the combination with ] was the 260th most commonly prescribed medication in the United States, with more than 1{{nbsp}}million prescriptions.<ref name="Top 300 of 2022">{{cite web | title=The Top 300 of 2022 | url=https://clincalc.com/DrugStats/Top300Drugs.aspx | website=ClinCalc | access-date=30 August 2024 | archive-date=30 August 2024 | archive-url=https://web.archive.org/web/20240830202410/https://clincalc.com/DrugStats/Top300Drugs.aspx | url-status=live }}</ref><ref>{{cite web | title = Dextromethorphan; Promethazine Drug Usage Statistics, United States, 2013 - 2022 | website = ClinCalc | url = https://clincalc.com/DrugStats/Drugs/DextromethorphanPromethazine | access-date = 30 August 2024 }}</ref> In 2022, the combination with ] and ] was the 265th most commonly prescribed medication in the United States, with more than 1{{nbsp}}million prescriptions.<ref name="Top 300 of 2022" /><ref>{{cite web | title = Brompheniramine; Dextromethorphan; Pseudoephedrine Drug Usage Statistics, United States, 2013 - 2022 | website = ClinCalc | url = https://clincalc.com/DrugStats/Drugs/BrompheniramineDextromethorphanPseudoephedrine | access-date = 30 August 2024 }}</ref> | |||
| {{TOC limit|3}} | |||
| ==Medical uses== | |||
| ] | |||
| ===Cough suppression=== | |||
| The primary use of dextromethorphan is as a ], for the temporary relief of cough caused by minor throat and bronchial irritation (such as commonly accompanies the ] and ]), or from inhaled particle irritants, as well as chronic cough at a higher dosage.<ref name="Young Smith 2011 pp. 224–230">{{cite journal | vauthors = Young EC, Smith JA | title = Pharmacologic therapy for cough | journal = Current Opinion in Pharmacology | volume = 11 | issue = 3 | pages = 224–230 | date = June 2011 | pmid = 21724464 | doi = 10.1016/j.coph.2011.06.003 | publisher = Elsevier BV }}</ref><ref name = AMH>{{cite book | title = Australian Medicines Handbook | year = 2013 | publisher = The Australian Medicines Handbook Unit Trust | isbn = 978-0-9805790-9-3 | place = Adelaide | editor = Rossi, S }}{{page needed|date=April 2015}}</ref> Dextromethorphan is available alone in the form of ] and ]s as well as in combination with other agents. | |||
| ===Pseudobulbar affect=== | |||
| In 2010, the FDA approved the combination drug ] under the brand name Nuedexta<ref name="Nuedexta FDA label">{{cite web | title=Nuedexta- dextromethorphan hydrobromide and quinidine sulfate capsule, gelatin coated | website=DailyMed | date=23 June 2019 | url=https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=484e0918-3442-49dc-8ccf-177f1f3ee9f3 | access-date=23 October 2020}}</ref> for the treatment of ] (uncontrollable laughing/crying). Dextromethorphan is the active therapeutic agent in the combination; ] merely serves to inhibit the ] ] of dextromethorphan and thereby increase its circulating concentrations via inhibition of ].<ref name="NguyenThomas2016">{{cite journal | vauthors = Nguyen L, Thomas KL, Lucke-Wold BP, Cavendish JZ, Crowe MS, Matsumoto RR | title = Dextromethorphan: An update on its utility for neurological and neuropsychiatric disorders | journal = Pharmacology & Therapeutics | volume = 159 | pages = 1–22 | date = March 2016 | pmid = 26826604 | doi = 10.1016/j.pharmthera.2016.01.016 }}</ref> | |||
| ===Major depressive disorder === | |||
| The combination medicine ] is approved for ] under the brand name Auvelity.<ref name="Auvelity Label">{{cite web | title=Auvelity (dextromethorphan hydrobromide/bupropion hydrochloride) | website=Axsome Therapeutics | url=https://www.axsome.com/auvelity-prescribing-information.pdf | access-date=19 August 2022 | archive-date=21 August 2022 | archive-url=https://web.archive.org/web/20220821043349/https://www.axsome.com/auvelity-prescribing-information.pdf | url-status=live }}</ref><ref name="pmid36301443">{{cite journal |vauthors=Keam SJ |title=Dextromethorphan/Bupropion: First Approval |journal=] |volume=36 |issue=11 |pages=1229–1238 |date=November 2022 |pmid=36301443 |doi=10.1007/s40263-022-00968-4|s2cid=253158902 |url=https://figshare.com/articles/online_resource/Dextromethorphan_Bupropion_First_Approval/21320970 }}</ref> | |||
| ==Contraindications== | |||
| {{Empty section|date=November 2024}} | |||
| ==Adverse effects== | |||
| Side effects of dextromethorphan at normal therapeutic doses can include:<ref name = MSR/><ref name = AMH/><ref name="nhtsa">{{cite web|url=http://www.nhtsa.dot.gov/PEOPLE/injury/research/job185drugs/dextromethorphan.htm|title=Dextromethorphan|work=National Highway Traffic Safety Administration (NHTSA)|archive-url=https://web.archive.org/web/20080801054711/http://www.nhtsa.dot.gov/PEOPLE/injury/research/job185drugs/dextromethorphan.htm|archive-date=2008-08-01}}</ref> | |||
| {{Columns-list|colwidth=20em| | |||
| * Body ]/] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ]}} | |||
| A rare side effect is ].<ref name = AMH/> | |||
| ===Neurotoxicity=== | |||
| Dextromethorphan was once thought to cause ] when administered ]; however, this was later proven inconclusive, due to lack of research on humans. Tests were performed on rats, giving them 50 mg or more every day for as long as a month. Neurotoxic changes, including vacuolation, have been observed in ] and ] of rats administered other ]s such as PCP, but not with dextromethorphan.<ref>{{cite journal | vauthors = Olney JW, Labruyere J, Price MT | title = Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs | journal = Science | volume = 244 | issue = 4910 | pages = 1360–1362 | date = June 1989 | pmid = 2660263 | doi = 10.1126/science.2660263 | bibcode = 1989Sci...244.1360O }}</ref><ref>{{cite journal | vauthors = Carliss RD, Radovsky A, Chengelis CP, O'Neill TP, Shuey DL | title = Oral administration of dextromethorphan does not produce neuronal vacuolation in the rat brain | journal = Neurotoxicology | volume = 28 | issue = 4 | pages = 813–818 | date = July 2007 | pmid = 17573115 | doi = 10.1016/j.neuro.2007.03.009 | bibcode = 2007NeuTx..28..813C }}</ref> | |||
| ===Dependence and withdrawal=== | |||
| In many documented cases{{Citation needed|date=August 2020}}, dextromethorphan has produced ] in people who used it recreationally. It is considered less addictive than other common cough suppressants, such as the opiate ].<ref name = MSR/> Since dextromethorphan also acts as a serotonin reuptake inhibitor, users report that regular recreational use over a long period of time can cause withdrawal symptoms similar to those of ]. Additionally, disturbances have been reported in sleep, senses, movement, mood, and thinking. | |||
| ==Overdose== | |||
| Adverse effects of dextromethorphan in ] at doses 3 to 10 times the recommended therapeutic dose:<ref name="webmd">{{cite web|url=http://www.webmd.com/parenting/teen-abuse-cough-medicine-9/teens-and-dxm-drug-abuse?page=3|title=Teen Drug Abuse: Cough Medicine and DXM (Dextromethorphan)|publisher=webmd|archive-url=https://web.archive.org/web/20171121023716/https://www.webmd.com/parenting/features/teens-and-dxm-drug-abuse|archive-date=2017-11-21|url-status=live}}</ref>{{Failed verification|date=July 2018}} | |||
| {{Columns-list|colwidth=20em| | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * Glassy eyes | |||
| * ] | |||
| }} | |||
| At doses 11 to 75 times the recommended therapeutic dose:<ref name = webmd/>{{Failed verification|date=July 2018}}<ref name="Dextromethorphan in Cough Syrup: Th">{{cite journal | vauthors = Martinak B, Bolis RA, Black JR, Fargason RE, Birur B | title = Dextromethorphan in Cough Syrup: The Poor Man's Psychosis | journal = Psychopharmacology Bulletin | volume = 47 | issue = 4 | pages = 59–63 | date = September 2017 | pmid = 28936010 | pmc = 5601090 }}</ref> | |||
| {{Columns-list|colwidth=20em| | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] (teeth grinding) | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * Severe ] | |||
| * ] | |||
| }} | |||
| Episodic acute ] can occur when high doses of dextromethorphan are taken for recreational use, and an abundance of psychiatric symptoms can result, including ] and other PCP-like symptoms.<ref name="Dextromethorphan in Cough Syrup: Th"/> | |||
| ==Interactions== | |||
| ] may result from the combined use of dextromethorphan and ] antidepressants such as ] (SSRIs) or ] (MAOIs).<ref>{{cite journal | vauthors = Dy P, Arcega V, Ghali W, Wolfe W | title = Serotonin syndrome caused by drug to drug interaction between escitalopram and dextromethorphan | journal = BMJ Case Reports | volume = 2017 | pages = bcr–2017–221486 | date = August 2017 | pmid = 28784915 | pmc = 5747823 | doi = 10.1136/bcr-2017-221486 }}</ref> Further research is needed to determine whether doses of dextromethorphan beyond those normally used therapeutically are needed to produce this effect.<ref name="Schwartz, Pizon, Brooks 2008"/> In any case, dextromethorphan should not be taken with MAOIs due to the possibility of this complication.<ref name="nhtsa" /> Serotonin syndrome is a potentially life-threatening condition that can occur rapidly, due to a buildup of an excessive amount of ] in the body. | |||
| Combining ] with dextromethorphan significantly increases the risk of overdose and other severe health complications, according to the ].<ref>{{cite web |title=Harmful Interactions {{!}} National Institute on Alcohol Abuse and Alcoholism (NIAAA) |url=https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/harmful-interactions-mixing-alcohol-with-medicines |website=www.niaaa.nih.gov}}</ref> | |||
| ], including dextromethorphan, through the inhibition of the ] system in the liver, and can lead to excessive accumulation of the drug which both increases and prolongs effects. ] and grapefruit juices (especially white grapefruit juice, but also including other citrus fruits such as ] and ], as well as a number of noncitrus fruits<ref name="GANFYD.org">{{cite web|url=http://www.ganfyd.org/index.php?title=Inhibitors_of_CYP3A4|title=Inhibitors of CYP3A4|publisher=ganfyd.org|archive-url=https://web.archive.org/web/20170720174834/http://www.ganfyd.org/index.php?title=Inhibitors_of_CYP3A4|archive-date=2017-07-20|url-status=live|access-date=23 August 2013}}</ref>) generally are recommended to be avoided while using dextromethorphan and numerous other medications. | |||
| ==Pharmacology== | |||
| ===Pharmacodynamics=== | |||
| {| class="wikitable sortable floatright" style="font-size:small;" | |||
| |+ Dextromethorphan and metabolite<ref name="PDSP">{{cite web | title = PDSP K<sub>i</sub> Database | work = Psychoactive Drug Screening Program (PDSP)|author1-link=Bryan Roth | vauthors = Roth BL, Driscol J | publisher = University of North Carolina at Chapel Hill and the United States National Institute of Mental Health | access-date = 14 August 2017 | url = https://pdsp.unc.edu/databases/pdsp.php?knowID=0&kiKey=&receptorDD=&receptor=&speciesDD=&species=&sourcesDD=&source=&hotLigandDD=&hotLigand=&testLigandDD=&testFreeRadio=testFreeRadio&testLigand=dextromethorphan&referenceDD=&reference=&KiGreater=&KiLess=&kiAllRadio=all&doQuery=Submit+Query}}</ref><ref name="NguyenThomas2016"/><ref name="pmid17689532">{{cite journal | vauthors = Werling LL, Keller A, Frank JG, Nuwayhid SJ | title = A comparison of the binding profiles of dextromethorphan, memantine, fluoxetine and amitriptyline: treatment of involuntary emotional expression disorder | journal = Experimental Neurology | volume = 207 | issue = 2 | pages = 248–257 | date = October 2007 | pmid = 17689532 | doi = 10.1016/j.expneurol.2007.06.013 | s2cid = 38476281 }}</ref><ref name="pmid27139517">{{cite journal | vauthors = Taylor CP, Traynelis SF, Siffert J, Pope LE, Matsumoto RR | title = Pharmacology of dextromethorphan: Relevance to dextromethorphan/quinidine (Nuedexta) clinical use | journal = Pharmacology & Therapeutics | volume = 164 | pages = 170–182 | date = August 2016 | pmid = 27139517 | doi = 10.1016/j.pharmthera.2016.04.010 | doi-access = free | title-link = doi }}</ref> | |||
| |- | |||
| ! Site !! {{abbr|DXM|Dextromethorphan}} !! {{abbrlink|DXO|Dextrorphan}} !! Species !! Ref | |||
| |- | |||
| | ] || 2,120–8,945 || 486–906 || Rat || <ref name="NguyenThomas2016"/> | |||
| |- | |||
| | ] || 142–652 || 118–481 || Rat || <ref name="NguyenThomas2016"/> | |||
| |- | |||
| | ] || 11,060–22,864 || 11,325–15,582 || Rat || <ref name="NguyenThomas2016"/> | |||
| |- | |||
| | {{abbrlink|MOR|μ-Opioid receptor}} || 1,280<br />{{abbr|ND|No data}} || 420<br />>1,000 || Rat<br />Human || <ref name="NguyenThomas2016"/><br /><ref name="pmid7815359">{{cite journal | vauthors = Raynor K, Kong H, Mestek A, Bye LS, Tian M, Liu J, Yu L, Reisine T | title = Characterization of the cloned human mu opioid receptor | journal = The Journal of Pharmacology and Experimental Therapeutics | volume = 272 | issue = 1 | pages = 423–428 | date = January 1995 | pmid = 7815359 }}</ref> | |||
| |- | |||
| | {{abbrlink|DOR|δ-Opioid receptor}} || 11,500 || 34,700 || Rat || <ref name="NguyenThomas2016"/> | |||
| |- | |||
| | {{abbrlink|KOR|κ-Opioid receptor}} || 7,000 || 5,950 || Rat || <ref name="NguyenThomas2016"/> | |||
| |- | |||
| | {{abbrlink|SERT|Serotonin transporter}} || 23–40 || 401–484 || Rat || <ref name="NguyenThomas2016"/> | |||
| |- | |||
| | {{abbrlink|NET|Norepinephrine transporter}} || ≥240 || ≥340 || Rat || <ref name="NguyenThomas2016"/> | |||
| |- | |||
| | {{abbrlink|DAT|Dopamine transporter}} || >1,000 || >1,000 || Rat || <ref name="NguyenThomas2016"/> | |||
| |- | |||
| | ] || >1,000 || >1,000 || Rat || <ref name="NguyenThomas2016"/> | |||
| |- | |||
| | ]<sub>/</sub>] || 61% at 1 μM || 54% at 1 μM || Rat || <ref name="NguyenThomas2016"/> | |||
| |- | |||
| | ] || >1,000 || >1,000 || Rat || <ref name="NguyenThomas2016"/> | |||
| |- | |||
| | ] || >1,000 || >1,000 || Rat || <ref name="NguyenThomas2016"/> | |||
| |- | |||
| | ] || 60% at 1 μM || >1,000 || Rat || <ref name="NguyenThomas2016"/> | |||
| |- | |||
| | ] || >1,000 || 35% at 1 μM || Rat || <ref name="NguyenThomas2016"/> | |||
| |- | |||
| | ] || >1,000 || >1,000 || Rat || <ref name="NguyenThomas2016"/> | |||
| |- | |||
| | ] || >1,000 || 95% at 1 μM || Rat || <ref name="NguyenThomas2016"/> | |||
| |- | |||
| | {{abbrlink|mAChRs|Muscarinic acetylcholine receptors}} || >1,000 || 100% at 1 μM || Rat || <ref name="NguyenThomas2016"/> | |||
| |- | |||
| | {{abbrlink|nAChRs|Nicotinic acetylcholine receptors}} || 700–8,900<br />(]) || 1,300–29,600<br />(IC<sub>50</sub>) || Rat || <ref name="NguyenThomas2016"/> | |||
| |- | |||
| | {{abbrlink|VDSCs|Voltage-dependent sodium channels}} || >50,000 (IC<sub>50</sub>) || {{abbr|ND|No data}} || Rat || <ref name="pmid17346698">{{cite journal | vauthors = Lee JH, Shin EJ, Jeong SM, Lee BH, Yoon IS, Lee JH, Choi SH, Kim YH, Pyo MK, Lee SM, Chae JS, Rhim H, Oh JW, Kim HC, Nah SY | title = Effects of dextrorotatory morphinans on brain Na+ channels expressed in Xenopus oocytes | journal = European Journal of Pharmacology | volume = 564 | issue = 1–3 | pages = 7–17 | date = June 2007 | pmid = 17346698 | doi = 10.1016/j.ejphar.2007.01.088 }}</ref><ref name="pmid23139844">{{cite journal | vauthors = Gao XF, Yao JJ, He YL, Hu C, Mei YA | title = Sigma-1 receptor agonists directly inhibit Nav1.2/1.4 channels | journal = PLOS ONE | volume = 7 | issue = 11 | pages = e49384 | year = 2012 | pmid = 23139844 | pmc = 3489664 | doi = 10.1371/journal.pone.0049384 | doi-access = free | title-link = doi | bibcode = 2012PLoSO...749384G }}</ref> | |||
| |- class="sortbottom" | |||
| | colspan="5" style="width: 1px;" | Values are K<sub>i</sub> (nM), unless otherwise noted. The smaller the value, the more strongly the drug binds to the site. | |||
| |} | |||
| Dextromethorphan has been found to possess the following actions (<1 μM) using rat tissues:<ref name="NguyenThomas2016"/><ref name="pmid24648790" /> | |||
| * ] of the ] via the ]/{{abbrlink|PCP|phencyclidine}} site<ref name="pmid24648790">{{cite journal | vauthors = Burns JM, Boyer EW | title = Antitussives and substance abuse | journal = Substance Abuse and Rehabilitation | volume = 4 | pages = 75–82 | year = 2013 | pmid = 24648790 | pmc = 3931656 | doi = 10.2147/SAR.S36761 | doi-access = free | title-link = doi }}</ref> | |||
| * {{abbrlink|SERT|Serotonin transporter}} and {{abbrlink|NET|norepinephrine transporter}} ] (cf. ]) | |||
| * ] ] ] | |||
| * ] of ]s | |||
| * ] of the ] ]<sub>/</sub>], ] ], ], and ]s | |||
| Dextromethorphan is a ] of ], which is the actual mediator of most of its ] effects through acting as a more potent NMDA receptor antagonist than dextromethorphan itself.<ref name="pmid10064839">{{cite journal | vauthors = Chou YC, Liao JF, Chang WY, Lin MF, Chen CF | title = Binding of dimemorfan to sigma-1 receptor and its anticonvulsant and locomotor effects in mice, compared with dextromethorphan and dextrorphan | journal = Brain Research | volume = 821 | issue = 2 | pages = 516–519 | date = March 1999 | pmid = 10064839 | doi = 10.1016/S0006-8993(99)01125-7 | s2cid = 22762264 }}</ref> What role, if any, (+)-], dextromethorphan's other major metabolite, plays in its effects is not entirely clear.<ref>{{cite journal | vauthors = Schmider J, Greenblatt DJ, Fogelman SM, von Moltke LL, Shader RI | title = Metabolism of dextromethorphan in vitro: involvement of cytochromes P450 2D6 and 3A3/4, with a possible role of 2E1 | journal = Biopharmaceutics & Drug Disposition | volume = 18 | issue = 3 | pages = 227–240 | date = April 1997 | pmid = 9113345 | doi = 10.1002/(SICI)1099-081X(199704)18:3<227::AID-BDD18>3.0.CO;2-L | s2cid = 5638973 }}</ref> | |||
| ===Pharmacokinetics=== | |||
| Following oral administration, dextromethorphan is rapidly absorbed from the ], where it enters the ] and crosses the ].{{Citation needed|date=March 2011}} | |||
| At therapeutic doses, dextromethorphan acts ] (meaning that it acts on the ]) as opposed to locally (on the ]). It elevates the threshold for coughing, without inhibiting ]ry activity. Dextromethorphan is rapidly absorbed from the gastrointestinal tract and converted into the active metabolite dextrorphan in the liver by the cytochrome P450 enzyme ]. The average dose necessary for effective antitussive therapy is between 10 and 45 mg, depending on the individual. The International Society for the Study of Cough recommends "an adequate first dose of medication is 60 mg in the adult and repeat dosing should be infrequent rather than ] recommended."<ref name="MoriceCough">{{cite web|url=http://www.issc.info/cough.html|title=Cough| vauthors = Morice AH |publisher=International Society for the Study of Cough|archive-url=https://web.archive.org/web/20170509013127/http://www.issc.info/cough.html|archive-date=2017-05-09|url-status=live}}</ref> | |||
| Dextromethorphan has an ] of approximately 4 hours in individuals with an extensive metabolizer phenotype; this is increased to approximately 13 hours when dextromethorphan is given in combination with ].<ref name="pmid27139517" /> The ] after oral administration is about three to eight hours for dextromethorphan hydrobromide, and 10 to 12 hours for dextromethorphan polistirex.{{Citation needed|date=June 2023}} Around one in 10 of the Caucasian population has little or no CYP2D6 enzyme activity, leading to long-lived high drug levels.<ref name=MoriceCough/> | |||
| ====Metabolism==== | |||
| ] and ]) and UDP-glucuronosyl-transferase (UGT)<ref>{{cite journal | vauthors = Strauch K, Lutz U, Bittner N, Lutz WK | title = Dose-response relationship for the pharmacokinetic interaction of grapefruit juice with dextromethorphan investigated by human urinary metabolite profiles | journal = Food and Chemical Toxicology | volume = 47 | issue = 8 | pages = 1928–1935 | date = August 2009 | pmid = 19445995 | doi = 10.1016/j.fct.2009.05.004 }}</ref>]] | |||
| The first pass through the ] results in some of the drug being metabolized by O-demethylation into an active metabolite of dextromethorphan called dextrorphan, the 3-hydroxy derivative of dextromethorphan. The therapeutic activity of dextromethorphan is believed to be caused by both the drug and this metabolite. Dextromethorphan also undergoes N-demethylation (to 3-methoxymorphinan or MEM),<ref name="DXMdualprobe">{{cite journal | vauthors = Yu A, Haining RL | title = Comparative contribution to dextromethorphan metabolism by cytochrome P450 isoforms in vitro: can dextromethorphan be used as a dual probe for both CTP2D6 and CYP3A activities? | journal = Drug Metabolism and Disposition | volume = 29 | issue = 11 | pages = 1514–1520 | date = November 2001 | pmid = 11602530 | url = http://dmd.aspetjournals.org/cgi/pmidlookup?view=long&pmid=11602530 | access-date = 2015-04-26 | archive-date = 2020-03-12 | archive-url = https://web.archive.org/web/20200312134718/http://dmd.aspetjournals.org/content/29/11/1514.long | url-status = dead }}</ref> and partial conjugation with glucuronic acid and sulfate ions. Hours after dextromethorphan therapy, (in humans) the metabolites (+)-3-hydroxy-N-methylmorphinan, (+)-3-morphinan, and traces of the unchanged drug are detectable in the urine.<ref name="nhtsa" /> | |||
| A major metabolic catalyst involved is the cytochrome P450 enzyme known as 2D6, or CYP2D6. A significant portion of the population has a functional deficiency in this enzyme and are known as poor CYP2D6 metabolizers. O-demethylation of dextromethorphan to dextrorphan contributes to at least 80% of the dextrorphan formed during dextromethorphan metabolism.<ref name="DXMdualprobe"/> As CYP2D6 is a major ] in the inactivation of dextromethorphan, the duration of action and effects of dextromethorphan can be increased by as much as three times in such poor metabolizers.<ref name="pmid8841152">{{cite journal | vauthors = Capon DA, Bochner F, Kerry N, Mikus G, Danz C, Somogyi AA | title = The influence of CYP2D6 polymorphism and quinidine on the disposition and antitussive effect of dextromethorphan in humans | journal = Clinical Pharmacology and Therapeutics | volume = 60 | issue = 3 | pages = 295–307 | date = September 1996 | pmid = 8841152 | doi = 10.1016/S0009-9236(96)90056-9 | s2cid = 10147669 }}</ref> In one study on 252 Americans, 84.3% were found to be "fast" (extensive) metabolizers, 6.8% to be "intermediate" metabolizers, and 8.8% were "slow" metabolizers of dextromethorphan.<ref name="DXMpolymorphicmetabolism">{{cite journal | vauthors = Woodworth JR, Dennis SR, Moore L, Rotenberg KS | title = The polymorphic metabolism of dextromethorphan | journal = Journal of Clinical Pharmacology | volume = 27 | issue = 2 | pages = 139–143 | date = February 1987 | pmid = 3680565 | doi = 10.1002/j.1552-4604.1987.tb02174.x | s2cid = 37950361 }}</ref> A number of ]s for CYP2D6 are known, including several completely inactive variants. The distribution of alleles is uneven amongst ]. | |||
| A large number of medications are potent ]. Some types of medications known to inhibit CYP2D6 include certain SSRIs and ] ]s, some ], and the commonly available ] ]. Therefore, the potential for interactions exists between dextromethorphan and medications that inhibit this enzyme, particularly in slow metabolizers. | |||
| Dextromethorphan is also metabolized by ]. N-demethylation is primarily accomplished by CYP3A4, contributing to at least 90% of the MEM formed as a primary metabolite of dextromethorphan.<ref name="DXMdualprobe" /> | |||
| A number of other CYP enzymes are implicated as minor pathways of dextromethorphan metabolism. CYP2D6 is more effective than CYP3A4 at N-demethylation of dextromethorphan, but since the average individual has a much lower CYP2D6 content in the liver compared to CYP3A4, most N-demethylation of dextromethorphan is catalyzed by CYP3A4.<ref name="DXMdualprobe" /> | |||
| ==Chemistry== | ==Chemistry== | ||
| Dextromethorphan is the ] ] of ], which is the methyl ] of ], both ] ]s. It is named according to ] rules as (+)-3-methoxy-17-methyl-9α,13α,14α-]. As its pure form, dextromethorphan occurs as an odorless, opalescent white powder. It is freely soluble in ] and insoluble in ]; the hydrobromide salt is water-soluble up to 1.5 g/100 mL at 25 °C.<ref>{{cite web|url=http://www.inchem.org/documents/pims/pharm/pim179.htm|title=Dextromethorphan (PIM 179)|website=www.inchem.org|archive-url=https://web.archive.org/web/20170310190514/http://www.inchem.org/documents/pims/pharm/pim179.htm|archive-date=2017-03-10|url-status=live|access-date=2018-03-24}}</ref> Dextromethorphan is commonly available as the monohydrated hydrobromide salt, and is also available in extended-release formulations (sold as dextromethorphan polistirex) contain dextromethorphan bound to an ] based on ].{{cn|date=January 2024}} Dextromethorphan's ] in water is +27.6° (20 °C, Sodium D-line).{{Citation needed|date=March 2011}} | |||
| Dextromethorphan is a ] of the methyl ether ] ] of ], a narcotic (]) ] (and DXM itself is an enantiomeric isomer, that is, a mirror image in the 3D space, of ], a substance considered an opioid<ref>{{cite web |author=Shulgin, Alexander|year=2003| title=DXM (Dextromethorphan) | work= | url=http://www.cognitiveliberty.org/shulgin/adsarchive/dxm.htm | accessdate=2006-05-31}}</ref>). It is chemically named as ''3-methoxy-17-methyl-9(alpha), 13(alpha), 14(alpha)-morphinan hydrobromide monohydrate''. DXM occurs as white ], is sparingly soluble in water, and freely soluble in ]. The drug is ] in water (at 20 degrees ], Sodium D-line) with a specific rotation of +27.6 degrees. | |||
| == |
===Synthesis=== | ||
| Several routes exist for the synthesis of Dextromethorphan. Even though many of the syntheses have been known since the middle of the 20th century, researchers are still working today to further develop the synthesis of Dextromethorphan and, for example, to make it more ]. | |||
| The FDA approved dextromethorphan for over-the-counter sale as a cough suppressant in 1958. This filled the need for a cough suppressant lacking the abuse liability and addictive properties of ] phosphate, the most widely used cough medication at the time. The advantage of dextromethorphan preparations over those containing codeine (now prescription only in the ]) was the lack of physical addiction potential and sedative side-effects, although as with most cough supressants, studies show that its effectiveness is highly debatable. See also: ] | |||
| This includes the synthesis by means of ionic liquids.{{cn|date=August 2022}} | |||
| ==Pharmacodynamics== | |||
| At therapeutic doses, the drug acts ] to elevate the threshold for coughing, without inhibiting ] activity. Dextromethorphan is rapidly absorbed from the gastrointestinal tract, and exerts its activity within 15 to 60 minutes of ingestion. The duration of action after oral administration is approximately three to eight hours. Because administration of DXM can be accompanied by ] release, its use in ] children is very limited. | |||
| ====Racemate separation==== | |||
| The average dosage necessary for effective antitussive therapy is between 10mg and 30mg every four to six hours. | |||
| Since only one of the stereoisomers has the desired effect, the separation of a racemic mixture of hydroxy N- methyl morphinan using tartaric acid and subsequent methylation of the hydroxyl group is a suitable method. By using (D)-tartrate, the (+)-isomer remains as the product. | |||
| ] | |||
| This synthetic pathway was patented by ] in 1950. | |||
| ====Traditional synthesis==== | |||
| According to the ] committee on Drug Dependence, dextromethorphan, when used recreationally (see ]), does not produce ] but can generate slight psychological dependence in some users. | |||
| The traditional synthetic route uses Raney nickel and has been further improved over time, for example by the use of ibuprofen and AlCl<sub>3</sub>. | |||
| ] | |||
| ==Clinical pharmacology== | |||
| {{no references}} | |||
| Overall, it is a cost-effective method with moderate reaction conditions that is easy to handle and suitable for industrial production.{{cn|date=August 2022}} | |||
| Following oral administration, dextromethorphan is rapidly absorbed from the gastrointestinal tract, where it enters the bloodstream and crosses the ]. The first-pass through the hepatic portal vein results in some of the drug being metabolized into an active ] of dextromethorphan, ], the 3-hydroxy derivative of dextromethorphan. The therapeutic activity of dextromethorphan is believed to be caused by both the drug and this metabolite. Dextromethorphan is metabolized by various liver ]s and subsequently undergoes O-demethylation (producing dextrorphan), N-demethylation, and partial conjugation with glucuronic acid and sulfate ions. Hours after dextromethorphan therapy, (in humans) the metabolites (+)-3-hydroxy-N-methylmorphinan, (+)-3-morphinan, and traces of the unchanged drug are detectable in the ]. | |||
| ====Grewe's cyclization==== | |||
| One well known metabolic catalyst involved is a specific ] ] known as 2D6, or ]. A significant portion of the population has a functional deficiency in this enzyme (and are known as poor CYP2D6 metabolizers). As CYP2D6 is the primary ] in the inactivation of dextromethorphan, the duration of action and effects of dextromethorphan are significantly increased in such poor metabolizers. Deaths and hospitalizations have been reported in recreational use by poor CYP2D6 metabolizers.{{verify source}} | |||
| ] | |||
| Grewe's cyclization is easier to handle in terms of the chemicals used, produces higher yields and higher purity of the product.<ref>{{cite journal| vauthors = Meuzelaar GJ, Neeleman E, Maat L, Sheldon RA |date=2010-06-17|title=ChemInform Abstract: A Novel Synthesis of Substituted 1-Benzyloctahydroisoquinolines by Acid-Catalyzed Cyclization of N--N-styrylformamides. |journal= ChemInform |volume=30 | issue=5 |pages=no |doi=10.1002/chin.199905129 |issn=0931-7597}}</ref> | |||
| A large number of medications (including ]s) are potent inhibitors of CYP2D6 ''(see ] article)''. There exists, therefore, the potential of drug-drug interactions between dextromethorphan and concomitant medications. There have been reports of fatal consequences arising from such interactions. | |||
| ====Improved Grewe's cyclization==== | |||
| Dextromethorphan crosses the ], and the following pharmacological actions have been reported: | |||
| Formylation of octabase prior to cyclization avoids ether cleavage as a side reaction and yields higher than without N-substitution or N-methylation. In this example, the purification was done by formation of a brucine salt.{{cn|date=August 2022}} | |||
| ] | |||
| * ] ] ] | |||
| * ] ] inhibitor<ref name="http://www.nhtsa.dot.gov/people/injury/research/job185drugs/dextromethorphan.htm"></ref> | |||
| This process has also been patented by Roche. | |||
| * ] and ] receptor agonist (Zhou & Musacchio, 1991) | |||
| * α3β4 ] receptor antagonist<ref name="http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10869398"></ref> | |||
| * ] ] inhibitor<ref name="http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=1636059"></ref> | |||
| ==History== | ==History== | ||
| The ] parent compound ] was first described in a Swiss and US patent application from ] in 1946 and 1947, respectively; a patent was granted in 1950. A resolution of the two isomers of racemorphan with ] was published in 1952,<ref name="Morris">{{cite journal | vauthors = Morris H, Wallach J | title = From PCP to MXE: a comprehensive review of the non-medical use of dissociative drugs | journal = Drug Testing and Analysis | volume = 6 | issue = 7–8 | pages = 614–632 | year = 2014 | pmid = 24678061 | doi = 10.1002/dta.1620 }}</ref> and dextromethorphan was successfully tested in 1954 as part of ] and ]-funded research on nonaddictive substitutes for ].<ref>{{cite web|url=http://www.esd.whs.mil/Portals/54/Documents/FOID/Reading%20Room/NCB/02-A-0846_RELEASE.pdf|title=Memorandum for the Secretary of Defense|archive-url=https://web.archive.org/web/20170916010918/http://www.esd.whs.mil/Portals/54/Documents/FOID/Reading%20Room/NCB/02-A-0846_RELEASE.pdf|archive-date=2017-09-16|url-status=live|access-date=2013-07-28}}</ref>{{Primary source inline|date=July 2024}} Dextromethorphan was approved by the FDA in 1958 as an ] antitussive.<ref name=Morris/> As had been initially hoped, dextromethorphan was a solution for some of the problems associated with the use of ] as a cough suppressant, such as sedation and ], but like the dissociative anesthetics ] and ], dextromethorphan later became associated with nonmedical use.<ref name="Morris"/><ref name="cesar">{{cite web|url=http://www.cesar.umd.edu/cesar/drugs/dxm.asp|title=Dextromethorphan (DXM)|publisher=Cesar.umd.edu|archive-url=https://web.archive.org/web/20180106073947/http://www.cesar.umd.edu/cesar/drugs/dxm.asp|archive-date=2018-01-06|access-date=2013-07-28}}</ref> | |||
| Dextromethorphan was first patented with {{US patent|2,676,177}}, and was approved for over-the-counter purchase as an antitussive in ]. | |||
| During the 1960s and 1970s, dextromethorphan became available in an over-the-counter tablet form by the brand name Romilar. In 1973, Romilar was taken off the shelves after a burst in sales because of frequent misuse. A few years later, products with an unpleasant taste were introduced (such as Robitussin, Vicks-44, and Dextrotussion), but later the same manufacturers began producing products with a better taste.<ref name="cesar"/> The advent of widespread internet access in the 1990s allowed users to rapidly disseminate information about dextromethorphan, and online discussion groups formed around use and acquisition of the drug. As early as 1996, dextromethorphan hydrobromide powder could be purchased in bulk from online retailers, allowing users to avoid consuming dextromethorphan in syrup preparations.<ref name=Morris/> | |||
| During the 1960s and 1970s, DXM became available in an ] tablet form by the brand name Romilar. It was put on the shelves in hopes of cutting down on ] cough remedies. | |||
| In 1973, Romilar was taken off the shelves after a burst in sales due to common recreational use. | |||
| It was then replaced by ], in an attempt to cut down on recreational usage. | |||
| FDA panels considered moving dextromethorphan to prescription status due to its potential for abuse, but voted against the recommendation in September 2010, citing lack of evidence that making it prescription-only would curb abuse.<ref>Nordqvist C. FDA panel: cough meds should stay over the counter. Sept. 14, 2010. Medical News Today Website. http://www.medicalnewstoday.com/articles/201227.php. Accessed May 18, 2020. </ref> Some states have restricted the sale of dextromethorphan to adults or put other restrictions on its purchase in place, similar to those for ]. As of January 1, 2012, dextromethorphan is prohibited for sale to minors in the State of California and in the State of Oregon as of January 1, 2018, except with a doctor's prescription.<ref name="urlwww.leginfo.ca.gov">{{cite web|url=http://www.leginfo.ca.gov/pub/11-12/bill/sen/sb_0501-0550/sb_514_bill_20110831_chaptered.pdf|title=Senate Bill No. 514|work=An act to add Sections 11110 and 11111 to the Health and Safety Code, relating to nonprescription drugs.|publisher=State of California, Legislative Counsel|archive-url=https://web.archive.org/web/20180308012049/http://www.leginfo.ca.gov/pub/11-12/bill/sen/sb_0501-0550/sb_514_bill_20110831_chaptered.pdf|archive-date=2018-03-08|url-status=live}}</ref> Several ] have also begun regulating sales of dextromethorphan to minors. | |||
| ==Fibromyalgia Treatment== | |||
| Dextromethorphan is currently being investigated as a potential treatment for ] symptoms. | |||
| In ], the ] (BPOM-RI) prohibited single-component dextromethorphan drug sales with or without prescription. Indonesia is the only country that makes single-component dextromethorphan illegal over the counter and by prescription<ref>{{cite web | title = BPOM Tetap Batalkan Izin Edar Obat Dekstrometorfan | trans-title = BPOM Still Cancels Dextromethorphan Drug Distribution Permit | language = Indonesian | date = 22 May 2014 | work = VIVAnews | url = http://nasional.news.viva.co.id/news/read/506418-bpom-tetap-batalkan-izin-edar-obat-dekstrometorfan | archive-url = https://web.archive.org/web/20150528120821/http://nasional.news.viva.co.id/news/read/506418-bpom-tetap-batalkan-izin-edar-obat-dekstrometorfan |archive-date=2015-05-28 }}</ref> and violators may be prosecuted by law. ] has threatened to revoke pharmacies' and drug stores' licenses if they still stock dextromethorphan, and will notify the police for criminal prosecution.<ref>{{cite web|url=http://daerah.sindonews.com/read/878465/21/bnn-ancam-tutup-apotek-penjual-dextromethorphan-1404129585%5B%5D|title=SINDOnews {{!}} Berita Daerah Dan Provinsi Di Indonesia|website=daerah.sindonews.com|language=id-ID|access-date=2017-12-10}} {{Dead link|date=March 2018}}</ref> As a result of this regulation, 130 medications have been withdrawn from the market, but those containing multicomponent dextromethorphan can still be sold over the counter.<ref>{{cite web|url=http://www.pom.go.id/files/edaran_dektrome_2013.pdf|title=Pimpinan dan Apoteker Penanggung Jawab|archive-url=https://web.archive.org/web/20170810021225/http://www.pom.go.id/files/edaran_dektrome_2013.pdf|archive-date=2017-08-10}}</ref><ref>{{cite web|url=http://www.pom.go.id/new/index.php/view/pers/231/Penjelasan-Terkait-Produk-Obat-Batuk-yang-Beredar--dan--Mengandung-Bahan-Dekstrometorfan-Tunggal-.html|title=Badan Pengawas Obat dan Makanan{{dash}}Republik Indonesia|website=www.pom.go.id|language=en-US|archive-url=https://web.archive.org/web/20170203184008/http://www.pom.go.id/new/index.php/view/pers/231/Penjelasan-Terkait-Produk-Obat-Batuk-yang-Beredar--dan--Mengandung-Bahan-Dekstrometorfan-Tunggal-.html|archive-date=2017-02-03|access-date=2017-12-10}}</ref> | |||
| ==Recreational use== | |||
| {{no references}} | |||
| {{main|non-medical use of dextromethorphan}} | |||
| ==Society and culture== | |||
| Since their introduction, preparations containing the ] drug dextromethorphan have been used in a manner ], often as a recreational drug or to induce intoxication (sometimes referred to as "robo-tripping"). Dextromethorphan has little to no psychological effect in the doses used medically, however alteration of consciousness generally occurs following ingestion of approximately 7 to 50 times the therapeutic dose over a relatively short period of time. <ref name=Jones>{{cite journal | author =Jones K, Taranto M | year =2006 | title = Illicit Drug Manual: Dextromethorphan ("Robo-tripping") | journal =collegehealth-e | volume =1 | issue =4 | pages =13-17 }} }}</ref> | |||
| ===Marketing=== | |||
| People who study the specific effects of psychotropic substances classify DXM as a ], a major subclass of hallucinogenic drugs, along with ] and ]. It generally does not produce withdrawal symptoms characteristic of ] substances, but ] has been reported by some users. | |||
| It may be used in ] labels and ]s, ] DM, Mucinex DM, Camydex-20 tablets, Robitussin, Komix DT, ], ], ], ], Delsym, ], Charcoal D, Cinfatós and others. It has been used in counterfeit medications.{{citation needed|date=August 2023}} | |||
| ===Recreational use=== | |||
| However, many chemical dependency treatment centers have been reporting many young people addicted to DXM. Their withdrawal symptoms were documented as being identical to those of the illegal opiate, ].<ref name="http://www.insightrecovery.org/"></ref> | |||
| ] | |||
| {{Main|Recreational use of dextromethorphan}} | |||
| ] preparations containing dextromethorphan have been used in manners inconsistent with their labeling, often as a recreational drug.<ref name="cesar" /> At doses much higher than medically recommended, dextromethorphan and its major metabolite, ], acts as an ], which produces dissociative ] states somewhat similar to ] and ].<ref name=dea1>{{cite web |url=http://www.deadiversion.usdoj.gov/drugs_concern/dextro_m/dextro_m.pdf |archive-url=https://web.archive.org/web/20121016221008/http://www.deadiversion.usdoj.gov/drugs_concern/dextro_m/dextro_m.pdf |url-status=dead |archive-date=2012-10-16 |title=Dextromethorphan |work=Drugs and Chemicals of Concern |publisher=] |date=August 2010 }}</ref> | |||
| DXM, when consumed in low recreational doses (usually under 100mg), is often described as having a buoyant, vaguely psychedelic effect similar to a mixture of ], opiates, and nitrous oxide. With higher doses, intense euphoria and vivid imagination may occur as bizarre feelings of ] increase. With very high doses, profound alterations in consciousness have been noted, and users often report out of body experiences or temporary psychosis. In 1981, a paper by Gosselin estimated the lethal dose between 50 and 500 mg/kg. | |||
| It may produce distortions of the visual field, feelings of ], distorted bodily perception, excitement, and a loss of sense of time. Some users report stimulant-like ], particularly in response to music. Dextromethorphan usually provides its recreational effects in a non-linear fashion, so that they are experienced in significantly varied stages. These stages are commonly referred to as "plateaus". These plateaus are numbered from one to four, with the first having the mildest effects to fourth being the most intense. Each plateau is said to come with different related effects and experiences.<ref name="Giannini_1997">{{cite book | vauthors = Giannini AJ | title = Drugs of abuse | date = 1997 | publisher = Practice Management Information Corp. | location = Los Angeles | isbn = 1570660530 | edition = 2nd }}{{Page needed|date=March 2011}}</ref> | |||
| Individual reactions to recreational doses of Dextromethorphan vary widely. Some find the effects of the drug to be immensely pleasurable, similar to a combination of opiates and hallucinogens, while others find that the drug produces dysphoria, panic, or dread. | |||
| The first plateau is said to induce music euphoria and mild stimulation, likened to that of ]. The second plateau is likened to a state of being on moderate amounts of ] and ] at the same time, featuring euphoria, sedation and minor hallucinations. The third plateau induces a significant dissociative state which can often cause anxiety in users. Reaching the fourth plateau is said to cause extreme sedation and a significant hallucinatory state as well as complete dissociation from reality. Teenagers tend to have a higher likelihood to use dextromethorphan-related drugs as they are easier to access; youths and young adults with psychiatric disorders are at risk of abusing the drug.<ref>{{cite journal| vauthors = Akerman SC, Hammel JL, Brunette MF |date=2010-12-20|title=Dextromethorphan Abuse and Dependence in Adolescents|journal=Journal of Dual Diagnosis|volume=6|issue=3–4|pages=266–278|doi=10.1080/15504263.2010.537515|s2cid=70666093}}</ref> | |||
| Physical side effects that can occur after ingestion of recreational doses of DXM include a blotchy skin rash, itching (sometimes referred to as "robo itch," short for "Robitussin itch"), and sweating. Many people vomit from recreational doses or feel ill for the first part of the “trip”. When taken in higher doses, side effects can include dilated pupils and loss of appetite, as well as shakiness. | |||
| == |
==Research== | ||
| The combination drug ] (AVP-923),<ref name="pmid20373255">{{cite journal | vauthors = Olney N, Rosen H | title = AVP-923, a combination of dextromethorphan hydrobromide and quinidine sulfate for the treatment of pseudobulbar affect and neuropathic pain | journal = IDrugs | volume = 13 | issue = 4 | pages = 254–265 | date = April 2010 | pmid = 20373255 | doi = }}</ref><ref name="pmid33615952">{{cite journal | vauthors = Khoury R, Marx C, Mirgati S, Velury D, Chakkamparambil B, Grossberg GT | title = AVP-786 as a promising treatment option for Alzheimer's Disease including agitation | journal = Expert Opinion on Pharmacotherapy | volume = 22 | issue = 7 | pages = 783–795 | date = May 2021 | pmid = 33615952 | doi = 10.1080/14656566.2021.1882995 | s2cid = 231987025 }}</ref> traditionally used to treat ], is under investigation for the treatment of a variety of other neurological and neuropsychiatric conditions including ] associated with ], among others.<ref name="NguyenThomas2016" /><ref>{{cite journal | vauthors = Murrough JW, Wade E, Sayed S, Ahle G, Kiraly DD, Welch A, Collins KA, Soleimani L, Iosifescu DV, Charney DS | title = Dextromethorphan/quinidine pharmacotherapy in patients with treatment resistant depression: A proof of concept clinical trial | journal = Journal of Affective Disorders | volume = 218 | pages = 277–283 | date = August 2017 | pmid = 28478356 | doi = 10.1016/j.jad.2017.04.072 | s2cid = 46777150 }}</ref> In 2013, a randomized clinical trial found that dextromethorphan may reduce the overall discomfort and duration of withdrawal symptoms associated with ]. When combined with ], dextromethorphan reduced the overall time needed for withdrawal symptoms to peak by 24 hours while reducing severity of symptoms compared to clonidine alone.<ref>{{cite journal | vauthors = Malek A, Amiri S, Habibi Asl B | title = The therapeutic effect of adding dextromethorphan to clonidine for reducing symptoms of opioid withdrawal: a randomized clinical trial | journal = ISRN Psychiatry | volume = 2013 | pages = 546030 | year = 2013 | pmid = 23864983 | pmc = 3706070 | doi = 10.1155/2013/546030 | doi-access = free | title-link = doi }}</ref> | |||
| * ] | |||
| **] | |||
| **] | |||
| **] | |||
| **'']'' | |||
| * ] | |||
| ==References== | ==References== | ||
| {{Reflist}} | |||
| <references/> | |||
| ==External links== | ==External links== | ||
| {{Commons category-inline}} | |||
| * | |||
| * | |||
| * | |||
| * | |||
| {{ChemicalSources}} | |||
| {{Antitussives}} | |||
| {{Navboxes | |||
| ] | |||
| | title = ] | |||
| ] | |||
| | titlestyle = background:#ccccff | |||
| ] | |||
| | list1 = | |||
| ] | |||
| {{Drug use}} | |||
| ] | |||
| {{Hallucinogens}} | |||
| ] | |||
| }} | |||
| {{Navboxes | |||
| | title = ] | |||
| | titlestyle = background:#ccccff | |||
| | list1 = | |||
| {{Ionotropic glutamate receptor modulators}} | |||
| {{Monoamine reuptake inhibitors}} | |||
| {{Nicotinic acetylcholine receptor modulators}} | |||
| {{Opioid receptor modulators}} | |||
| {{Sigma receptor modulators}} | |||
| }} | |||
| {{Portal bar | Medicine}} | |||
| {{Authority control}} | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 01:01, 25 December 2024
Cough suppressant, antidepressant, and dissociative drug Not to be confused with Dextrorphan, Dexamethasone, or Dextroamphetamine.Pharmaceutical compound
Dextromethorphan, sold under the brand name Robitussin among others, is a cough suppressant used in many cough and cold medicines. In 2022, the US Food and Drug Administration (FDA) approved the combination dextromethorphan/bupropion to serve as a rapid-acting antidepressant in people with major depressive disorder.
It is in the morphinan class of medications with sedative, dissociative, and stimulant properties (at lower doses). Dextromethorphan does not have a significant affinity for the mu-opioid receptor activity typical of morphinan compounds and exerts its therapeutic effects through several other receptors. In its pure form, dextromethorphan occurs as a white powder.
When exceeding approved dosages, dextromethorphan acts as a dissociative hallucinogen. It has multiple mechanisms of action, including actions as a nonselective serotonin reuptake inhibitor and a sigma-1 receptor agonist. Dextromethorphan and its major metabolite, dextrorphan, also block the NMDA receptor at high doses, which produces effects similar to other dissociative anesthetics such as ketamine, nitrous oxide, and phencyclidine.
It was patented in 1949 and approved for medical use in 1953. In 2022, the combination with promethazine was the 260th most commonly prescribed medication in the United States, with more than 1 million prescriptions. In 2022, the combination with brompheniramine and pseudoephedrine was the 265th most commonly prescribed medication in the United States, with more than 1 million prescriptions.
Medical uses

Cough suppression
The primary use of dextromethorphan is as a cough suppressant, for the temporary relief of cough caused by minor throat and bronchial irritation (such as commonly accompanies the flu and common cold), or from inhaled particle irritants, as well as chronic cough at a higher dosage. Dextromethorphan is available alone in the form of cough syrup and pills as well as in combination with other agents.
Pseudobulbar affect
In 2010, the FDA approved the combination drug dextromethorphan/quinidine under the brand name Nuedexta for the treatment of pseudobulbar affect (uncontrollable laughing/crying). Dextromethorphan is the active therapeutic agent in the combination; quinidine merely serves to inhibit the enzymatic degradation of dextromethorphan and thereby increase its circulating concentrations via inhibition of CYP2D6.
Major depressive disorder
The combination medicine dextromethorphan/bupropion is approved for major depressive disorder under the brand name Auvelity.
Contraindications
| This section is empty. You can help by adding to it. (November 2024) |
Adverse effects
Side effects of dextromethorphan at normal therapeutic doses can include:
- Body rash/itching
- Nausea
- Vomiting
- Drowsiness
- Dizziness
- Constipation
- Diarrhea
- Sedation
- Confusion
- Anxiety
- Closed-eye hallucinations
A rare side effect is respiratory depression.
Neurotoxicity
Dextromethorphan was once thought to cause Olney's lesions when administered intravenously; however, this was later proven inconclusive, due to lack of research on humans. Tests were performed on rats, giving them 50 mg or more every day for as long as a month. Neurotoxic changes, including vacuolation, have been observed in posterior cingulate and retrosplenial cortices of rats administered other NMDA receptor antagonists such as PCP, but not with dextromethorphan.
Dependence and withdrawal
In many documented cases, dextromethorphan has produced psychological dependence in people who used it recreationally. It is considered less addictive than other common cough suppressants, such as the opiate codeine. Since dextromethorphan also acts as a serotonin reuptake inhibitor, users report that regular recreational use over a long period of time can cause withdrawal symptoms similar to those of antidepressant discontinuation syndrome. Additionally, disturbances have been reported in sleep, senses, movement, mood, and thinking.
Overdose
Adverse effects of dextromethorphan in overdose at doses 3 to 10 times the recommended therapeutic dose:
- Nausea
- Restlessness
- Insomnia
- Rapid speech
- Dilated pupils
- Glassy eyes
- Dizziness
At doses 11 to 75 times the recommended therapeutic dose:
- Hallucinations
- Dissociation
- Vomiting
- Blurred vision
- Double vision
- Red eyes
- Dilated pupils
- Sweating
- Fever
- Bruxism (teeth grinding)
- Hypotension
- Hypertension
- Tachycardia
- Hypoventilation
- Diarrhea
- Urinary retention
- Muscle spasms
- Sedation
- Paresthesia
- Blackouts
- Inability to focus eyes
- Rash
- Severe itchiness
- Psychosis
Episodic acute psychosis can occur when high doses of dextromethorphan are taken for recreational use, and an abundance of psychiatric symptoms can result, including dissociation and other PCP-like symptoms.
Interactions
Serotonin syndrome may result from the combined use of dextromethorphan and serotonergic antidepressants such as selective serotonin reuptake inhibitor (SSRIs) or monoamine oxidase inhibitor (MAOIs). Further research is needed to determine whether doses of dextromethorphan beyond those normally used therapeutically are needed to produce this effect. In any case, dextromethorphan should not be taken with MAOIs due to the possibility of this complication. Serotonin syndrome is a potentially life-threatening condition that can occur rapidly, due to a buildup of an excessive amount of serotonin in the body.
Combining alcohol with dextromethorphan significantly increases the risk of overdose and other severe health complications, according to the NIAAA.
Compounds in grapefruit affect a number of drugs, including dextromethorphan, through the inhibition of the cytochrome P450 system in the liver, and can lead to excessive accumulation of the drug which both increases and prolongs effects. Grapefruit and grapefruit juices (especially white grapefruit juice, but also including other citrus fruits such as bergamot and lime, as well as a number of noncitrus fruits) generally are recommended to be avoided while using dextromethorphan and numerous other medications.
Pharmacology
Pharmacodynamics
| Site | DXM | DXOTooltip Dextrorphan | Species | Ref |
|---|---|---|---|---|
| NMDAR (MK-801) |
2,120–8,945 | 486–906 | Rat | |
| σ1 | 142–652 | 118–481 | Rat | |
| σ2 | 11,060–22,864 | 11,325–15,582 | Rat | |
| MORTooltip μ-Opioid receptor | 1,280 ND |
420 >1,000 |
Rat Human |
|
| DORTooltip δ-Opioid receptor | 11,500 | 34,700 | Rat | |
| KORTooltip κ-Opioid receptor | 7,000 | 5,950 | Rat | |
| SERTTooltip Serotonin transporter | 23–40 | 401–484 | Rat | |
| NETTooltip Norepinephrine transporter | ≥240 | ≥340 | Rat | |
| DATTooltip Dopamine transporter | >1,000 | >1,000 | Rat | |
| 5-HT1A | >1,000 | >1,000 | Rat | |
| 5-HT1B/1D | 61% at 1 μM | 54% at 1 μM | Rat | |
| 5-HT2A | >1,000 | >1,000 | Rat | |
| α1 | >1,000 | >1,000 | Rat | |
| α2 | 60% at 1 μM | >1,000 | Rat | |
| β | >1,000 | 35% at 1 μM | Rat | |
| D2 | >1,000 | >1,000 | Rat | |
| H1 | >1,000 | 95% at 1 μM | Rat | |
| mAChRsTooltip Muscarinic acetylcholine receptors | >1,000 | 100% at 1 μM | Rat | |
| nAChRsTooltip Nicotinic acetylcholine receptors | 700–8,900 (IC50) |
1,300–29,600 (IC50) |
Rat | |
| VDSCsTooltip Voltage-dependent sodium channels | >50,000 (IC50) | ND | Rat | |
| Values are Ki (nM), unless otherwise noted. The smaller the value, the more strongly the drug binds to the site. | ||||
Dextromethorphan has been found to possess the following actions (<1 μM) using rat tissues:
- Uncompetitive antagonist of the NMDA receptor via the MK-801/PCPTooltip phencyclidine site
- SERTTooltip Serotonin transporter and NETTooltip norepinephrine transporter blocker (cf. serotonin–norepinephrine reuptake inhibitor)
- Sigma σ1 receptor agonist
- Negative allosteric modulator of nicotinic acetylcholine receptors
- Ligand of the serotonin 5-HT1B/1D, histamine H1, α2-adrenergic, and muscarinic acetylcholine receptors
Dextromethorphan is a prodrug of dextrorphan, which is the actual mediator of most of its dissociative effects through acting as a more potent NMDA receptor antagonist than dextromethorphan itself. What role, if any, (+)-3-methoxymorphinan, dextromethorphan's other major metabolite, plays in its effects is not entirely clear.
Pharmacokinetics
Following oral administration, dextromethorphan is rapidly absorbed from the gastrointestinal tract, where it enters the bloodstream and crosses the blood–brain barrier.
At therapeutic doses, dextromethorphan acts centrally (meaning that it acts on the brain) as opposed to locally (on the respiratory tract). It elevates the threshold for coughing, without inhibiting ciliary activity. Dextromethorphan is rapidly absorbed from the gastrointestinal tract and converted into the active metabolite dextrorphan in the liver by the cytochrome P450 enzyme CYP2D6. The average dose necessary for effective antitussive therapy is between 10 and 45 mg, depending on the individual. The International Society for the Study of Cough recommends "an adequate first dose of medication is 60 mg in the adult and repeat dosing should be infrequent rather than qds recommended."
Dextromethorphan has an elimination half-life of approximately 4 hours in individuals with an extensive metabolizer phenotype; this is increased to approximately 13 hours when dextromethorphan is given in combination with quinidine. The duration of action after oral administration is about three to eight hours for dextromethorphan hydrobromide, and 10 to 12 hours for dextromethorphan polistirex. Around one in 10 of the Caucasian population has little or no CYP2D6 enzyme activity, leading to long-lived high drug levels.
Metabolism

The first pass through the hepatic portal vein results in some of the drug being metabolized by O-demethylation into an active metabolite of dextromethorphan called dextrorphan, the 3-hydroxy derivative of dextromethorphan. The therapeutic activity of dextromethorphan is believed to be caused by both the drug and this metabolite. Dextromethorphan also undergoes N-demethylation (to 3-methoxymorphinan or MEM), and partial conjugation with glucuronic acid and sulfate ions. Hours after dextromethorphan therapy, (in humans) the metabolites (+)-3-hydroxy-N-methylmorphinan, (+)-3-morphinan, and traces of the unchanged drug are detectable in the urine.
A major metabolic catalyst involved is the cytochrome P450 enzyme known as 2D6, or CYP2D6. A significant portion of the population has a functional deficiency in this enzyme and are known as poor CYP2D6 metabolizers. O-demethylation of dextromethorphan to dextrorphan contributes to at least 80% of the dextrorphan formed during dextromethorphan metabolism. As CYP2D6 is a major metabolic pathway in the inactivation of dextromethorphan, the duration of action and effects of dextromethorphan can be increased by as much as three times in such poor metabolizers. In one study on 252 Americans, 84.3% were found to be "fast" (extensive) metabolizers, 6.8% to be "intermediate" metabolizers, and 8.8% were "slow" metabolizers of dextromethorphan. A number of alleles for CYP2D6 are known, including several completely inactive variants. The distribution of alleles is uneven amongst ethnic groups.
A large number of medications are potent inhibitors of CYP2D6. Some types of medications known to inhibit CYP2D6 include certain SSRIs and tricyclic antidepressants, some antipsychotics, and the commonly available antihistamine diphenhydramine. Therefore, the potential for interactions exists between dextromethorphan and medications that inhibit this enzyme, particularly in slow metabolizers.
Dextromethorphan is also metabolized by CYP3A4. N-demethylation is primarily accomplished by CYP3A4, contributing to at least 90% of the MEM formed as a primary metabolite of dextromethorphan.
A number of other CYP enzymes are implicated as minor pathways of dextromethorphan metabolism. CYP2D6 is more effective than CYP3A4 at N-demethylation of dextromethorphan, but since the average individual has a much lower CYP2D6 content in the liver compared to CYP3A4, most N-demethylation of dextromethorphan is catalyzed by CYP3A4.
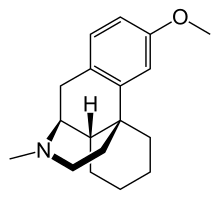
Chemistry
Dextromethorphan is the dextrorotatory enantiomer of levomethorphan, which is the methyl ether of levorphanol, both opioid analgesics. It is named according to IUPAC rules as (+)-3-methoxy-17-methyl-9α,13α,14α-morphinan. As its pure form, dextromethorphan occurs as an odorless, opalescent white powder. It is freely soluble in chloroform and insoluble in water; the hydrobromide salt is water-soluble up to 1.5 g/100 mL at 25 °C. Dextromethorphan is commonly available as the monohydrated hydrobromide salt, and is also available in extended-release formulations (sold as dextromethorphan polistirex) contain dextromethorphan bound to an ion-exchange resin based on polystyrene sulfonic acid. Dextromethorphan's specific rotation in water is +27.6° (20 °C, Sodium D-line).
Synthesis
Several routes exist for the synthesis of Dextromethorphan. Even though many of the syntheses have been known since the middle of the 20th century, researchers are still working today to further develop the synthesis of Dextromethorphan and, for example, to make it more environmentally friendly.
This includes the synthesis by means of ionic liquids.
Racemate separation
Since only one of the stereoisomers has the desired effect, the separation of a racemic mixture of hydroxy N- methyl morphinan using tartaric acid and subsequent methylation of the hydroxyl group is a suitable method. By using (D)-tartrate, the (+)-isomer remains as the product.
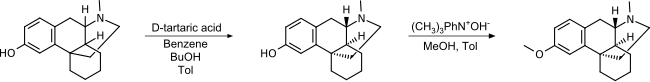
This synthetic pathway was patented by Roche in 1950.
Traditional synthesis
The traditional synthetic route uses Raney nickel and has been further improved over time, for example by the use of ibuprofen and AlCl3.

Overall, it is a cost-effective method with moderate reaction conditions that is easy to handle and suitable for industrial production.
Grewe's cyclization

Grewe's cyclization is easier to handle in terms of the chemicals used, produces higher yields and higher purity of the product.
Improved Grewe's cyclization
Formylation of octabase prior to cyclization avoids ether cleavage as a side reaction and yields higher than without N-substitution or N-methylation. In this example, the purification was done by formation of a brucine salt.

This process has also been patented by Roche.
History
The racemic parent compound racemorphan was first described in a Swiss and US patent application from Hoffmann-La Roche in 1946 and 1947, respectively; a patent was granted in 1950. A resolution of the two isomers of racemorphan with tartaric acid was published in 1952, and dextromethorphan was successfully tested in 1954 as part of US Navy and CIA-funded research on nonaddictive substitutes for codeine. Dextromethorphan was approved by the FDA in 1958 as an over-the-counter antitussive. As had been initially hoped, dextromethorphan was a solution for some of the problems associated with the use of codeine phosphate as a cough suppressant, such as sedation and opiate dependence, but like the dissociative anesthetics phencyclidine and ketamine, dextromethorphan later became associated with nonmedical use.
During the 1960s and 1970s, dextromethorphan became available in an over-the-counter tablet form by the brand name Romilar. In 1973, Romilar was taken off the shelves after a burst in sales because of frequent misuse. A few years later, products with an unpleasant taste were introduced (such as Robitussin, Vicks-44, and Dextrotussion), but later the same manufacturers began producing products with a better taste. The advent of widespread internet access in the 1990s allowed users to rapidly disseminate information about dextromethorphan, and online discussion groups formed around use and acquisition of the drug. As early as 1996, dextromethorphan hydrobromide powder could be purchased in bulk from online retailers, allowing users to avoid consuming dextromethorphan in syrup preparations.
FDA panels considered moving dextromethorphan to prescription status due to its potential for abuse, but voted against the recommendation in September 2010, citing lack of evidence that making it prescription-only would curb abuse. Some states have restricted the sale of dextromethorphan to adults or put other restrictions on its purchase in place, similar to those for pseudoephedrine. As of January 1, 2012, dextromethorphan is prohibited for sale to minors in the State of California and in the State of Oregon as of January 1, 2018, except with a doctor's prescription. Several other states have also begun regulating sales of dextromethorphan to minors.
In Indonesia, the National Agency of Drug and Food Control (BPOM-RI) prohibited single-component dextromethorphan drug sales with or without prescription. Indonesia is the only country that makes single-component dextromethorphan illegal over the counter and by prescription and violators may be prosecuted by law. National Anti-Narcotics Agency (BNN RI) has threatened to revoke pharmacies' and drug stores' licenses if they still stock dextromethorphan, and will notify the police for criminal prosecution. As a result of this regulation, 130 medications have been withdrawn from the market, but those containing multicomponent dextromethorphan can still be sold over the counter.
Society and culture
Marketing
It may be used in generic labels and store brands, Benylin DM, Mucinex DM, Camydex-20 tablets, Robitussin, Komix DT, NyQuil, Dimetapp, Vicks, Coricidin, Delsym, TheraFlu, Charcoal D, Cinfatós and others. It has been used in counterfeit medications.
Recreational use

Over-the-counter preparations containing dextromethorphan have been used in manners inconsistent with their labeling, often as a recreational drug. At doses much higher than medically recommended, dextromethorphan and its major metabolite, dextrorphan, acts as an NMDA receptor antagonist, which produces dissociative hallucinogenic states somewhat similar to ketamine and phencyclidine.
It may produce distortions of the visual field, feelings of dissociation, distorted bodily perception, excitement, and a loss of sense of time. Some users report stimulant-like euphoria, particularly in response to music. Dextromethorphan usually provides its recreational effects in a non-linear fashion, so that they are experienced in significantly varied stages. These stages are commonly referred to as "plateaus". These plateaus are numbered from one to four, with the first having the mildest effects to fourth being the most intense. Each plateau is said to come with different related effects and experiences.
The first plateau is said to induce music euphoria and mild stimulation, likened to that of MDMA. The second plateau is likened to a state of being on moderate amounts of alcohol and cannabis at the same time, featuring euphoria, sedation and minor hallucinations. The third plateau induces a significant dissociative state which can often cause anxiety in users. Reaching the fourth plateau is said to cause extreme sedation and a significant hallucinatory state as well as complete dissociation from reality. Teenagers tend to have a higher likelihood to use dextromethorphan-related drugs as they are easier to access; youths and young adults with psychiatric disorders are at risk of abusing the drug.
Research
The combination drug dextromethorphan/quinidine (AVP-923), traditionally used to treat pseudobulbar affect, is under investigation for the treatment of a variety of other neurological and neuropsychiatric conditions including agitation associated with Alzheimer's disease, among others. In 2013, a randomized clinical trial found that dextromethorphan may reduce the overall discomfort and duration of withdrawal symptoms associated with opioid use disorder. When combined with clonidine, dextromethorphan reduced the overall time needed for withdrawal symptoms to peak by 24 hours while reducing severity of symptoms compared to clonidine alone.
References
- "Dextromethorphan Monograph for Professionals". Drugs.com.
- Windhab LG, Gastberger S, Hulka LM, Baumgartner MR, Soyka M, Müller TJ, et al. (2020). "Dextromethorphan Abuse Among Opioid-Dependent Patients". Clinical Neuropharmacology. 43 (5): 127–133. doi:10.1097/WNF.0000000000000403. PMID 32947422. S2CID 221798401.
- McCarthy B (2023-11-30). "Dextromethorphan-bupropion (Auvelity) for the Treatment of Major Depressive Disorder". Clinical Psychopharmacology and Neuroscience. 21 (4): 609–616. doi:10.9758/cpn.23.1081. PMC 10591164. PMID 37859435.
- Kukanich B, Papich MG (October 2004). "Plasma profile and pharmacokinetics of dextromethorphan after intravenous and oral administration in healthy dogs". Journal of Veterinary Pharmacology and Therapeutics. 27 (5): 337–341. doi:10.1111/j.1365-2885.2004.00608.x. PMID 15500572.
- ^ "Balminil DM, Benylin DM (dextromethorphan) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 15 April 2014.
- Dicpinigaitis P (2022-09-12). "The Current and Emerging Treatment Landscape for Chronic Cough". The American Journal of Managed Care. Uncovering the Economic Burden of Chronic Cough and the Promising Role of Emerging Targeted Therapies. 28 (9): S159–S165. doi:10.37765/ajmc.2022.89244. PMID 36198074. S2CID 252736111.
By sales, dextromethorphan is the most widely used OTC antitussive drug in the United States, and approximately 85% to 90% of OTC cough medicines contain dextromethorphan
- Majeed A, Xiong J, Teopiz KM, Ng J, Ho R, Rosenblat JD, et al. (March 2021). "Efficacy of dextromethorphan for the treatment of depression: a systematic review of preclinical and clinical trials". Expert Opinion on Emerging Drugs. 26 (1): 63–74. doi:10.1080/14728214.2021.1898588. PMID 33682569. S2CID 232141396.
- ^ Taylor CP, Traynelis SF, Siffert J, Pope LE, Matsumoto RR (August 2016). "Pharmacology of dextromethorphan: Relevance to dextromethorphan/quinidine (Nuedexta) clinical use". Pharmacology & Therapeutics. 164: 170–182. doi:10.1016/j.pharmthera.2016.04.010. PMID 27139517.
- "Reference Tables: Description and Solubility – D". Archived from the original on 2017-07-04. Retrieved 2011-05-06.
- ^ Schwartz AR, Pizon AF, Brooks DE (September 2008). "Dextromethorphan-induced serotonin syndrome". Clinical Toxicology. 46 (8): 771–773. doi:10.1080/15563650701668625. PMID 19238739. S2CID 37817922.
- Shin EJ, Nah SY, Chae JS, Bing G, Shin SW, Yen TP, et al. (May 2007). "Dextromethorphan attenuates trimethyltin-induced neurotoxicity via sigma1 receptor activation in rats". Neurochemistry International. 50 (6): 791–799. doi:10.1016/j.neuint.2007.01.008. PMID 17386960. S2CID 43230896.
- Shin EJ, Nah SY, Kim WK, Ko KH, Jhoo WK, Lim YK, et al. (April 2005). "The dextromethorphan analog dimemorfan attenuates kainate-induced seizures via sigma1 receptor activation: comparison with the effects of dextromethorphan". British Journal of Pharmacology. 144 (7): 908–918. doi:10.1038/sj.bjp.0705998. PMC 1576070. PMID 15723099.
- Fischer J, Ganellin CR (2006). Analogue-based Drug Discovery. John Wiley & Sons. p. 527. ISBN 9783527607495.
- ^ "The Top 300 of 2022". ClinCalc. Archived from the original on 30 August 2024. Retrieved 30 August 2024.
- "Dextromethorphan; Promethazine Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- "Brompheniramine; Dextromethorphan; Pseudoephedrine Drug Usage Statistics, United States, 2013 - 2022". ClinCalc. Retrieved 30 August 2024.
- Young EC, Smith JA (June 2011). "Pharmacologic therapy for cough". Current Opinion in Pharmacology. 11 (3). Elsevier BV: 224–230. doi:10.1016/j.coph.2011.06.003. PMID 21724464.
- ^ Rossi, S, ed. (2013). Australian Medicines Handbook. Adelaide: The Australian Medicines Handbook Unit Trust. ISBN 978-0-9805790-9-3.
- "Nuedexta- dextromethorphan hydrobromide and quinidine sulfate capsule, gelatin coated". DailyMed. 23 June 2019. Retrieved 23 October 2020.
- ^ Nguyen L, Thomas KL, Lucke-Wold BP, Cavendish JZ, Crowe MS, Matsumoto RR (March 2016). "Dextromethorphan: An update on its utility for neurological and neuropsychiatric disorders". Pharmacology & Therapeutics. 159: 1–22. doi:10.1016/j.pharmthera.2016.01.016. PMID 26826604.
- "Auvelity (dextromethorphan hydrobromide/bupropion hydrochloride)" (PDF). Axsome Therapeutics. Archived (PDF) from the original on 21 August 2022. Retrieved 19 August 2022.
- Keam SJ (November 2022). "Dextromethorphan/Bupropion: First Approval". CNS Drugs. 36 (11): 1229–1238. doi:10.1007/s40263-022-00968-4. PMID 36301443. S2CID 253158902.
- ^ "Dextromethorphan". National Highway Traffic Safety Administration (NHTSA). Archived from the original on 2008-08-01.
- Olney JW, Labruyere J, Price MT (June 1989). "Pathological changes induced in cerebrocortical neurons by phencyclidine and related drugs". Science. 244 (4910): 1360–1362. Bibcode:1989Sci...244.1360O. doi:10.1126/science.2660263. PMID 2660263.
- Carliss RD, Radovsky A, Chengelis CP, O'Neill TP, Shuey DL (July 2007). "Oral administration of dextromethorphan does not produce neuronal vacuolation in the rat brain". Neurotoxicology. 28 (4): 813–818. Bibcode:2007NeuTx..28..813C. doi:10.1016/j.neuro.2007.03.009. PMID 17573115.
- ^ "Teen Drug Abuse: Cough Medicine and DXM (Dextromethorphan)". webmd. Archived from the original on 2017-11-21.
- ^ Martinak B, Bolis RA, Black JR, Fargason RE, Birur B (September 2017). "Dextromethorphan in Cough Syrup: The Poor Man's Psychosis". Psychopharmacology Bulletin. 47 (4): 59–63. PMC 5601090. PMID 28936010.
- Dy P, Arcega V, Ghali W, Wolfe W (August 2017). "Serotonin syndrome caused by drug to drug interaction between escitalopram and dextromethorphan". BMJ Case Reports. 2017: bcr–2017–221486. doi:10.1136/bcr-2017-221486. PMC 5747823. PMID 28784915.
- "Harmful Interactions | National Institute on Alcohol Abuse and Alcoholism (NIAAA)". www.niaaa.nih.gov.
- "Inhibitors of CYP3A4". ganfyd.org. Archived from the original on 2017-07-20. Retrieved 23 August 2013.
- Roth BL, Driscol J. "PDSP Ki Database". Psychoactive Drug Screening Program (PDSP). University of North Carolina at Chapel Hill and the United States National Institute of Mental Health. Retrieved 14 August 2017.
- Werling LL, Keller A, Frank JG, Nuwayhid SJ (October 2007). "A comparison of the binding profiles of dextromethorphan, memantine, fluoxetine and amitriptyline: treatment of involuntary emotional expression disorder". Experimental Neurology. 207 (2): 248–257. doi:10.1016/j.expneurol.2007.06.013. PMID 17689532. S2CID 38476281.
- Raynor K, Kong H, Mestek A, Bye LS, Tian M, Liu J, et al. (January 1995). "Characterization of the cloned human mu opioid receptor". The Journal of Pharmacology and Experimental Therapeutics. 272 (1): 423–428. PMID 7815359.
- Lee JH, Shin EJ, Jeong SM, Lee BH, Yoon IS, Lee JH, et al. (June 2007). "Effects of dextrorotatory morphinans on brain Na+ channels expressed in Xenopus oocytes". European Journal of Pharmacology. 564 (1–3): 7–17. doi:10.1016/j.ejphar.2007.01.088. PMID 17346698.
- Gao XF, Yao JJ, He YL, Hu C, Mei YA (2012). "Sigma-1 receptor agonists directly inhibit Nav1.2/1.4 channels". PLOS ONE. 7 (11): e49384. Bibcode:2012PLoSO...749384G. doi:10.1371/journal.pone.0049384. PMC 3489664. PMID 23139844.
- ^ Burns JM, Boyer EW (2013). "Antitussives and substance abuse". Substance Abuse and Rehabilitation. 4: 75–82. doi:10.2147/SAR.S36761. PMC 3931656. PMID 24648790.
- Chou YC, Liao JF, Chang WY, Lin MF, Chen CF (March 1999). "Binding of dimemorfan to sigma-1 receptor and its anticonvulsant and locomotor effects in mice, compared with dextromethorphan and dextrorphan". Brain Research. 821 (2): 516–519. doi:10.1016/S0006-8993(99)01125-7. PMID 10064839. S2CID 22762264.
- Schmider J, Greenblatt DJ, Fogelman SM, von Moltke LL, Shader RI (April 1997). "Metabolism of dextromethorphan in vitro: involvement of cytochromes P450 2D6 and 3A3/4, with a possible role of 2E1". Biopharmaceutics & Drug Disposition. 18 (3): 227–240. doi:10.1002/(SICI)1099-081X(199704)18:3<227::AID-BDD18>3.0.CO;2-L. PMID 9113345. S2CID 5638973.
- ^ Morice AH. "Cough". International Society for the Study of Cough. Archived from the original on 2017-05-09.
- Strauch K, Lutz U, Bittner N, Lutz WK (August 2009). "Dose-response relationship for the pharmacokinetic interaction of grapefruit juice with dextromethorphan investigated by human urinary metabolite profiles". Food and Chemical Toxicology. 47 (8): 1928–1935. doi:10.1016/j.fct.2009.05.004. PMID 19445995.
- ^ Yu A, Haining RL (November 2001). "Comparative contribution to dextromethorphan metabolism by cytochrome P450 isoforms in vitro: can dextromethorphan be used as a dual probe for both CTP2D6 and CYP3A activities?". Drug Metabolism and Disposition. 29 (11): 1514–1520. PMID 11602530. Archived from the original on 2020-03-12. Retrieved 2015-04-26.
- Capon DA, Bochner F, Kerry N, Mikus G, Danz C, Somogyi AA (September 1996). "The influence of CYP2D6 polymorphism and quinidine on the disposition and antitussive effect of dextromethorphan in humans". Clinical Pharmacology and Therapeutics. 60 (3): 295–307. doi:10.1016/S0009-9236(96)90056-9. PMID 8841152. S2CID 10147669.
- Woodworth JR, Dennis SR, Moore L, Rotenberg KS (February 1987). "The polymorphic metabolism of dextromethorphan". Journal of Clinical Pharmacology. 27 (2): 139–143. doi:10.1002/j.1552-4604.1987.tb02174.x. PMID 3680565. S2CID 37950361.
- "Dextromethorphan (PIM 179)". www.inchem.org. Archived from the original on 2017-03-10. Retrieved 2018-03-24.
- Meuzelaar GJ, Neeleman E, Maat L, Sheldon RA (2010-06-17). "ChemInform Abstract: A Novel Synthesis of Substituted 1-Benzyloctahydroisoquinolines by Acid-Catalyzed Cyclization of N--N-styrylformamides". ChemInform. 30 (5): no. doi:10.1002/chin.199905129. ISSN 0931-7597.
- ^ Morris H, Wallach J (2014). "From PCP to MXE: a comprehensive review of the non-medical use of dissociative drugs". Drug Testing and Analysis. 6 (7–8): 614–632. doi:10.1002/dta.1620. PMID 24678061.
- "Memorandum for the Secretary of Defense" (PDF). Archived (PDF) from the original on 2017-09-16. Retrieved 2013-07-28.
- ^ "Dextromethorphan (DXM)". Cesar.umd.edu. Archived from the original on 2018-01-06. Retrieved 2013-07-28.
- Nordqvist C. FDA panel: cough meds should stay over the counter. Sept. 14, 2010. Medical News Today Website. http://www.medicalnewstoday.com/articles/201227.php. Accessed May 18, 2020.
- "Senate Bill No. 514" (PDF). An act to add Sections 11110 and 11111 to the Health and Safety Code, relating to nonprescription drugs. State of California, Legislative Counsel. Archived (PDF) from the original on 2018-03-08.
- "BPOM Tetap Batalkan Izin Edar Obat Dekstrometorfan" [BPOM Still Cancels Dextromethorphan Drug Distribution Permit]. VIVAnews (in Indonesian). 22 May 2014. Archived from the original on 2015-05-28.
- "SINDOnews | Berita Daerah Dan Provinsi Di Indonesia". daerah.sindonews.com (in Indonesian). Retrieved 2017-12-10.
- "Pimpinan dan Apoteker Penanggung Jawab" (PDF). Archived from the original (PDF) on 2017-08-10.
- "Badan Pengawas Obat dan Makanan – Republik Indonesia". www.pom.go.id. Archived from the original on 2017-02-03. Retrieved 2017-12-10.
- "Dextromethorphan" (PDF). Drugs and Chemicals of Concern. Drug Enforcement Administration. August 2010. Archived from the original (PDF) on 2012-10-16.
- Giannini AJ (1997). Drugs of abuse (2nd ed.). Los Angeles: Practice Management Information Corp. ISBN 1570660530.
- Akerman SC, Hammel JL, Brunette MF (2010-12-20). "Dextromethorphan Abuse and Dependence in Adolescents". Journal of Dual Diagnosis. 6 (3–4): 266–278. doi:10.1080/15504263.2010.537515. S2CID 70666093.
- Olney N, Rosen H (April 2010). "AVP-923, a combination of dextromethorphan hydrobromide and quinidine sulfate for the treatment of pseudobulbar affect and neuropathic pain". IDrugs. 13 (4): 254–265. PMID 20373255.
- Khoury R, Marx C, Mirgati S, Velury D, Chakkamparambil B, Grossberg GT (May 2021). "AVP-786 as a promising treatment option for Alzheimer's Disease including agitation". Expert Opinion on Pharmacotherapy. 22 (7): 783–795. doi:10.1080/14656566.2021.1882995. PMID 33615952. S2CID 231987025.
- Murrough JW, Wade E, Sayed S, Ahle G, Kiraly DD, Welch A, et al. (August 2017). "Dextromethorphan/quinidine pharmacotherapy in patients with treatment resistant depression: A proof of concept clinical trial". Journal of Affective Disorders. 218: 277–283. doi:10.1016/j.jad.2017.04.072. PMID 28478356. S2CID 46777150.
- Malek A, Amiri S, Habibi Asl B (2013). "The therapeutic effect of adding dextromethorphan to clonidine for reducing symptoms of opioid withdrawal: a randomized clinical trial". ISRN Psychiatry. 2013: 546030. doi:10.1155/2013/546030. PMC 3706070. PMID 23864983.
External links
![]() Media related to Dextromethorphan at Wikimedia Commons
Media related to Dextromethorphan at Wikimedia Commons
| Recreational uses | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Pharmacodynamics | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||