| Revision as of 20:26, 28 December 2017 editMm9656 (talk | contribs)56 edits →Modern Synthetic ApplicationsTag: Visual edit← Previous edit | Latest revision as of 17:03, 23 December 2024 edit undoDirac66 (talk | contribs)Extended confirmed users17,914 editsm Undid revision 1264792856 by 106.219.195.203 (talk) Restore formatting of referenceTag: Undo | ||
| (61 intermediate revisions by 29 users not shown) | |||
| Line 1: | Line 1: | ||
| {{short description|Chemical reaction in which two molecules are combined and a small molecule, usually water, is lost}} | |||
| ⚫ | |||
| ] | |||
| ⚫ | |||
| ] | |||
| <references /> | |||
| In ], a '''condensation reaction''' is a type of ] in which two ]s are ] to form a single molecule, usually with the loss of a small molecule such as ].<ref>{{cite book |title=Book: Introductory Chemistry (CK-12) |date=12 August 2020 |publisher=Chemistry Libre Texts |url=https://chem.libretexts.org/Bookshelves/Introductory_Chemistry/Book%3A_Introductory_Chemistry_(CK-12)/25%3A_Organic_Chemistry/25.18%3A_Condensation_Reactions |access-date=9 January 2021 |chapter=25.18 Condensation Reactions}}</ref> If water is lost, the reaction is also known as a ]. However other molecules can also be lost, such as ], ], ] and ].<ref>{{cite journal|url=https://goldbook.iupac.org/html/C/C01238.html|title=Condensation Reaction|website=IUPAC Compendium of Chemical Terminology (Gold Book)|year=2014|publisher=IUPAC|doi=10.1351/goldbook.C01238|access-date=7 December 2017|doi-access=free}}</ref> | |||
| == '''<u>Mechanistic Details</u>''' == | |||
| The numerous variations of condensation reactions which correspond to numerous mechanisms. | |||
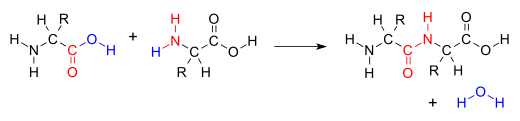
| ⚫ | The addition of the two molecules typically proceeds in a step-wise fashion to the addition product, usually in ], and with loss of a water molecule (hence the name ]).<ref>{{Cite journal|last=Fakirov|first=S.|date=2019-02-01|title=Condensation Polymers: Their Chemical Peculiarities Offer Great Opportunities|journal=Progress in Polymer Science|volume=89|pages=1–18|doi=10.1016/j.progpolymsci.2018.09.003|s2cid=105101288|issn=0079-6700}}</ref> The reaction may otherwise involve the ]s of the molecule, and is a versatile class of reactions that can occur in ]ic or ] conditions or in the presence of a ]. This class of reactions is a vital part of life as it is essential to the formation of ]s between ]s and to the ].<ref>{{Cite book|title=Fundamentals of Biochemistry|url=https://archive.org/details/fundamentalsbioc00voet|url-access=limited|last1=Voet|first1=Donald|last2=Voet|first2=Judith|last3=Pratt|first3=Chriss|publisher=John Wiley & Sons, Inc.|year=2008|isbn=978-0470-12930-2|location=Hoboken, NJ|pages=}}</ref> | ||
| This reaction takes between a carbonyl compound and a activated methylene compound or with a nitromethane group, in mildly basic conditions. The final product of the reaction is an alkene with two geminal acceptor groups or one nitro group.<ref>{{Cite book|title=Advanced Organic Chemistry|last=Bruckner|first=Reinhard|publisher=Harcourt Academic Press|year=2002|isbn=0-12-138110-2|location=San Diego, California|pages=414}}</ref> | |||
| ] | |||
| ].]] | |||
| ==== <u>Crossed Claisen Condensation Reaction Mechanism</u> ==== | |||
| The crossed claisen reaction results in the acylation of an ester enolate with another ester. These reactions are only possible when one ester has no alpha hydrogens. The reaction conditions call for a highly basic solution and a rapid acidic workup to achieve the final compound. | |||
| ] | |||
| ⚫ | Many variations of condensation reactions exist. Common examples include the ] and the ], which both form water as a by-product, as well as the ] and the ] (intramolecular Claisen condensation), which form alcohols as by-products.<ref name=":0">{{cite book|title=Advanced Organic Chemistry|url=https://archive.org/details/advancedorganicc00bruc|url-access=limited|last1=Bruckner|first1=Reinhard|date=2002|publisher=Harcourt Academic Press|isbn=0-12-138110-2|edition=First|location=San Diego, California|pages=–427}}</ref> | ||
| == '''<u>Modern Synthetic Applications</u>''' == | |||
| Condensation reactions are used throughout the field of synthetic and medicinal chemistry to synthesize target compounds. Their familiarity and predictable nature make them a widely used synthetic tool. In this section are several examples of some recent uses of condensation reactions in the fields of medicinal and synthetic chemistry. | |||
| ] | |||
| A team of chemists led by Young Lok Choi and Jung-Nyoung Heo successfully synthesized dibenzoheptenones by combining a suzuki-miyaura coupling reaction with an aldol condensation reaction.<ref>{{Cite journal|last=Kim|first=Joa|last2=Kim|first2=Young|last3=Jung-Nyoung|first3=Heo|date=Winter 2017|title=Total Synthesis of Aristolactams via a One-Pot Suzuki−Miyaura Coupling/Aldol Condensation Cascade Reaction|url=http://pubs.acs.org/doi/abs/10.1021/ol801291k|journal=Journal of Organic Letters|volume=10|pages=3543-3546|via=}}</ref> These dibenzoheptenone form the skeleton for important medicinal drugs such as colchicine (anti-inflamatory used to treat gout). | |||
| == Synthesis of prebiotic molecules == | |||
| ] | |||
| {{main|Abiogenesis}} | |||
| Condensation reactions have also recently been used to initiate tandem C-N bond formation to form heterocycles. In a paper published in 2017 by a team led by Yan-Xiao Jiao and Gui-Fa Su* they managed to form quinoxilines which is a structure that is used throughout the pharmaceutical and technological industry.<ref>{{Cite journal|last=Jiao|first=Yan-Xiao|last2=Wu|first2=Ling-Ling|last3=Su|first3=Gui-FA|date=Winter 2017|title=Tandem C–N Bond Formation through Condensation and Metal-Free N-Arylation: Protocol for Synthesizing Diverse Functionalized Quinoxalines|url=http://pubs.acs.org/doi/abs/10.1021/acs.joc.7b00011|journal=Journal of Organic Chemistry|volume=82|pages=4407-4417|via=}}</ref> | |||
| Condensation reactions likely played major roles in the synthesis of the first biotic molecules including early ]s and ]s. In fact, condensation reactions would be required at multiple steps in ] oligomerization: the condensation of ]s and ]s, ] ], and ] polymerization.<ref name=":02">{{Cite book |last=Fiore |first=Michele |title=Prebiotic Chemistry and Life's Origin |publisher=Royal Society of Chemistry |year=2022 |isbn=9781839164804 |location=United Kingdom |pages=124–144}}</ref> | |||
| ==See also== | ==See also== | ||
| *] | * ] | ||
| *], the opposite of a condensation reaction | * ], the opposite of a condensation reaction | ||
| *]s | * ]s | ||
| ==References== | ==References== | ||
| {{Reflist}} | {{Reflist}} | ||
| {{Authority control}} | |||
| ⚫ | ] | ||
| ⚫ | ] | ||
Latest revision as of 17:03, 23 December 2024
Chemical reaction in which two molecules are combined and a small molecule, usually water, is lostIn organic chemistry, a condensation reaction is a type of chemical reaction in which two molecules are combined to form a single molecule, usually with the loss of a small molecule such as water. If water is lost, the reaction is also known as a dehydration synthesis. However other molecules can also be lost, such as ammonia, ethanol, acetic acid and hydrogen sulfide.
The addition of the two molecules typically proceeds in a step-wise fashion to the addition product, usually in equilibrium, and with loss of a water molecule (hence the name condensation). The reaction may otherwise involve the functional groups of the molecule, and is a versatile class of reactions that can occur in acidic or basic conditions or in the presence of a catalyst. This class of reactions is a vital part of life as it is essential to the formation of peptide bonds between amino acids and to the biosynthesis of fatty acids.
Many variations of condensation reactions exist. Common examples include the aldol condensation and the Knoevenagel condensation, which both form water as a by-product, as well as the Claisen condensation and the Dieckman condensation (intramolecular Claisen condensation), which form alcohols as by-products.
Synthesis of prebiotic molecules
Main article: AbiogenesisCondensation reactions likely played major roles in the synthesis of the first biotic molecules including early peptides and nucleic acids. In fact, condensation reactions would be required at multiple steps in RNA oligomerization: the condensation of nucleobases and sugars, nucleoside phosphorylation, and nucleotide polymerization.
See also
- Anabolism
- Hydrolysis, the opposite of a condensation reaction
- Condensed tannins
References
- "25.18 Condensation Reactions". Book: Introductory Chemistry (CK-12). Chemistry Libre Texts. 12 August 2020. Retrieved 9 January 2021.
- "Condensation Reaction". IUPAC Compendium of Chemical Terminology (Gold Book). IUPAC. 2014. doi:10.1351/goldbook.C01238. Retrieved 7 December 2017.
- Fakirov, S. (2019-02-01). "Condensation Polymers: Their Chemical Peculiarities Offer Great Opportunities". Progress in Polymer Science. 89: 1–18. doi:10.1016/j.progpolymsci.2018.09.003. ISSN 0079-6700. S2CID 105101288.
- Voet, Donald; Voet, Judith; Pratt, Chriss (2008). Fundamentals of Biochemistry. Hoboken, NJ: John Wiley & Sons, Inc. pp. 88. ISBN 978-0470-12930-2.
- Bruckner, Reinhard (2002). Advanced Organic Chemistry (First ed.). San Diego, California: Harcourt Academic Press. pp. 414–427. ISBN 0-12-138110-2.
- Fiore, Michele (2022). Prebiotic Chemistry and Life's Origin. United Kingdom: Royal Society of Chemistry. pp. 124–144. ISBN 9781839164804.
