| Revision as of 15:00, 22 December 2004 view sourceGene Nygaard (talk | contribs)Extended confirmed users90,047 edits →Other units of energy: expansion← Previous edit | Latest revision as of 17:19, 26 December 2024 view source Orpaul (talk | contribs)Extended confirmed users513 editsm →History | ||
| Line 1: | Line 1: | ||
| {{Short description|Physical quantity}} | |||
| :''This article is about the scientific concept. Energy use by humans is discussed in ]''. | |||
| {{About|the scalar physical quantity|an overview of and topical guide to energy|Outline of energy|other uses}} | |||
| {{redirect|Energetic}} | |||
| {{pp-semi-indef}} | |||
| {{pp-move}} | |||
| {{Use British English|date=March 2013}} | |||
| {{CS1 config|mode=cs1}} | |||
| {{Infobox physical quantity | |||
| | name = Energy | |||
| | image = Energy Arc (central electrode of a Plasma Lamp).jpg | |||
| | caption = A ], using ] to create ], ], ], ] and a faint ] | |||
| | unit = ] | |||
| | otherunits = ], ], ], ], ], ] | |||
| | symbols = ''E'' | |||
| | baseunits = J = kg⋅m<sup>2</sup>⋅s<sup>−2</sup> | |||
| | dimension = '''M''' '''L'''<sup>2</sup> '''T'''<sup>−2</sup> | |||
| | extensive = yes | |||
| | conserved = ] | |||
| | derivations = | |||
| |image_upright=1.15}} | |||
| {{Thermodynamics}} | |||
| '''Energy''' ({{etymology|grc|''{{Wikt-lang|grc|ἐνέργεια}}'' ({{grc-transl|ἐνέργεια}})|activity}}) is the ] ] that is ] to a ] or to a ], recognizable in the performance of ] and in the form of ] and ]. Energy is a ]—the law of ] states that energy can be ] in form, but not created or destroyed. The unit of measurement for energy in the ] (SI) is the ] (J). | |||
| '''Energy''', generally and qualitatively speaking, is the property (or the quantity of the property) of doing things or supplying power. The expressions energy and power have different meaning in different scientific and non-scientific fields. Physics aims to explain quantitatively this property and gives a definition that makes it possible to consider energy as a description of the whole state and the different ways jobs are done are unified in this treatment. | |||
| Forms of energy include the ] of a moving object, the ] stored by an object (for instance due to its position in a ]), the ] stored in a solid object, ] associated with ]s, the ] carried by ], the ] contained within a ], and ] associated with an object's ]. These are not mutually exclusive. | |||
| Energy is a fundamental quantity that every ] ] possesses; it allows us to predict how much ] the system could be made to do, or how much ] it can exchange. In the past, energy was discussed in terms of easily ] effects it has on the ] of ]s or changes in ] of various systems. Basically, if something changes, some sort of energy was involved in that change. As it was realized that energy could be ] in objects, the concept of energy came to embrace the idea of the potential for change as well as change itself. Such effects (both potential and realized) come in many different forms; examples are the electrical energy stored in a battery, the chemical energy stored in a piece of food, the thermal energy of a hot water heater, or the kinetic energy of a moving train. To simply say energy is "change or the potential for change", however, misses many important examples of energy as it exists in the physical world. Energy can be used not only to produce observable change, it also is used to prevent change in which case unaided observation of this kind of energy can be difficult. For example, looking at a statue holding a 50 pound weight, the presence of energy needed to do so may not be observable. However, if you are holding up the fifty pound weight instead of the statue the need for energy to accomplish this becomes apparent. You can feel the gravitational force on you both when you are moving the weight up and when you are not moving it. Energy can be readily transformed from one form into another; for instance, using a battery to power an electrical heater converts electrical energy into thermal energy. In the previous example of holding the fifty pound weight, the work you perform to raise the weight is observed as kinetic energy of motion which is converted to potential energy and added to the weight's potential energy as you continue to hold the weight up against the pull of gravity. Letting go of the weight once again transforms this stored potential energy back into kinetic energy as the weight falls under the force of gravity. The law of ] states that the total amount of energy, corresponding to the sum of a system's constituent energy components, remains constant. Scientists have also defined several forms of energy that are not easily measured by the unaided observer. | |||
| All ] constantly take in and release energy. The Earth's ] and ]s processes are driven primarily by ].<ref>{{cite web |url=https://energyeducation.ca/encyclopedia/Earth%27s_energy_flow |title=Earth's energy flow |website=Energy Education |access-date=2024-08-28}}</ref> The ] provides the energy required for human civilization to function, which it obtains from ]s such as ]s, ], and ]. | |||
| ==Units== | |||
| == Forms == | |||
| ===] and related units=== | |||
| ] strike, 500 ]s of ] is converted into the same amount of energy in other forms, mostly ], ] and ].]] | |||
| ] is energy of microscopic constituents of matter, which may include both ] and ].]] | |||
| The total energy of a ] can be subdivided and classified into ], ], or combinations of the two in various ways. Kinetic energy is determined by the ] of an object – or the ] of the object's components – while ] reflects the potential of an object to have motion, generally being based upon the object's position within a ] or what is stored within the field itself.<ref>{{Cite journal |last=Bobrowsky |first=Matt |title=SCIENCE 101: Q: What Is Energy? |url=https://www.jstor.org/stable/27133353 |access-date=February 5, 2024 |journal=] |date=2021 |volume=59 |issue=1 |pages=61–65 |language=en |doi=10.1080/19434812.2021.12291716 |jstor=27133353 |s2cid=266084433 |issn=0036-8148}}</ref> | |||
| The ] unit for both energy and work is the ] (J), named in honour of ] and his experiments on the ]. In slightly more fundamental terms, ] is equal to 1 ]-] and, in terms of ]s, 1 J is equal to 1 ] ]<small><sup>2</sup></small> ]<small><sup>−2</sup></small>. | |||
| While these two categories are sufficient to describe all forms of energy, it is often convenient to refer to particular combinations of potential and kinetic energy as its own form. For example, the sum of translational and ] kinetic and potential energy within a system is referred to as ], whereas nuclear energy refers to the combined potentials within an atomic nucleus from either the ] or the ], among other examples.<ref>{{Cite web |title=Nuclear Energy {{!}} Definition, Formula & Examples {{!}} nuclear-power.com |url=https://www.nuclear-power.com/nuclear-power/nuclear-energy/ |access-date=2022-07-06 |website=Nuclear Power |language=en-us |archive-date=2022-07-06 |archive-url=https://web.archive.org/web/20220706153815/https://www.nuclear-power.com/nuclear-power/nuclear-energy/ |url-status=live }}</ref> | |||
| An energy unit that is used in ] is the ] (eV). One eV is equivalent to ]. | |||
| {| class="wikitable plainrowheaders" | |||
| (Note that ] has the same units as energy, but there is no deeper connection between the two concepts.) | |||
| |+Some forms of energy (that an object or system can have as a measurable property) | |||
| !scope="col"|Type of energy | |||
| !scope="col"|Description | |||
| |- | |||
| !scope="row"|] | |||
| |the sum of ] translational and rotational kinetic and potential energies | |||
| |- | |||
| !scope="row"|] | |||
| |potential energy due to or stored in electric fields | |||
| |- | |||
| !scope="row"|] | |||
| |potential energy due to or stored in magnetic fields | |||
| |- | |||
| !scope="row"|] | |||
| |potential energy due to or stored in gravitational fields | |||
| |- | |||
| !scope="row"|] | |||
| |potential energy due to chemical bonds | |||
| |- | |||
| !scope="row"|] | |||
| |potential energy that ] an electron to its atom or molecule | |||
| |- | |||
| !scope="row"|] | |||
| |potential energy that ] ] to form the ] (and nuclear reactions) | |||
| |- | |||
| !scope="row"|] | |||
| |potential energy that ] ]s to form ]s | |||
| |- | |||
| !scope="row"|] | |||
| |potential energy due to the deformation of a material (or its container) exhibiting a restorative force as it returns to its original shape | |||
| |- | |||
| !scope="row"|] | |||
| |kinetic and potential energy in an elastic material due to a propagating ] of matter | |||
| |- | |||
| !scope="row"|] | |||
| |kinetic and potential energy in a material due to a sound propagated wave (a particular type of mechanical wave) | |||
| |- | |||
| !scope="row"|] | |||
| |] stored in the fields of waves propagated by ], including ] | |||
| |- | |||
| !scope="row"|] | |||
| |potential energy ] an object's ] | |||
| |- | |||
| !scope="row"|] | |||
| |kinetic energy of the ] motion of particles, a kind of disordered equivalent of mechanical energy | |||
| |- | |||
| |} | |||
| == |
== History == | ||
| {{Main|History of energy|timeline of thermodynamics, statistical mechanics, and random processes|}} | |||
| ], the first person to use the term "energy" in the modern sense]] | |||
| The word ''energy'' derives from the {{langx|grc|ἐνέργεια|]|activity, operation}},<ref>{{cite web |url=http://www.etymonline.com/index.php?term=energy |title=Energy |work=Online Etymology Dictionary |last=Harper |first=Douglas |access-date=May 1, 2007 |url-status=live |archive-url=https://web.archive.org/web/20071011122441/http://etymonline.com/index.php?term=energy |archive-date=October 11, 2007 }}</ref> which possibly appears for the first time in the work of ] in the 4th century BC. In contrast to the modern definition, energeia was a qualitative philosophical concept, broad enough to include ideas such as happiness and pleasure. | |||
| In the late 17th century, ] proposed the idea of the {{langx|la|]}}, or living force, which defined as the product of the mass of an object and its velocity squared; he believed that total ''vis viva'' was conserved. To account for slowing due to friction, Leibniz theorized that thermal energy consisted of the motions of the constituent parts of matter, although it would be more than a century until this was generally accepted. The modern analog of this property, ], differs from ''vis viva'' only by a factor of two. Writing in the early 18th century, ] proposed the concept of ] in the marginalia of her French language translation of Newton's '']'', which represented the first formulation of a conserved measurable quantity that was distinct from ], and which would later be called "energy". | |||
| In ] units, one ] is 1 ] ]<small><sup>2</sup></small> ]<small><sup>−2</sup></small>, equal to ]. | |||
| In 1807, ] was possibly the first to use the term "energy" instead of ''vis viva'', in its modern sense.<ref>{{Cite book| last = Smith | first = Crosbie | title = The Science of Energy – a Cultural History of Energy Physics in Victorian Britain | publisher = The University of Chicago Press | year = 1998 | isbn = 978-0-226-76420-7}}</ref> ] described "]" in 1829 in its modern sense, and in 1853, ] coined the term "]". The law of ] was also first postulated in the early 19th century, and applies to any ]. It was argued for some years whether heat was a physical substance, dubbed the ], or merely a physical quantity, such as ]. In 1845 ] discovered the link between mechanical work and the generation of heat. | |||
| The ]/] for both energy and work include the ] (1.3558 J), the ] (Btu) which has various values in the range of 1055 J, and the ]-hour (2.6845 MJ). | |||
| These developments led to the theory of conservation of energy, formalized largely by William Thomson (]) as the field of ]. Thermodynamics aided the rapid development of explanations of chemical processes by ], ], and ]. It also led to a mathematical formulation of the concept of ] by Clausius and to the introduction of laws of ] by ]. According to ], the conservation of energy is a consequence of the fact that the laws of physics do not change over time.<ref name="jphysics">{{Cite book |last1=Lofts |first1=G. |title=Jacaranda Physics 1 |last2=O'Keeffe |first2=D. |publisher=John Wiley & Sons Australia Limited |year=2004 |isbn=978-0-7016-3777-4 |edition=2 |location=Milton, Queensland, Australia |page=286 |chapter=11 – Mechanical Interactions |display-authors=etal}}</ref> Thus, since 1918, theorists have understood that the law of conservation of energy is the direct mathematical consequence of the ] of the quantity ] to energy, namely time. | |||
| The energy unit used for everyday ], particularly for utility bills, is the ] (kW h), and one kW h is equivalent to ] (3600 kJ or 3.6 MJ). | |||
| == Units of measure == | |||
| The ] is mainly used in nutrition and equals the amount of ] necessary to raise the ] of one ] of ] by 1 degree ], at a ] of 1 ]. This amount of heat depends somewhat on the initial temperature of the water, which results in various different units sharing the name of "calorie" but having slightly different energy values. It is approximately equal to ]. | |||
| ] | |||
| {{Main|Units of energy}} | |||
| In the ] (SI), the unit of energy is the ]. It is a ] that is equal to the energy expended, or ] done, in applying a force of one ] through a distance of one metre. However energy can also be expressed in many other units not part of the SI, such as ]s, ]s, ]s, ]s and ]s, which require a conversion factor when expressed in SI units. | |||
| The calories used for ] in nutrition are the large calories based on the kilogram rather than the gram, often identified as ''food calories''. These are sometimes called kilocalories with that calorie being the small calorie based on the gram, and as a result the prefixes are generally avoided for the large calories (i.e., 1 kcal is 4.184 kJ, never 4.184 MJ, even if "calories" are also used for the other, larger unit in the same document or the same nutrition label). Food calories are sometimes noted as ''C''alories (1000 calories) or simply abbreviated Cal with the capital C, but that convention is more often found in chemistry or phphysics textbooks which do not use these large calories than it is in real-world applications by those who do use these calories. | |||
| The SI unit of ], defined as energy per unit of time, is the ], which is a joule per second. Thus, one joule is one watt-second, and 3600 joules equal one watt-hour. The ] energy unit is the ] and the ] unit is the ]. Other energy units such as the ], ] or thermodynamic ] (based on the temperature change of water in a heating process), and ] are used in specific areas of science and commerce. | |||
| ==Transfer of energy== | |||
| In 1843, ] ] ], ] of the unit of measure, discovered that the ] ] lost by a descending weight attached via a string was equal to the ] gained by the water through ] with the paddle. | |||
| ===Work=== | |||
| == Scientific use == | |||
| ''Main article: ].'' | |||
| === Classical mechanics === | |||
| ''Work'' is a measure of energy expended in applying force over a distance. Performing work requires energy, and thus the amount of energy in a system limits the maximum amount of work that a system could conceivably perform. | |||
| {{Classical mechanics}} | |||
| {{Main|Mechanics|Mechanical work|Thermodynamics}} | |||
| In classical mechanics, energy is a conceptually and mathematically useful property, as it is a ]. Several formulations of mechanics have been developed using energy as a core concept. | |||
| :<math> E = \int \mathbf{F} \cdot \mathrm{d}\mathbf{s}</math> | |||
| ], a function of energy, is force times distance. | |||
| The equation above says that the energy used in the process of performing work (<math>E</math>) is equal to the integral of the ] of the ] (<math>\mathbf{F}</math>) on a body and the ] of the body's ] (<math>\mathbf{s}</math>). | |||
| : <math> W = \int_C \mathbf{F} \cdot \mathrm{d} \mathbf{s}</math> | |||
| This says that the work (<math>W</math>) is equal to the ] of the ] '''F''' along a path ''C''; for details see the ] article. Work and thus energy is ]. For example, consider a ball being hit by a bat. In the center-of-mass reference frame, the bat does no work on the ball. But, in the reference frame of the person swinging the bat, considerable work is done on the ball. | |||
| In most simple physics models, this is assumed to be the same quantity as the work that is actually performed on the body in question. In reality, however, not all energy given by the above equation is transferred into a recoverable form: for example, energy may be converted into heat which cannot then be converted into another useful form of energy. Thus, in practice, the amount of energy in a system available for performing work may be much less than the total amount of energy in the system. | |||
| The total energy of a system is sometimes called the ], after ]. The classical equations of motion can be written in terms of the Hamiltonian, even for highly complex or abstract systems. These classical equations have direct analogs in nonrelativistic quantum mechanics.<ref> MIT OpenCourseWare website 18.013A Chapter 16.3 Accessed February 2007</ref> | |||
| ===Heat=== | |||
| ''Main article: ].'' | |||
| Another energy-related concept is called the ], after ]. This formalism is as fundamental as the Hamiltonian, and both can be used to derive the equations of motion or be derived from them. It was invented in the context of ], but is generally useful in modern physics. The Lagrangian is defined as the kinetic energy ''minus'' the potential energy. Usually, the Lagrange formalism is mathematically more convenient than the Hamiltonian for non-conservative systems (such as systems with friction). | |||
| ''Heat'' is an amount of energy which is usually linked with a change in temperature or in a change in phase of matter. In chemistry, heat is the amount of energy which is absorbed or released by a given chemical reaction. | |||
| The relationship between heat and energy is similar to that between work and energy. Heat flows from areas of high temperature to areas of low temperature. All objects (matter) have a certain amount of internal energy that is related to the random motion of their atoms or molecules. This internal energy is directly proportional to the temperature of the object. When two bodies of different temperature come in to thermal contact, they will exchange internal energy until the temperature is equalised. The amount of energy transferred is the amount of heat exchanged. It is a common misconception to confuse heat with internal energy, but there is a difference: the change of the internal energy is the heat that flows from the surroundings into the system plus the work performed by the surroundings on the system. | |||
| ] (1918) states that any differentiable symmetry of the action of a physical system has a corresponding conservation law. Noether's theorem has become a fundamental tool of modern theoretical physics and the calculus of variations. A generalisation of the seminal formulations on constants of motion in Lagrangian and Hamiltonian mechanics (1788 and 1833, respectively), it does not apply to systems that cannot be modeled with a Lagrangian; for example, dissipative systems with continuous symmetries need not have a corresponding conservation law. | |||
| ==Conservation of energy== | |||
| === Chemistry === | |||
| The first law of ] says that the total inflow of energy into a system must equal the total outflow of energy from the system, plus the change in the energy contained within the system. This law is used in all branches of physics. ] relates the conservation of energy to the time invariance of physical laws. | |||
| <!-- courtesy note per ]: redirect ] links here --> | |||
| In the context of ], ] is an attribute of a substance as a consequence of its atomic, molecular, or aggregate structure. Since a chemical transformation is accompanied by a change in one or more of these kinds of structure, it is usually accompanied by a decrease, and sometimes an increase, of the total energy of the substances involved. Some energy may be transferred between the surroundings and the reactants in the form of heat or light; thus the products of a reaction have sometimes more but usually less energy than the reactants. A reaction is said to be ] or ] if the final state is lower on the energy scale than the initial state; in the less common case of ] reactions the situation is the reverse. | |||
| ]s are usually not possible unless the reactants surmount an energy barrier known as the ]. The ''speed'' of a chemical reaction (at a given temperature ''T'') is related to the activation energy ''E'' by the Boltzmann's population factor e<sup>−''E''/''kT''</sup>; that is, the probability of a molecule to have energy greater than or equal to ''E'' at a given temperature ''T''. This exponential dependence of a reaction rate on temperature is known as the ]. The activation energy necessary for a chemical reaction can be provided in the form of thermal energy. | |||
| == Kinetic energy == | |||
| === Biology === | |||
| ''Main article: ].'' | |||
| <!-- courtesy note per ]: redirect ] links here --> | |||
| {{Main|Bioenergetics|Food energy}} | |||
| ]]] | |||
| {{anchor|Biology}}In ], energy is an attribute of all biological systems, from the biosphere to the smallest living organism. Within an organism it is responsible for growth and development of a biological ] or ] of a biological organism. Energy used in ] is stored in substances such as ]s (including sugars), ]s, and ]s stored by ]. In human terms, the ] (H-e) (Human energy conversion) indicates, for a given amount of energy expenditure, the relative quantity of energy needed for human ], using as a standard an average human energy expenditure of 12,500 kJ per day and a ] of 80 watts. | |||
| For example, if our bodies run (on average) at 80 watts, then a light bulb running at 100 watts is running at 1.25 human equivalents (100 ÷ 80) i.e. 1.25 H-e. For a difficult task of only a few seconds' duration, a person can put out thousands of watts, many times the 746 watts in one official horsepower. For tasks lasting a few minutes, a fit human can generate perhaps 1,000 watts. For an activity that must be sustained for an hour, output drops to around 300; for an activity kept up all day, 150 watts is about the maximum.<ref>{{cite web |url=http://www.uic.edu/aa/college/gallery400/notions/human%20energy.htm |title=Retrieved on May-29-09 |publisher=Uic.edu |access-date=2010-12-12 |url-status=live |archive-url=https://web.archive.org/web/20100604191319/http://www.uic.edu/aa/college/gallery400/notions/human%20energy.htm |archive-date=2010-06-04 }}</ref> The human equivalent assists understanding of energy flows in physical and biological systems by expressing energy units in human terms: it provides a "feel" for the use of a given amount of energy.<ref>Bicycle calculator – speed, weight, wattage etc. {{cite web |url=http://bikecalculator.com/ |title=Bike Calculator |access-date=2009-05-29 |url-status=live |archive-url=https://web.archive.org/web/20090513091201/http://bikecalculator.com/ |archive-date=2009-05-13 }}.</ref> | |||
| ] is the portion of energy associated with the motion of a body. | |||
| Sunlight's radiant energy is also captured by plants as ''chemical potential energy'' in ], when carbon dioxide and water (two low-energy compounds) are converted into carbohydrates, lipids, proteins and oxygen. Release of the energy stored during photosynthesis as heat or light may be triggered suddenly by a spark in a forest fire, or it may be made available more slowly for animal or human metabolism when organic molecules are ingested and ] is triggered by ] action. | |||
| :<math>E_k = \int \mathbf{v} \cdot \mathrm{d}\mathbf{p}</math> | |||
| All living creatures rely on an external source of energy to be able to grow and reproduce – radiant energy from the Sun in the case of green plants and chemical energy (in some form) in the case of animals. The daily 1500–2000 ] (6–8 MJ) recommended for a human adult are taken as food molecules, mostly carbohydrates and fats, of which ] (C<sub>6</sub>H<sub>12</sub>O<sub>6</sub>) and ] (C<sub>57</sub>H<sub>110</sub>O<sub>6</sub>) are convenient examples. The food molecules are oxidized to ] and ] in the ] | |||
| The equation above says that the kinetic energy (<math>E_k</math>) is equal to the integral of the ] of the ] (<math>\mathbf{v}</math>) of a body and the ] of the body's ] (<math>\mathbf{p}</math>). | |||
| <chem display="block">C6H12O6 + 6O2 -> 6CO2 + 6H2O</chem> | |||
| <chem display="block">C57H110O6 + (81 1/2) O2 -> 57CO2 + 55H2O</chem> | |||
| and some of the energy is used to convert ] into ]: | |||
| {{block indent|em=1.6|text=ADP + HPO<sub>4</sub><sup>2−</sup> → ATP + H<sub>2</sub>O}} | |||
| The rest of the chemical energy of the carbohydrate or fat are converted into heat: the ATP is used as a sort of "energy currency", and some of the chemical energy it contains is used for other ] when ATP reacts with OH groups and eventually splits into ADP and phosphate (at each stage of a ], some chemical energy is converted into heat). Only a tiny fraction of the original chemical energy is used for ]:<ref group=note>These examples are solely for illustration, as it is not the energy available for work which limits the performance of the athlete but the ] output (in case of a sprinter) and the ] (in case of a weightlifter).</ref> | |||
| : gain in kinetic energy of a sprinter during a 100 m race: 4 kJ | |||
| : gain in gravitational potential energy of a 150 kg weight lifted through 2 metres: 3 kJ | |||
| : daily food intake of a normal adult: 6–8 MJ | |||
| It would appear that living organisms are remarkably ] in their use of the energy they receive (chemical or radiant energy); most ]s manage higher efficiencies. In growing organisms the energy that is converted to heat serves a vital purpose, as it allows the organism tissue to be highly ordered with regard to the molecules it is built from. The ] states that energy (and matter) tends to become more evenly spread out across the universe: to concentrate energy (or matter) in one specific place, it is necessary to spread out a greater amount of energy (as heat) across the remainder of the universe ("the surroundings").<ref group=note>]s are another example of highly ordered systems that exist in nature: in this case too, the order is associated with the transfer of a large amount of heat (known as the ]) to the surroundings.</ref> Simpler organisms can achieve higher energy efficiencies than more complex ones, but the complex organisms can occupy ]s that are not available to their simpler brethren. The conversion of a portion of the chemical energy to heat at each step in a metabolic pathway is the physical reason behind the pyramid of biomass observed in ]. As an example, to take just the first step in the ]: of the estimated 124.7 Pg/a of carbon that is ] by ], 64.3 Pg/a (52%) are used for the metabolism of green plants,<ref>Ito, Akihito; Oikawa, Takehisa (2004). " {{webarchive|url=https://web.archive.org/web/20061002083948/http://www.terrapub.co.jp/e-library/kawahata/pdf/343.pdf |date=2006-10-02 }}" in Shiyomi, M. et al. (Eds.) ''Global Environmental Change in the Ocean and on Land.'' pp. 343–58.</ref> i.e. reconverted into carbon dioxide and heat. | |||
| For non-] velocities, that is velocities much smaller than the ], we can use the Newtonian approximation | |||
| === Earth sciences === | |||
| :<math>E_k = \begin{matrix} \frac{1}{2} \end{matrix} mv^2</math> | |||
| In ], ], ], ]es, and ]s are phenomena that can be explained in terms of energy transformations in the Earth's interior,<ref>{{cite web |url=http://okfirst.ocs.ou.edu/train/meteorology/EnergyBudget.html |title=Earth's Energy Budget |publisher=Okfirst.ocs.ou.edu |access-date=2010-12-12 |url-status=live |archive-url=https://web.archive.org/web/20080827194704/http://okfirst.ocs.ou.edu/train/meteorology/EnergyBudget.html |archive-date=2008-08-27 }}</ref> while ] phenomena like wind, rain, ], snow, lightning, ]es and ] are all a result of energy transformations in our ] brought about by ]. | |||
| Sunlight is the main input to ] which accounts for its temperature and climate stability. Sunlight may be stored as gravitational potential energy after it strikes the Earth, as (for example when) water evaporates from oceans and is deposited upon mountains (where, after being released at a hydroelectric dam, it can be used to drive turbines or generators to produce electricity). Sunlight also drives most weather phenomena, save a few exceptions, like those generated by volcanic events for example. An example of a solar-mediated weather event is a hurricane, which occurs when large unstable areas of warm ocean, heated over months, suddenly give up some of their thermal energy to power a few days of violent air movement. | |||
| where | |||
| In a slower process, ] of atoms in the core of the Earth releases heat. This thermal energy drives ] and may lift mountains, via ]. This slow lifting represents a kind of gravitational potential ] of the thermal energy, which may later be transformed into active kinetic energy during landslides, after a triggering event. Earthquakes also release stored elastic potential energy in rocks, a store that has been produced ultimately from the same radioactive heat sources. Thus, according to present understanding, familiar events such as landslides and earthquakes release energy that has been stored as potential energy in the Earth's gravitational field or elastic strain (mechanical potential energy) in rocks. Prior to this, they represent release of energy that has been stored in heavy atoms since the collapse of long-destroyed supernova stars (which created these atoms). | |||
| ''E''<sub>k</sub> is kinetic energy | |||
| === Cosmology === | |||
| ''m'' is mass of the body | |||
| <!-- courtesy note per ]: redirect ] links here --> | |||
| In ] the phenomena of ]s, ], ], ]s and ]s are the universe's highest-output energy transformations of matter. All ] phenomena (including solar activity) are driven by various kinds of energy transformations. Energy in such transformations is either from gravitational collapse of matter (usually molecular hydrogen) into various classes of astronomical objects (stars, black holes, etc.), or from nuclear fusion (of lighter elements, primarily hydrogen). | |||
| The ] of hydrogen in the Sun also releases another store of potential energy which was created at the time of the ]. At that time, according to theory, space expanded and the universe cooled too rapidly for hydrogen to completely fuse into heavier elements. This meant that hydrogen represents a store of potential energy that can be released by fusion. Such a fusion process is triggered by heat and pressure generated from gravitational collapse of hydrogen clouds when they produce stars, and some of the fusion energy is then transformed into sunlight. | |||
| ''v'' is velocity of the body | |||
| {{anchor|Physics}}<!-- courtesy note per ]: ] --> | |||
| === Quantum mechanics === | |||
| At near-light velocities, we use the relativistic formula: | |||
| {{Main|Energy operator}} | |||
| In ], energy is defined in terms of the ] | |||
| (Hamiltonian) as a time derivative of the ]. The ] equates the energy operator to the full energy of a particle or a system. Its results can be considered as a definition of measurement of energy in quantum mechanics. The Schrödinger equation describes the space- and time-dependence of a slowly changing (non-relativistic) ] of quantum systems. The solution of this equation for a bound system is discrete (a set of permitted states, each characterized by an ]) which results in the concept of ]. In the solution of the Schrödinger equation for any oscillator (vibrator) and for electromagnetic waves in a vacuum, the resulting energy states are related to the frequency by ]: <math>E = h\nu</math> (where <math>h</math> is the ] and <math>\nu</math> the frequency). In the case of an electromagnetic wave these energy states are called quanta of light or ]s. | |||
| === Relativity === | |||
| :<math>E_k = m c^2 (\gamma - 1) = \gamma m c^2 - m c^2 \;\!</math> | |||
| When calculating kinetic energy (] to accelerate a ] from zero ] to some finite speed) relativistically – using ] instead of ] – Einstein discovered an unexpected by-product of these calculations to be an energy term which does not vanish at zero speed. He called it ]: energy which every massive body must possess even when being at rest. The amount of energy is directly proportional to the mass of the body: | |||
| :<math>\gamma = \frac{1}{\sqrt{1 - (v/c)^2}} </math> | |||
| <math display="block"> E_0 = m_0 c^2 ,</math> | |||
| where | |||
| * ''m''<sub>0</sub> is the ] of the body, | |||
| * ''c'' is the ] in vacuum, | |||
| * <math>E_0</math> is the rest energy. | |||
| For example, consider ]–] annihilation, in which the rest energy of these two individual particles (equivalent to their rest mass) is converted to the radiant energy of the photons produced in the process. In this system the ] and ] (electrons and positrons) are destroyed and changed to non-matter (the photons). However, the total mass and total energy do not change during this interaction. The photons each have no rest mass but nonetheless have radiant energy which exhibits the same inertia as did the two original particles. This is a reversible process – the inverse process is called ] – in which the rest mass of particles is created from the radiant energy of two (or more) annihilating photons. | |||
| where | |||
| In general relativity, the ] serves as the source term for the gravitational field, in rough analogy to the way mass serves as the source term in the non-relativistic Newtonian approximation.<ref name="MTW"/> | |||
| ''v'' is the velocity of the body | |||
| Energy and mass are manifestations of one and the same underlying physical property of a system. This property is responsible for the inertia and strength of gravitational interaction of the system ("mass manifestations"), and is also responsible for the potential ability of the system to perform work or heating ("energy manifestations"), subject to the limitations of other physical laws. | |||
| ''m'' is its rest mass | |||
| In ], energy is a scalar quantity, the ] to time. In ] energy is also a scalar (although not a ] but a time component of the ]).<ref name="MTW">{{Cite book |author=Misner |first1=Charles W. |title=Gravitation |last2=Thorne |first2=Kip S. |last3=Wheeler |first3=John Archibald |publisher=W.H. Freeman |year=1973 |isbn=978-0-7167-0344-0 |location=San Francisco}}</ref> In other words, energy is invariant with respect to rotations of ], but not invariant with respect to rotations of ] (= ]). | |||
| ''c'' is the speed of light in a vacuum, which is approximately 300,000 kilometers per second | |||
| == Transformation == | |||
| <math>\gamma m c^2 \,</math> is the ''total energy'' of the body | |||
| {{Main|Energy transformation}} | |||
| {| class="wikitable" style="text-align:center;" | |||
| |+Some forms of ] of energy ("energy in transit") from one object or system to another | |||
| ! Type of transfer ]!! Description | |||
| |- | |||
| |] | |||
| |equal amount of ] in transit spontaneously towards a lower-] object | |||
| |- | |||
| |] | |||
| |equal amount of energy in transit due to a displacement in the direction of an applied ] | |||
| |- | |||
| |Transfer of material | |||
| |equal amount of energy carried by ] that is moving from one system to another | |||
| |- | |||
| |} | |||
| ] transforms the energy of pressurized steam into electrical energy.]] | |||
| <math>m c^2 \,</math> is again the rest mass energy. | |||
| Energy may be ] between different forms at various ]. Items that transform between these forms are called ]s. Examples of transducers include a ] (from ] to ]), a dam (from ] to ] of moving water (and the blades of a ]) and ultimately to ] through an ]), and a ] (from heat to work). | |||
| Examples of energy transformation include generating ] from heat energy via a steam turbine, or lifting an object against gravity using electrical energy driving a crane motor. Lifting against gravity performs mechanical work on the object and stores gravitational potential energy in the object. If the object falls to the ground, gravity does mechanical work on the object which transforms the potential energy in the gravitational field to the kinetic energy released as heat on impact with the ground. The Sun transforms ] to other forms of energy; its total mass does not decrease due to that itself (since it still contains the same total energy even in different forms) but its mass does decrease when the energy escapes out to its surroundings, largely as ]. | |||
| In the form of a ], the relativistic formula for can be written as: | |||
| There are strict limits to how efficiently heat can be converted into ] in a cyclic process, e.g. in a heat engine, as described by ] and the ]. However, some energy transformations can be quite efficient. The direction of transformations in energy (what kind of energy is transformed to what other kind) is often determined by ] (equal energy spread among all available ]) considerations. In practice all energy transformations are permitted on a small scale, but certain larger transformations are not permitted because it is statistically unlikely that energy or matter will randomly move into more concentrated forms or smaller spaces. | |||
| :<math>E_k = \frac{1}{2} mv^2 - \frac{3}{8} \frac{mv^4} {c^2} + \cdots </math> | |||
| Energy transformations in the universe over time are characterized by various kinds of potential energy, that has been available since the ], being "released" (transformed to more active types of energy such as kinetic or radiant energy) when a triggering mechanism is available. Familiar examples of such processes include ], a process ultimately using the gravitational potential energy released from the ] of ]e to "store" energy in the creation of heavy isotopes (such as ] and ]), and ], a process in which energy is released that was originally stored in these heavy elements, before they were incorporated into the Solar System and the Earth. This energy is triggered and released in nuclear ]s or in civil nuclear power generation. Similarly, in the case of a ], ] energy is transformed to ] and ] in a very short time. | |||
| Hence, the second and higher terms in the series correspond with the "inaccuracy" of the Newtonian approximation for kinetic energy in relation to the relativistic formula. | |||
| Yet another example is that of a ]. At its highest points the ] is zero and the ] is at its maximum. At its lowest point the ] is at its maximum and is equal to the decrease in ]. If one (unrealistically) assumes that there is no ] or other losses, the conversion of energy between these processes would be perfect, and the ] would continue swinging forever. | |||
| == Potential energy == | |||
| Energy is also transferred from potential energy (<math>E_p</math>) to kinetic energy (<math>E_k</math>) and then back to potential energy constantly. This is referred to as conservation of energy. In this ], energy cannot be created or destroyed; therefore, the initial energy and the final energy will be equal to each other. This can be demonstrated by the following: | |||
| ''Main article: ].'' | |||
| {{NumBlk||<math display="block">E_{pi} + E_{ki} = E_{pF} + E_{kF}</math>|{{EquationRef|4}}}} | |||
| The equation can then be simplified further since <math>E_p = mgh</math> (mass times acceleration due to gravity times the height) and <math display="inline">E_k = \frac{1}{2} mv^2</math> (half mass times velocity squared). Then the total amount of energy can be found by adding <math>E_p + E_k = E_\text{total}</math>. | |||
| While ] is the portion of a ]'s energy associated with motion, ] is the energy of a system associated with the spatial configuration of the system's components and their interaction(s) with each other. | |||
| === Conservation of energy and mass in transformation === | |||
| In an isolated system consisting of two stationary objects lying on the x-axis that exert a force <math>f(x)</math> on each other, the potential energy is most generally defined as | |||
| Energy gives rise to weight when it is trapped in a system with zero momentum, where it can be weighed. It is also equivalent to mass, and this mass is always associated with it. Mass is also equivalent to a certain amount of energy, and likewise always appears associated with it, as described in ]. The formula ''E'' = ''mc''<sup>2</sup>, derived by ] (1905) quantifies the relationship between ] and energy within the concept of special relativity. In different theoretical frameworks, similar formulas were derived by ] (1881), ] (1900), ] (1904) and others (see ] for further information). | |||
| Part of the rest energy (equivalent to rest mass) of ] may be converted to other forms of energy (still exhibiting mass), but neither energy nor mass can be destroyed; rather, both remain constant during any process. However, since <math>c^2</math> is extremely large relative to ordinary human scales, the conversion of an everyday amount of rest mass (for example, 1 kg) from rest energy to other forms of energy (such as kinetic energy, thermal energy, or the radiant energy carried by light and other radiation) can liberate tremendous amounts of energy (~ {{val|9|e=16|u=joules}}, equivalent to 21 megatons of TNT), as can be seen in ]s and nuclear weapons. | |||
| :<math>E_p = -\int f(x) \, dx</math> | |||
| Conversely, the mass equivalent of an everyday amount energy is minuscule, which is why a loss of energy (loss of mass) from most systems is difficult to measure on a weighing scale, unless the energy loss is very large. Examples of large transformations between rest energy (of matter) and other forms of energy (e.g., kinetic energy into particles with rest mass) are found in ] and ]. Often, however, the complete conversion of matter (such as atoms) to non-matter (such as photons) is forbidden by ]s. | |||
| where the force between the objects varies only with distance <math>x</math> and is integrated along the line connecting the two objects. | |||
| === Reversible and non-reversible transformations === | |||
| To further illustrate the relationship between force and potential energy, consider the same system of two objects situated along the x-axis. If the potential energy due to one of the objects at any point <math>x</math> is <math>U(x)</math>, then the force on the that object <math>x</math> is | |||
| Thermodynamics divides energy transformation into two kinds: ] and ]es. An irreversible process is one in which energy is dissipated (spread) into empty energy states available in a volume, from which it cannot be recovered into more concentrated forms (fewer quantum states), without degradation of even more energy. A reversible process is one in which this sort of dissipation does not happen. For example, conversion of energy from one type of potential field to another is reversible, as in the pendulum system described above. | |||
| In processes where heat is generated, quantum states of lower energy, present as possible excitations in fields between atoms, act as a reservoir for part of the energy, from which it cannot be recovered, in order to be converted with 100% efficiency into other forms of energy. In this case, the energy must partly stay as thermal energy and cannot be completely recovered as usable energy, except at the price of an increase in some other kind of heat-like increase in disorder in quantum states, in the universe (such as an expansion of matter, or a randomization in a crystal). | |||
| :<math>f(x) = -\frac{dU(x)}{dx}</math> | |||
| As the universe evolves with time, more and more of its energy becomes trapped in irreversible states (i.e., as heat or as other kinds of increases in disorder). This has led to the hypothesis of the inevitable thermodynamic ]. In this heat death the energy of the universe does not change, but the fraction of energy which is available to do work through a ], or be transformed to other usable forms of energy (through the use of generators attached to heat engines), continues to decrease. | |||
| This relationship demonstrates that the force between the objects is in the direction of decreasing potential energy, and the magnitude of the force is proportional to the extent to which potential energy decreases. A large force is associated with a large decrease in potential energy, while a small force is associated with a small decrease in potential energy. Notice how the force on an object depends entirely on its potential energy. | |||
| == Conservation of energy == | |||
| These two relationships – the definition of potential energy based on force, and the dependence of force on potential energy – show how the concepts of force and potential energy are intimately linked: if two objects do not exert forces on each other, there is no potential energy between them. If two objects do exert forces on each other, then potential energy naturally arises in the system as part of the system's total energy. Since potential energy arises from forces, any change in the system's spatial configuration will either increase or decrease the system's potential energy as the objects are repositioned. | |||
| {{Main|Conservation of energy}} | |||
| The fact that energy can be neither created nor destroyed is called the law of ]. In the form of the ], this states that a ]'s energy is constant unless energy is transferred in or out as ] or ], and that no energy is lost in transfer. The total inflow of energy into a system must equal the total outflow of energy from the system, plus the change in the energy contained within the system. Whenever one measures (or calculates) the total energy of a system of particles whose interactions do not depend explicitly on time, it is found that the total energy of the system always remains constant.<ref>Charles Kittel, Walter D. Knight and Malvin A. Ruderman. Berkeley Physics Course, Vol. 1.</ref> | |||
| While heat can always be fully converted into work in a reversible isothermal expansion of an ideal gas, for cyclic processes of practical interest in ]s the ] states that the system doing work always loses some energy as ]. This creates a limit to the amount of heat energy that can do work in a cyclic process, a limit called the ]. Mechanical and other forms of energy can be transformed in the other direction into ] without such limitations.<ref name="thermo-laws"/> The total energy of a system can be calculated by adding up all forms of energy in the system. | |||
| When a system moves to a lower potential energy state, energy is either released in some form or converted into another form of energy, such as kinetic energy. The potential energy can be "stored" as gravitational energy, elastic energy, chemical energy, rest mass energy or electrical energy, but arises in all cases from the spatial positioning and interaction of objects within a system. Unlike kinetic energy, which exists in any moving body, potential energy exists in any body which is interacting with another object. | |||
| ] said during a 1961 lecture:<ref name="RPF1"/> | |||
| For example a mass released above the ] initially has potential energy resulting from the ] of the Earth, which is transferred to kinetic energy as the gravitational force acts on the object and its potential energy is decreased as it falls. | |||
| {{Blockquote|There is a fact, or if you wish, a ''law'', governing all natural phenomena that are known to date. There is no known exception to this law – it is exact so far as we know. The law is called the '']''. It states that there is a certain quantity, which we call energy, that does not change in manifold changes which nature undergoes. That is a most abstract idea, because it is a mathematical principle; it says that there is a numerical quantity which does not change when something happens. It is not a description of a mechanism, or anything concrete; it is just a strange fact that we can calculate some number and when we finish watching nature go through her tricks and calculate the number again, it is the same.|'']''}} | |||
| Most kinds of energy (with gravitational energy being a notable exception)<ref>{{cite web|url=http://www.physics.ucla.edu/~cwp/articles/noether.asg/noether.html |title=E. Noether's Discovery of the Deep Connection Between Symmetries and Conservation Laws |publisher=UCLA Physics & Astronomy |date=December 1996 |first1=Nina |last1=Byers |access-date=2010-12-12 |url-status=dead |archive-url=https://web.archive.org/web/20110514080739/http://www.physics.ucla.edu/~cwp/articles/noether.asg/noether.html |archive-date=2011-05-14 }}</ref> are subject to strict local conservation laws as well. In this case, energy can only be exchanged between adjacent regions of space, and all observers agree as to the volumetric density of energy in any given space. There is also a global law of conservation of energy, stating that the total energy of the universe cannot change; this is a corollary of the local law, but not vice versa.<ref name="thermo-laws">. {{webarchive|url=https://web.archive.org/web/20061215201900/http://www.av8n.com/physics/thermo-laws.htm|date=2006-12-15}} including careful definitions of energy, free energy, et cetera.</ref><ref name="RPF1">{{Cite book|first=Richard|last=Feynman|title=The Feynman Lectures on Physics; Volume 1 |chapter=Ch. 4: Conservation of Energy |chapter-url=https://feynmanlectures.caltech.edu/I_04.html#Ch4-S1-p2|year=1964|publisher=Addison Wesley|location=US|isbn=978-0-201-02115-8|access-date=2022-05-04|archive-date=2022-07-30|archive-url=https://web.archive.org/web/20220730093042/https://www.feynmanlectures.caltech.edu/I_04.html#Ch4-S1-p2|url-status=live}}</ref> | |||
| Equation: | |||
| :<math>E_p = mgh \;</math> | |||
| This law is a fundamental principle of physics. As shown rigorously by ], the conservation of energy is a mathematical consequence of ] of time,<ref>{{cite web |url=http://ptolemy.eecs.berkeley.edu/eecs20/week9/timeinvariance.html |title=Time Invariance |publisher=Ptolemy Project |work=EECS20N |access-date=2010-12-12 |url-status=live |archive-url=https://web.archive.org/web/20110717210455/http://ptolemy.eecs.berkeley.edu/eecs20/week9/timeinvariance.html |archive-date=2011-07-17 }}</ref> a property of most phenomena below the cosmic scale that makes them independent of their locations on the time coordinate. Put differently, yesterday, today, and tomorrow are physically indistinguishable. This is because energy is the quantity which is ] to time. This mathematical entanglement of energy and time also results in the uncertainty principle – it is impossible to define the exact amount of energy during any definite time interval (though this is practically significant only for very short time intervals). The uncertainty principle should not be confused with ] – rather it provides mathematical limits to which energy can in principle be defined and measured. | |||
| where ''m'' is the mass, ''h'' is the height and ''g'' is the value of ] due to gravity at the Earth's surface (see ]). | |||
| Each of the basic forces of nature is associated with a different type of potential energy, and all types of potential energy (like all other types of energy) appear as system ], whenever present. For example, a compressed spring will be slightly more massive than before it was compressed. Likewise, whenever energy is transferred between systems by any mechanism, an associated mass is transferred with it. | |||
| ==Internal energy== | |||
| In ] energy is expressed using the ]. On any time scales, the uncertainty in the energy is by | |||
| ''Main article: ].'' | |||
| : <math>\Delta E \Delta t \ge \frac { \hbar } {2 } </math> | |||
| which is similar in form to the ] (but not really mathematically equivalent thereto, since ''H'' and ''t'' are not dynamically conjugate variables, neither in classical nor in quantum mechanics). | |||
| In ], this inequality permits a qualitative understanding of ], which carry ]. The exchange of virtual particles with real particles is responsible for the creation of all known ] (more accurately known as ]). ] are also responsible for the electrostatic interaction between ]s (which results in ]), for ] radiative decay of excited atomic and nuclear states, for the ], for the ] and some other observable phenomena. | |||
| ''Internal energy'' is the ] associated with the motion of ]s, and the ] associated with the ], ] and ] energy of ]s within molecules. ], like energy, is a quantifiable ] of a system. | |||
| == Energy |
== Energy transfer == | ||
| {{redirect|Energy transfer|the pipeline company|Energy Transfer Partners}} | |||
| The energy is a characteristic of the state of the system. | |||
| If the system is moved to a different configuration and then put back to the previous configuration the system will have the same energy as it was previously. For this to be true all the forces (or fields) should be conservative. In the case there are non-conservative forces, the so-called principle of conservation of energy loses its importance. Usually it is needed to take in consideration some energy channel that was previously neglected (like friction) to know the reason of otherwise unexplainable loss of energy. | |||
| === Closed systems === | |||
| ==Examples== | |||
| Energy transfer can be considered for the special case of systems which are ] to transfers of matter. The portion of the energy which is transferred by ]s over a distance is measured as the ] the source system does on the receiving system. The portion of the energy which does not do work during the transfer is called ].<ref group=note>Although heat is "wasted" energy for a specific energy transfer (see: ]), it can often be harnessed to do useful work in subsequent interactions. However, the maximum energy that can be "recycled" from such recovery processes is limited by the ].</ref> Energy can be transferred between systems in a variety of ways. Examples include the transmission of ] via photons, physical collisions which transfer ],<ref group=note>The mechanism for most macroscopic physical collisions is actually ], but it is very common to simplify the interaction by ignoring the mechanism of collision and just calculate the beginning and end result.</ref> ],<ref>{{cite book | title=The Physics of Energy | first1=Robert L. | last1=Jaffe | first2=Washington | last2=Taylor | date=2018 | isbn=9781107016651 | page=611 | publisher=Cambridge University Press | url=https://books.google.com/books?id=drZDDwAAQBAJ&pg=PA611 | access-date=2022-05-22 | archive-date=2022-07-30 | archive-url=https://web.archive.org/web/20220730093040/https://www.google.com/books/edition/The_Physics_of_Energy/drZDDwAAQBAJ?gbpv=1&pg=PA611 | url-status=live }}</ref> and the conductive transfer of ]. | |||
| Energy is strictly conserved and is also locally conserved wherever it can be defined. In thermodynamics, for closed systems, the process of energy transfer is described by the ]:<ref group=note>There are several ]. Here, the signs in this equation follow the IUPAC convention.</ref> | |||
| An example of the conversion and conservation of energy is a ]. At its highest points the kinetic energy is zero and the potential gravitational energy is at its maximum. At its lowest point the kinetic energy is at its maximum and is equal to the decrease of potential energy. If one unrealistically assumes that there is no ], the energy will be conserved and the pendulum will continue swinging forever. | |||
| {{NumBlk|:|<math>\Delta{}E = W + Q </math>|{{EquationRef|1}}}} | |||
| Another example is a ] in which potential chemical energy is converted to kinetic energy and heat in a very short time. | |||
| where <math>E</math> is the amount of energy transferred, <math>W</math> represents the work done on or by the system, and <math>Q</math> represents the heat flow into or out of the system. As a simplification, the heat term, <math>Q</math>, can sometimes be ignored, especially for fast processes involving gases, which are poor conductors of heat, or when the ] of the transfer is high. For such ]es, | |||
| ==See also== | |||
| {{NumBlk|:|<math>\Delta{}E = W</math>|{{EquationRef|2}}}} | |||
| *] | |||
| *] | |||
| *] | |||
| *] | |||
| *] | |||
| *] | |||
| *] | |||
| *] | |||
| *] | |||
| *] | |||
| *] | |||
| *] | |||
| This simplified equation is the one used to define the ], for example. | |||
| === Energy Agencies === | |||
| * ]. | |||
| == |
=== Open systems === | ||
| Beyond the constraints of closed systems, ] can gain or lose energy in association with matter transfer (this process is illustrated by injection of an air-fuel mixture into a car engine, a system which gains in energy thereby, without addition of either work or heat). Denoting this energy by <math>E_\text{matter}</math>, one may write | |||
| {{NumBlk|:|<math>\Delta E = W + Q + E_\text{matter} .</math>|{{EquationRef|3}}}} | |||
| * | |||
| * | |||
| == |
== Thermodynamics == | ||
| === Internal energy === | |||
| *]. ''Six Easy Pieces: Essentials of Physics Explained by Its Most Brilliant Teacher''. Helix Book. See the chapter "conservation of energy" for Feynman's explanation of what energy is and how to think about it. | |||
| ] is the sum of all microscopic forms of energy of a system. It is the energy needed to create the system. It is related to the potential energy, e.g., molecular structure, crystal structure, and other geometric aspects, as well as the motion of the particles, in form of kinetic energy. Thermodynamics is chiefly concerned with changes in internal energy and not its absolute value, which is impossible to determine with thermodynamics alone.<ref name=klotz>I. Klotz, R. Rosenberg, ''Chemical Thermodynamics – Basic Concepts and Methods'', 7th ed., Wiley (2008), p. 39</ref> | |||
| === First law of thermodynamics === | |||
| The ] asserts that the total energy of a system and its surroundings (but not necessarily ]) is always conserved<ref name="KK">{{Cite book|author=Kittel and Kroemer|title=Thermal Physics |year=1980|publisher=W.H. Freeman |location=New York| isbn=978-0-7167-1088-2}}</ref> and that heat flow is a form of energy transfer. For homogeneous systems, with a well-defined temperature and pressure, a commonly used corollary of the first law is that, for a system subject only to ] forces and heat transfer (e.g., a cylinder-full of gas) without chemical changes, the differential change in the internal energy of the system (with a ''gain'' in energy signified by a positive quantity) is given as | |||
| : <math>\mathrm{d}E = T\mathrm{d}S - P\mathrm{d}V\,,</math> | |||
| where the first term on the right is the heat transferred into the system, expressed in terms of ] ''T'' and ] ''S'' (in which entropy increases and its change d''S'' is positive when heat is added to the system), and the last term on the right hand side is identified as work done on the system, where pressure is ''P'' and volume ''V'' (the negative sign results since compression of the system requires work to be done on it and so the volume change, d''V'', is negative when work is done on the system). | |||
| This equation is highly specific, ignoring all chemical, electrical, nuclear, and gravitational forces, effects such as ] of any form of energy other than heat and ''PV''-work. The general formulation of the first law (i.e., conservation of energy) is valid even in situations in which the system is not homogeneous. For these cases the change in internal energy of a ''closed'' system is expressed in a general form by | |||
| : <math>\mathrm{d}E=\delta Q+\delta W</math> | |||
| where <math>\delta Q</math> is the heat supplied to the system and <math>\delta W</math> is the work applied to the system. | |||
| === Equipartition of energy === | |||
| The energy of a mechanical ] (a mass on a spring) is alternately ] and ]. At two points in the oscillation ] it is entirely kinetic, and at two points it is entirely potential. Over a whole cycle, or over many cycles, average energy is equally split between kinetic and potential. This is an example of the ]: the total energy of a system with many degrees of freedom is equally split among all available degrees of freedom, on average. | |||
| This principle is vitally important to understanding the behavior of a quantity closely related to energy, called ]. Entropy is a measure of evenness of a ] of energy between parts of a system. When an isolated system is given more degrees of freedom (i.e., given new available ]s that are the same as existing states), then total energy spreads over all available degrees equally without distinction between "new" and "old" degrees. This mathematical result is part of the ]. The second law of thermodynamics is simple only for systems which are near or in a physical ]. For non-equilibrium systems, the laws governing the systems' behavior are still debatable. One of the guiding principles for these systems is the principle of ].<ref>{{cite journal|last1=Onsager|first1=L.|title=Reciprocal relations in irreversible processes.|journal=Phys. Rev. |volume=37|issue=4|date=1931|pages=405–26|bibcode=1931PhRv...37..405O|doi=10.1103/PhysRev.37.405|doi-access=free}}</ref><ref>{{cite journal |last1=Martyushev |first1=L. M. |last2=Seleznev |first2=V. D. |date=2006 |title=Maximum entropy production principle in physics, chemistry and biology |journal=Physics Reports |volume=426 |issue=1 |pages=1–45 |bibcode=2006PhR...426....1M |doi=10.1016/j.physrep.2005.12.001}}</ref> It states that nonequilibrium systems behave in such a way as to maximize their entropy production.<ref>{{cite journal|last1=Belkin|first1=A.|last2=et.|first2=al.|title=Self-Assembled Wiggling Nano-Structures and the Principle of Maximum Entropy Production|journal=Sci. Rep. |volume=5|pages=8323|date=2015|issue=1 |doi=10.1038/srep08323|pmid=25662746|pmc=4321171|bibcode=2015NatSR...5.8323B}}</ref> | |||
| == See also == | |||
| {{Portal|Energy|Physics|Renewable energy}} | |||
| {{cols}} | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| {{colend}} | |||
| {{clear}} | |||
| == Notes == | |||
| {{reflist|group=note}} | |||
| == References == | == References == | ||
| {{reflist}} | |||
| == Further reading == | |||
| {{refbegin}} | |||
| * {{cite book |first=G. N. |last=Alekseev |title=Energy and Entropy |url=https://archive.org/details/EnergyAndEntropy |year=1986 |publisher=Mir Publishers |location=Moscow, Russia |ref=none}} | |||
| * ''The ]'' (A '']'' Book), San Francisco, California, W. H. Freeman and Company, 1970.{{ISBN|0-7167-0945-7}}. This book, originally a 1970 '']'' issue, covers virtually every major concern and concept since debated regarding materials and ]s, ] trends, and ]. | |||
| * {{citation |title=Light and Matter |last=Crowell |first=Benjamin |year=2011 |chapter=ch. 11 |publisher=Light and Matter |location=Fullerton, California |chapter-url=http://www.lightandmatter.com/lm |access-date=2017-04-12 |archive-date=2011-05-19 |archive-url=https://web.archive.org/web/20110519093054/http://lightandmatter.com/lm/ |url-status=live |ref=none}} | |||
| * ''Energy and Power'' (A '']'' Book), San Francisco, California, W. H. Freeman and Company, 1971.{{ISBN|0-7167-0938-4}}. | |||
| * {{cite web |last=Ross |first=John S. |title=Work, Power, Kinetic Energy |url=http://www.physnet.org/modules/pdf_modules/m20.pdf |work=Project PHYSNET |publisher=Michigan State University |date=23 April 2002 |access-date=10 April 2009 |archive-date=26 April 2011 |archive-url=https://web.archive.org/web/20110426160837/http://www.physnet.org/modules/pdf_modules/m20.pdf |url-status=live |ref=none}} | |||
| * Santos, Gildo M. "Energy in Brazil: a historical overview," ''The Journal of Energy History'' (2018), .{{Webarchive|url=https://web.archive.org/web/20190209180117/http://www.energyhistory.eu/en/panorama/energy-brazil-historical-overview |date=2019-02-09 |ref=none}} | |||
| * {{cite book |author=Smil |first=Vaclav |title=Energy in nature and society: general energetics of complex systems |year=2008 |publisher=MIT Press |location=Cambridge, Massachusetts |isbn=978-0-262-19565-2 |ref=none}} | |||
| * {{cite book |author1=Walding |first=Richard |author2=Rapkins, Greg |author3=Rossiter, Glenn |title=New Century Senior Physics |date=1999 |publisher=Oxford University Press |location=Melbourne, Australia |isbn=978-0-19-551084-3 |ref=none}} | |||
| {{refend}} | |||
| === Journals === | |||
| *] (1952). ''Relativity: The Special and the General Theory (Fifteenth Edition)''. ISBN 0-517-88441-0 | |||
| * | |||
| == External links== | |||
| ] | |||
| {{Sister project links|d=Q11379|collapsible=collapsed}} | |||
| ] | |||
| {{Prone to spam|date=July 2013}} | |||
| ---- | |||
| <!-- {{No more links}} | |||
| Apart from its usage in physics the concept of energy is also widely used in the many movements and beliefs that comprise ] but in contrast to physics its ] is not practical and reliable, or is even left completely undefined. | |||
| Please be cautious adding more external links. | |||
| Misplaced Pages is not a collection of links and should not be used for advertising. | |||
| Excessive or inappropriate links will be removed. | |||
| See ] and ] for details. | |||
| If there are already suitable links, propose additions or replacements on | |||
| the article's talk page, or submit your link to the relevant category at | |||
| the "long dead (2017)" Open Directory Project (dmoz.org) and link there using {{Dmoz}}. | |||
| --> | |||
| * ({{Webarchive|url=https://web.archive.org/web/20160827224418/http://www.biocab.org/Heat.html |date=2016-08-27 }}) – BioCab | |||
| {{Footer energy|state=collapsed}} | |||
| <!-- interwiki --> | |||
| {{Nature nav}} | |||
| {{Natural resources}} | |||
| {{Authority control}} | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
| ] | |||
Latest revision as of 17:19, 26 December 2024
Physical quantity This article is about the scalar physical quantity. For an overview of and topical guide to energy, see Outline of energy. For other uses, see Energy (disambiguation). "Energetic" redirects here. For other uses, see Energetic (disambiguation).
| Energy | |
|---|---|
 A plasma globe, using electrical energy to create plasma, light, heat, movement and a faint sound A plasma globe, using electrical energy to create plasma, light, heat, movement and a faint sound | |
| Common symbols | E |
| SI unit | joule |
| Other units | kW⋅h, BTU, calorie, eV, erg, foot-pound |
| In SI base units | J = kg⋅m⋅s |
| Extensive? | yes |
| Conserved? | yes |
| Dimension | M L T |
| Thermodynamics | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 The classical Carnot heat engine The classical Carnot heat engine | |||||||||||||||||||||
| Branches | |||||||||||||||||||||
| Laws | |||||||||||||||||||||
Systems
|
|||||||||||||||||||||
System propertiesNote: Conjugate variables in italics
|
|||||||||||||||||||||
Material properties
|
|||||||||||||||||||||
| Equations | |||||||||||||||||||||
| Potentials | |||||||||||||||||||||
|
|||||||||||||||||||||
| Scientists | |||||||||||||||||||||
| Other | |||||||||||||||||||||
Energy (from Ancient Greek ἐνέργεια (enérgeia) 'activity') is the quantitative property that is transferred to a body or to a physical system, recognizable in the performance of work and in the form of heat and light. Energy is a conserved quantity—the law of conservation of energy states that energy can be converted in form, but not created or destroyed. The unit of measurement for energy in the International System of Units (SI) is the joule (J).
Forms of energy include the kinetic energy of a moving object, the potential energy stored by an object (for instance due to its position in a field), the elastic energy stored in a solid object, chemical energy associated with chemical reactions, the radiant energy carried by electromagnetic radiation, the internal energy contained within a thermodynamic system, and rest energy associated with an object's rest mass. These are not mutually exclusive.
All living organisms constantly take in and release energy. The Earth's climate and ecosystems processes are driven primarily by radiant energy from the sun. The energy industry provides the energy required for human civilization to function, which it obtains from energy resources such as fossil fuels, nuclear fuel, and renewable energy.
Forms


The total energy of a system can be subdivided and classified into potential energy, kinetic energy, or combinations of the two in various ways. Kinetic energy is determined by the movement of an object – or the composite motion of the object's components – while potential energy reflects the potential of an object to have motion, generally being based upon the object's position within a field or what is stored within the field itself.
While these two categories are sufficient to describe all forms of energy, it is often convenient to refer to particular combinations of potential and kinetic energy as its own form. For example, the sum of translational and rotational kinetic and potential energy within a system is referred to as mechanical energy, whereas nuclear energy refers to the combined potentials within an atomic nucleus from either the nuclear force or the weak force, among other examples.
| Type of energy | Description |
|---|---|
| Mechanical | the sum of macroscopic translational and rotational kinetic and potential energies |
| Electric | potential energy due to or stored in electric fields |
| Magnetic | potential energy due to or stored in magnetic fields |
| Gravitational | potential energy due to or stored in gravitational fields |
| Chemical | potential energy due to chemical bonds |
| Ionization | potential energy that binds an electron to its atom or molecule |
| Nuclear | potential energy that binds nucleons to form the atomic nucleus (and nuclear reactions) |
| Chromodynamic | potential energy that binds quarks to form hadrons |
| Elastic | potential energy due to the deformation of a material (or its container) exhibiting a restorative force as it returns to its original shape |
| Mechanical wave | kinetic and potential energy in an elastic material due to a propagating oscillation of matter |
| Sound wave | kinetic and potential energy in a material due to a sound propagated wave (a particular type of mechanical wave) |
| Radiant | potential energy stored in the fields of waves propagated by electromagnetic radiation, including light |
| Rest | potential energy due to an object's rest mass |
| Thermal | kinetic energy of the microscopic motion of particles, a kind of disordered equivalent of mechanical energy |
History
Main articles: History of energy and timeline of thermodynamics, statistical mechanics, and random processes
The word energy derives from the Ancient Greek: ἐνέργεια, romanized: energeia, lit. 'activity, operation', which possibly appears for the first time in the work of Aristotle in the 4th century BC. In contrast to the modern definition, energeia was a qualitative philosophical concept, broad enough to include ideas such as happiness and pleasure.
In the late 17th century, Gottfried Wilhelm Leibniz proposed the idea of the Latin: vis viva, or living force, which defined as the product of the mass of an object and its velocity squared; he believed that total vis viva was conserved. To account for slowing due to friction, Leibniz theorized that thermal energy consisted of the motions of the constituent parts of matter, although it would be more than a century until this was generally accepted. The modern analog of this property, kinetic energy, differs from vis viva only by a factor of two. Writing in the early 18th century, Émilie du Châtelet proposed the concept of conservation of energy in the marginalia of her French language translation of Newton's Principia Mathematica, which represented the first formulation of a conserved measurable quantity that was distinct from momentum, and which would later be called "energy".
In 1807, Thomas Young was possibly the first to use the term "energy" instead of vis viva, in its modern sense. Gustave-Gaspard Coriolis described "kinetic energy" in 1829 in its modern sense, and in 1853, William Rankine coined the term "potential energy". The law of conservation of energy was also first postulated in the early 19th century, and applies to any isolated system. It was argued for some years whether heat was a physical substance, dubbed the caloric, or merely a physical quantity, such as momentum. In 1845 James Prescott Joule discovered the link between mechanical work and the generation of heat.
These developments led to the theory of conservation of energy, formalized largely by William Thomson (Lord Kelvin) as the field of thermodynamics. Thermodynamics aided the rapid development of explanations of chemical processes by Rudolf Clausius, Josiah Willard Gibbs, and Walther Nernst. It also led to a mathematical formulation of the concept of entropy by Clausius and to the introduction of laws of radiant energy by Jožef Stefan. According to Noether's theorem, the conservation of energy is a consequence of the fact that the laws of physics do not change over time. Thus, since 1918, theorists have understood that the law of conservation of energy is the direct mathematical consequence of the translational symmetry of the quantity conjugate to energy, namely time.
Units of measure

In the International System of Units (SI), the unit of energy is the joule. It is a derived unit that is equal to the energy expended, or work done, in applying a force of one newton through a distance of one metre. However energy can also be expressed in many other units not part of the SI, such as ergs, calories, British thermal units, kilowatt-hours and kilocalories, which require a conversion factor when expressed in SI units.
The SI unit of power, defined as energy per unit of time, is the watt, which is a joule per second. Thus, one joule is one watt-second, and 3600 joules equal one watt-hour. The CGS energy unit is the erg and the imperial and US customary unit is the foot pound. Other energy units such as the electronvolt, food calorie or thermodynamic kcal (based on the temperature change of water in a heating process), and BTU are used in specific areas of science and commerce.
In 1843, English physicist James Prescott Joule, namesake of the unit of measure, discovered that the gravitational potential energy lost by a descending weight attached via a string was equal to the internal energy gained by the water through friction with the paddle.
Scientific use
Classical mechanics
| Part of a series on |
| Classical mechanics |
|---|
| Second law of motion |
| Branches |
| Fundamentals |
| Formulations |
| Core topics |
| Rotation |
| Scientists |
In classical mechanics, energy is a conceptually and mathematically useful property, as it is a conserved quantity. Several formulations of mechanics have been developed using energy as a core concept.
Work, a function of energy, is force times distance.
This says that the work () is equal to the line integral of the force F along a path C; for details see the mechanical work article. Work and thus energy is frame dependent. For example, consider a ball being hit by a bat. In the center-of-mass reference frame, the bat does no work on the ball. But, in the reference frame of the person swinging the bat, considerable work is done on the ball.
The total energy of a system is sometimes called the Hamiltonian, after William Rowan Hamilton. The classical equations of motion can be written in terms of the Hamiltonian, even for highly complex or abstract systems. These classical equations have direct analogs in nonrelativistic quantum mechanics.
Another energy-related concept is called the Lagrangian, after Joseph-Louis Lagrange. This formalism is as fundamental as the Hamiltonian, and both can be used to derive the equations of motion or be derived from them. It was invented in the context of classical mechanics, but is generally useful in modern physics. The Lagrangian is defined as the kinetic energy minus the potential energy. Usually, the Lagrange formalism is mathematically more convenient than the Hamiltonian for non-conservative systems (such as systems with friction).
Noether's theorem (1918) states that any differentiable symmetry of the action of a physical system has a corresponding conservation law. Noether's theorem has become a fundamental tool of modern theoretical physics and the calculus of variations. A generalisation of the seminal formulations on constants of motion in Lagrangian and Hamiltonian mechanics (1788 and 1833, respectively), it does not apply to systems that cannot be modeled with a Lagrangian; for example, dissipative systems with continuous symmetries need not have a corresponding conservation law.
Chemistry
In the context of chemistry, energy is an attribute of a substance as a consequence of its atomic, molecular, or aggregate structure. Since a chemical transformation is accompanied by a change in one or more of these kinds of structure, it is usually accompanied by a decrease, and sometimes an increase, of the total energy of the substances involved. Some energy may be transferred between the surroundings and the reactants in the form of heat or light; thus the products of a reaction have sometimes more but usually less energy than the reactants. A reaction is said to be exothermic or exergonic if the final state is lower on the energy scale than the initial state; in the less common case of endothermic reactions the situation is the reverse.
Chemical reactions are usually not possible unless the reactants surmount an energy barrier known as the activation energy. The speed of a chemical reaction (at a given temperature T) is related to the activation energy E by the Boltzmann's population factor e; that is, the probability of a molecule to have energy greater than or equal to E at a given temperature T. This exponential dependence of a reaction rate on temperature is known as the Arrhenius equation. The activation energy necessary for a chemical reaction can be provided in the form of thermal energy.
Biology
Main articles: Bioenergetics and Food energy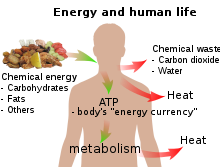
In biology, energy is an attribute of all biological systems, from the biosphere to the smallest living organism. Within an organism it is responsible for growth and development of a biological cell or organelle of a biological organism. Energy used in respiration is stored in substances such as carbohydrates (including sugars), lipids, and proteins stored by cells. In human terms, the human equivalent (H-e) (Human energy conversion) indicates, for a given amount of energy expenditure, the relative quantity of energy needed for human metabolism, using as a standard an average human energy expenditure of 12,500 kJ per day and a basal metabolic rate of 80 watts.
For example, if our bodies run (on average) at 80 watts, then a light bulb running at 100 watts is running at 1.25 human equivalents (100 ÷ 80) i.e. 1.25 H-e. For a difficult task of only a few seconds' duration, a person can put out thousands of watts, many times the 746 watts in one official horsepower. For tasks lasting a few minutes, a fit human can generate perhaps 1,000 watts. For an activity that must be sustained for an hour, output drops to around 300; for an activity kept up all day, 150 watts is about the maximum. The human equivalent assists understanding of energy flows in physical and biological systems by expressing energy units in human terms: it provides a "feel" for the use of a given amount of energy.
Sunlight's radiant energy is also captured by plants as chemical potential energy in photosynthesis, when carbon dioxide and water (two low-energy compounds) are converted into carbohydrates, lipids, proteins and oxygen. Release of the energy stored during photosynthesis as heat or light may be triggered suddenly by a spark in a forest fire, or it may be made available more slowly for animal or human metabolism when organic molecules are ingested and catabolism is triggered by enzyme action.
All living creatures rely on an external source of energy to be able to grow and reproduce – radiant energy from the Sun in the case of green plants and chemical energy (in some form) in the case of animals. The daily 1500–2000 Calories (6–8 MJ) recommended for a human adult are taken as food molecules, mostly carbohydrates and fats, of which glucose (C6H12O6) and stearin (C57H110O6) are convenient examples. The food molecules are oxidized to carbon dioxide and water in the mitochondria and some of the energy is used to convert ADP into ATP:
ADP + HPO4 → ATP + H2OThe rest of the chemical energy of the carbohydrate or fat are converted into heat: the ATP is used as a sort of "energy currency", and some of the chemical energy it contains is used for other metabolism when ATP reacts with OH groups and eventually splits into ADP and phosphate (at each stage of a metabolic pathway, some chemical energy is converted into heat). Only a tiny fraction of the original chemical energy is used for work:
- gain in kinetic energy of a sprinter during a 100 m race: 4 kJ
- gain in gravitational potential energy of a 150 kg weight lifted through 2 metres: 3 kJ
- daily food intake of a normal adult: 6–8 MJ
It would appear that living organisms are remarkably inefficient (in the physical sense) in their use of the energy they receive (chemical or radiant energy); most machines manage higher efficiencies. In growing organisms the energy that is converted to heat serves a vital purpose, as it allows the organism tissue to be highly ordered with regard to the molecules it is built from. The second law of thermodynamics states that energy (and matter) tends to become more evenly spread out across the universe: to concentrate energy (or matter) in one specific place, it is necessary to spread out a greater amount of energy (as heat) across the remainder of the universe ("the surroundings"). Simpler organisms can achieve higher energy efficiencies than more complex ones, but the complex organisms can occupy ecological niches that are not available to their simpler brethren. The conversion of a portion of the chemical energy to heat at each step in a metabolic pathway is the physical reason behind the pyramid of biomass observed in ecology. As an example, to take just the first step in the food chain: of the estimated 124.7 Pg/a of carbon that is fixed by photosynthesis, 64.3 Pg/a (52%) are used for the metabolism of green plants, i.e. reconverted into carbon dioxide and heat.
Earth sciences
In geology, continental drift, mountain ranges, volcanoes, and earthquakes are phenomena that can be explained in terms of energy transformations in the Earth's interior, while meteorological phenomena like wind, rain, hail, snow, lightning, tornadoes and hurricanes are all a result of energy transformations in our atmosphere brought about by solar energy.
Sunlight is the main input to Earth's energy budget which accounts for its temperature and climate stability. Sunlight may be stored as gravitational potential energy after it strikes the Earth, as (for example when) water evaporates from oceans and is deposited upon mountains (where, after being released at a hydroelectric dam, it can be used to drive turbines or generators to produce electricity). Sunlight also drives most weather phenomena, save a few exceptions, like those generated by volcanic events for example. An example of a solar-mediated weather event is a hurricane, which occurs when large unstable areas of warm ocean, heated over months, suddenly give up some of their thermal energy to power a few days of violent air movement.
In a slower process, radioactive decay of atoms in the core of the Earth releases heat. This thermal energy drives plate tectonics and may lift mountains, via orogenesis. This slow lifting represents a kind of gravitational potential energy storage of the thermal energy, which may later be transformed into active kinetic energy during landslides, after a triggering event. Earthquakes also release stored elastic potential energy in rocks, a store that has been produced ultimately from the same radioactive heat sources. Thus, according to present understanding, familiar events such as landslides and earthquakes release energy that has been stored as potential energy in the Earth's gravitational field or elastic strain (mechanical potential energy) in rocks. Prior to this, they represent release of energy that has been stored in heavy atoms since the collapse of long-destroyed supernova stars (which created these atoms).
Cosmology
In cosmology and astronomy the phenomena of stars, nova, supernova, quasars and gamma-ray bursts are the universe's highest-output energy transformations of matter. All stellar phenomena (including solar activity) are driven by various kinds of energy transformations. Energy in such transformations is either from gravitational collapse of matter (usually molecular hydrogen) into various classes of astronomical objects (stars, black holes, etc.), or from nuclear fusion (of lighter elements, primarily hydrogen).
The nuclear fusion of hydrogen in the Sun also releases another store of potential energy which was created at the time of the Big Bang. At that time, according to theory, space expanded and the universe cooled too rapidly for hydrogen to completely fuse into heavier elements. This meant that hydrogen represents a store of potential energy that can be released by fusion. Such a fusion process is triggered by heat and pressure generated from gravitational collapse of hydrogen clouds when they produce stars, and some of the fusion energy is then transformed into sunlight.
Quantum mechanics
Main article: Energy operatorIn quantum mechanics, energy is defined in terms of the energy operator (Hamiltonian) as a time derivative of the wave function. The Schrödinger equation equates the energy operator to the full energy of a particle or a system. Its results can be considered as a definition of measurement of energy in quantum mechanics. The Schrödinger equation describes the space- and time-dependence of a slowly changing (non-relativistic) wave function of quantum systems. The solution of this equation for a bound system is discrete (a set of permitted states, each characterized by an energy level) which results in the concept of quanta. In the solution of the Schrödinger equation for any oscillator (vibrator) and for electromagnetic waves in a vacuum, the resulting energy states are related to the frequency by Planck's relation: (where is the Planck constant and the frequency). In the case of an electromagnetic wave these energy states are called quanta of light or photons.
Relativity
When calculating kinetic energy (work to accelerate a massive body from zero speed to some finite speed) relativistically – using Lorentz transformations instead of Newtonian mechanics – Einstein discovered an unexpected by-product of these calculations to be an energy term which does not vanish at zero speed. He called it rest energy: energy which every massive body must possess even when being at rest. The amount of energy is directly proportional to the mass of the body: where
- m0 is the rest mass of the body,
- c is the speed of light in vacuum,
- is the rest energy.
For example, consider electron–positron annihilation, in which the rest energy of these two individual particles (equivalent to their rest mass) is converted to the radiant energy of the photons produced in the process. In this system the matter and antimatter (electrons and positrons) are destroyed and changed to non-matter (the photons). However, the total mass and total energy do not change during this interaction. The photons each have no rest mass but nonetheless have radiant energy which exhibits the same inertia as did the two original particles. This is a reversible process – the inverse process is called pair creation – in which the rest mass of particles is created from the radiant energy of two (or more) annihilating photons.
In general relativity, the stress–energy tensor serves as the source term for the gravitational field, in rough analogy to the way mass serves as the source term in the non-relativistic Newtonian approximation.
Energy and mass are manifestations of one and the same underlying physical property of a system. This property is responsible for the inertia and strength of gravitational interaction of the system ("mass manifestations"), and is also responsible for the potential ability of the system to perform work or heating ("energy manifestations"), subject to the limitations of other physical laws.
In classical physics, energy is a scalar quantity, the canonical conjugate to time. In special relativity energy is also a scalar (although not a Lorentz scalar but a time component of the energy–momentum 4-vector). In other words, energy is invariant with respect to rotations of space, but not invariant with respect to rotations of spacetime (= boosts).
Transformation
Main article: Energy transformation| Type of transfer process | Description |
|---|---|
| Heat | equal amount of thermal energy in transit spontaneously towards a lower-temperature object |
| Work | equal amount of energy in transit due to a displacement in the direction of an applied force |
| Transfer of material | equal amount of energy carried by matter that is moving from one system to another |

Energy may be transformed between different forms at various efficiencies. Items that transform between these forms are called transducers. Examples of transducers include a battery (from chemical energy to electric energy), a dam (from gravitational potential energy to kinetic energy of moving water (and the blades of a turbine) and ultimately to electric energy through an electric generator), and a heat engine (from heat to work).
Examples of energy transformation include generating electric energy from heat energy via a steam turbine, or lifting an object against gravity using electrical energy driving a crane motor. Lifting against gravity performs mechanical work on the object and stores gravitational potential energy in the object. If the object falls to the ground, gravity does mechanical work on the object which transforms the potential energy in the gravitational field to the kinetic energy released as heat on impact with the ground. The Sun transforms nuclear potential energy to other forms of energy; its total mass does not decrease due to that itself (since it still contains the same total energy even in different forms) but its mass does decrease when the energy escapes out to its surroundings, largely as radiant energy.
There are strict limits to how efficiently heat can be converted into work in a cyclic process, e.g. in a heat engine, as described by Carnot's theorem and the second law of thermodynamics. However, some energy transformations can be quite efficient. The direction of transformations in energy (what kind of energy is transformed to what other kind) is often determined by entropy (equal energy spread among all available degrees of freedom) considerations. In practice all energy transformations are permitted on a small scale, but certain larger transformations are not permitted because it is statistically unlikely that energy or matter will randomly move into more concentrated forms or smaller spaces.
Energy transformations in the universe over time are characterized by various kinds of potential energy, that has been available since the Big Bang, being "released" (transformed to more active types of energy such as kinetic or radiant energy) when a triggering mechanism is available. Familiar examples of such processes include nucleosynthesis, a process ultimately using the gravitational potential energy released from the gravitational collapse of supernovae to "store" energy in the creation of heavy isotopes (such as uranium and thorium), and nuclear decay, a process in which energy is released that was originally stored in these heavy elements, before they were incorporated into the Solar System and the Earth. This energy is triggered and released in nuclear fission bombs or in civil nuclear power generation. Similarly, in the case of a chemical explosion, chemical potential energy is transformed to kinetic and thermal energy in a very short time.
Yet another example is that of a pendulum. At its highest points the kinetic energy is zero and the gravitational potential energy is at its maximum. At its lowest point the kinetic energy is at its maximum and is equal to the decrease in potential energy. If one (unrealistically) assumes that there is no friction or other losses, the conversion of energy between these processes would be perfect, and the pendulum would continue swinging forever.
Energy is also transferred from potential energy () to kinetic energy () and then back to potential energy constantly. This is referred to as conservation of energy. In this isolated system, energy cannot be created or destroyed; therefore, the initial energy and the final energy will be equal to each other. This can be demonstrated by the following:
| (4) |
The equation can then be simplified further since (mass times acceleration due to gravity times the height) and (half mass times velocity squared). Then the total amount of energy can be found by adding .
Conservation of energy and mass in transformation
Energy gives rise to weight when it is trapped in a system with zero momentum, where it can be weighed. It is also equivalent to mass, and this mass is always associated with it. Mass is also equivalent to a certain amount of energy, and likewise always appears associated with it, as described in mass–energy equivalence. The formula E = mc, derived by Albert Einstein (1905) quantifies the relationship between relativistic mass and energy within the concept of special relativity. In different theoretical frameworks, similar formulas were derived by J.J. Thomson (1881), Henri Poincaré (1900), Friedrich Hasenöhrl (1904) and others (see Mass–energy equivalence#History for further information).
Part of the rest energy (equivalent to rest mass) of matter may be converted to other forms of energy (still exhibiting mass), but neither energy nor mass can be destroyed; rather, both remain constant during any process. However, since is extremely large relative to ordinary human scales, the conversion of an everyday amount of rest mass (for example, 1 kg) from rest energy to other forms of energy (such as kinetic energy, thermal energy, or the radiant energy carried by light and other radiation) can liberate tremendous amounts of energy (~ 9×10 joules, equivalent to 21 megatons of TNT), as can be seen in nuclear reactors and nuclear weapons.
Conversely, the mass equivalent of an everyday amount energy is minuscule, which is why a loss of energy (loss of mass) from most systems is difficult to measure on a weighing scale, unless the energy loss is very large. Examples of large transformations between rest energy (of matter) and other forms of energy (e.g., kinetic energy into particles with rest mass) are found in nuclear physics and particle physics. Often, however, the complete conversion of matter (such as atoms) to non-matter (such as photons) is forbidden by conservation laws.
Reversible and non-reversible transformations
Thermodynamics divides energy transformation into two kinds: reversible processes and irreversible processes. An irreversible process is one in which energy is dissipated (spread) into empty energy states available in a volume, from which it cannot be recovered into more concentrated forms (fewer quantum states), without degradation of even more energy. A reversible process is one in which this sort of dissipation does not happen. For example, conversion of energy from one type of potential field to another is reversible, as in the pendulum system described above.
In processes where heat is generated, quantum states of lower energy, present as possible excitations in fields between atoms, act as a reservoir for part of the energy, from which it cannot be recovered, in order to be converted with 100% efficiency into other forms of energy. In this case, the energy must partly stay as thermal energy and cannot be completely recovered as usable energy, except at the price of an increase in some other kind of heat-like increase in disorder in quantum states, in the universe (such as an expansion of matter, or a randomization in a crystal).
As the universe evolves with time, more and more of its energy becomes trapped in irreversible states (i.e., as heat or as other kinds of increases in disorder). This has led to the hypothesis of the inevitable thermodynamic heat death of the universe. In this heat death the energy of the universe does not change, but the fraction of energy which is available to do work through a heat engine, or be transformed to other usable forms of energy (through the use of generators attached to heat engines), continues to decrease.
Conservation of energy
Main article: Conservation of energyThe fact that energy can be neither created nor destroyed is called the law of conservation of energy. In the form of the first law of thermodynamics, this states that a closed system's energy is constant unless energy is transferred in or out as work or heat, and that no energy is lost in transfer. The total inflow of energy into a system must equal the total outflow of energy from the system, plus the change in the energy contained within the system. Whenever one measures (or calculates) the total energy of a system of particles whose interactions do not depend explicitly on time, it is found that the total energy of the system always remains constant.
While heat can always be fully converted into work in a reversible isothermal expansion of an ideal gas, for cyclic processes of practical interest in heat engines the second law of thermodynamics states that the system doing work always loses some energy as waste heat. This creates a limit to the amount of heat energy that can do work in a cyclic process, a limit called the available energy. Mechanical and other forms of energy can be transformed in the other direction into thermal energy without such limitations. The total energy of a system can be calculated by adding up all forms of energy in the system.
Richard Feynman said during a 1961 lecture:
There is a fact, or if you wish, a law, governing all natural phenomena that are known to date. There is no known exception to this law – it is exact so far as we know. The law is called the conservation of energy. It states that there is a certain quantity, which we call energy, that does not change in manifold changes which nature undergoes. That is a most abstract idea, because it is a mathematical principle; it says that there is a numerical quantity which does not change when something happens. It is not a description of a mechanism, or anything concrete; it is just a strange fact that we can calculate some number and when we finish watching nature go through her tricks and calculate the number again, it is the same.
— The Feynman Lectures on Physics
Most kinds of energy (with gravitational energy being a notable exception) are subject to strict local conservation laws as well. In this case, energy can only be exchanged between adjacent regions of space, and all observers agree as to the volumetric density of energy in any given space. There is also a global law of conservation of energy, stating that the total energy of the universe cannot change; this is a corollary of the local law, but not vice versa.
This law is a fundamental principle of physics. As shown rigorously by Noether's theorem, the conservation of energy is a mathematical consequence of translational symmetry of time, a property of most phenomena below the cosmic scale that makes them independent of their locations on the time coordinate. Put differently, yesterday, today, and tomorrow are physically indistinguishable. This is because energy is the quantity which is canonical conjugate to time. This mathematical entanglement of energy and time also results in the uncertainty principle – it is impossible to define the exact amount of energy during any definite time interval (though this is practically significant only for very short time intervals). The uncertainty principle should not be confused with energy conservation – rather it provides mathematical limits to which energy can in principle be defined and measured.
Each of the basic forces of nature is associated with a different type of potential energy, and all types of potential energy (like all other types of energy) appear as system mass, whenever present. For example, a compressed spring will be slightly more massive than before it was compressed. Likewise, whenever energy is transferred between systems by any mechanism, an associated mass is transferred with it.
In quantum mechanics energy is expressed using the Hamiltonian operator. On any time scales, the uncertainty in the energy is by
which is similar in form to the Heisenberg Uncertainty Principle (but not really mathematically equivalent thereto, since H and t are not dynamically conjugate variables, neither in classical nor in quantum mechanics).
In particle physics, this inequality permits a qualitative understanding of virtual particles, which carry momentum. The exchange of virtual particles with real particles is responsible for the creation of all known fundamental forces (more accurately known as fundamental interactions). Virtual photons are also responsible for the electrostatic interaction between electric charges (which results in Coulomb's law), for spontaneous radiative decay of excited atomic and nuclear states, for the Casimir force, for the Van der Waals force and some other observable phenomena.
Energy transfer
"Energy transfer" redirects here. For the pipeline company, see Energy Transfer Partners.Closed systems
Energy transfer can be considered for the special case of systems which are closed to transfers of matter. The portion of the energy which is transferred by conservative forces over a distance is measured as the work the source system does on the receiving system. The portion of the energy which does not do work during the transfer is called heat. Energy can be transferred between systems in a variety of ways. Examples include the transmission of electromagnetic energy via photons, physical collisions which transfer kinetic energy, tidal interactions, and the conductive transfer of thermal energy.
Energy is strictly conserved and is also locally conserved wherever it can be defined. In thermodynamics, for closed systems, the process of energy transfer is described by the first law:
| (1) |
where is the amount of energy transferred, represents the work done on or by the system, and represents the heat flow into or out of the system. As a simplification, the heat term, , can sometimes be ignored, especially for fast processes involving gases, which are poor conductors of heat, or when the thermal efficiency of the transfer is high. For such adiabatic processes,
| (2) |
This simplified equation is the one used to define the joule, for example.
Open systems
Beyond the constraints of closed systems, open systems can gain or lose energy in association with matter transfer (this process is illustrated by injection of an air-fuel mixture into a car engine, a system which gains in energy thereby, without addition of either work or heat). Denoting this energy by , one may write
| (3) |
Thermodynamics
Internal energy
Internal energy is the sum of all microscopic forms of energy of a system. It is the energy needed to create the system. It is related to the potential energy, e.g., molecular structure, crystal structure, and other geometric aspects, as well as the motion of the particles, in form of kinetic energy. Thermodynamics is chiefly concerned with changes in internal energy and not its absolute value, which is impossible to determine with thermodynamics alone.
First law of thermodynamics
The first law of thermodynamics asserts that the total energy of a system and its surroundings (but not necessarily thermodynamic free energy) is always conserved and that heat flow is a form of energy transfer. For homogeneous systems, with a well-defined temperature and pressure, a commonly used corollary of the first law is that, for a system subject only to pressure forces and heat transfer (e.g., a cylinder-full of gas) without chemical changes, the differential change in the internal energy of the system (with a gain in energy signified by a positive quantity) is given as
where the first term on the right is the heat transferred into the system, expressed in terms of temperature T and entropy S (in which entropy increases and its change dS is positive when heat is added to the system), and the last term on the right hand side is identified as work done on the system, where pressure is P and volume V (the negative sign results since compression of the system requires work to be done on it and so the volume change, dV, is negative when work is done on the system).
This equation is highly specific, ignoring all chemical, electrical, nuclear, and gravitational forces, effects such as advection of any form of energy other than heat and PV-work. The general formulation of the first law (i.e., conservation of energy) is valid even in situations in which the system is not homogeneous. For these cases the change in internal energy of a closed system is expressed in a general form by
where is the heat supplied to the system and is the work applied to the system.
Equipartition of energy
The energy of a mechanical harmonic oscillator (a mass on a spring) is alternately kinetic and potential energy. At two points in the oscillation cycle it is entirely kinetic, and at two points it is entirely potential. Over a whole cycle, or over many cycles, average energy is equally split between kinetic and potential. This is an example of the equipartition principle: the total energy of a system with many degrees of freedom is equally split among all available degrees of freedom, on average.
This principle is vitally important to understanding the behavior of a quantity closely related to energy, called entropy. Entropy is a measure of evenness of a distribution of energy between parts of a system. When an isolated system is given more degrees of freedom (i.e., given new available energy states that are the same as existing states), then total energy spreads over all available degrees equally without distinction between "new" and "old" degrees. This mathematical result is part of the second law of thermodynamics. The second law of thermodynamics is simple only for systems which are near or in a physical equilibrium state. For non-equilibrium systems, the laws governing the systems' behavior are still debatable. One of the guiding principles for these systems is the principle of maximum entropy production. It states that nonequilibrium systems behave in such a way as to maximize their entropy production.
See also
- Combustion
- Efficient energy use
- Energy democracy
- Energy crisis
- Energy recovery
- Energy recycling
- Index of energy articles
- Index of wave articles
- List of low-energy building techniques
- Orders of magnitude (energy)
- Power station
- Sustainable energy
- Transfer energy
- Waste-to-energy
- Waste-to-energy plant
- Zero-energy building
Notes
- These examples are solely for illustration, as it is not the energy available for work which limits the performance of the athlete but the power output (in case of a sprinter) and the force (in case of a weightlifter).
- Crystals are another example of highly ordered systems that exist in nature: in this case too, the order is associated with the transfer of a large amount of heat (known as the lattice energy) to the surroundings.
- Although heat is "wasted" energy for a specific energy transfer (see: waste heat), it can often be harnessed to do useful work in subsequent interactions. However, the maximum energy that can be "recycled" from such recovery processes is limited by the second law of thermodynamics.
- The mechanism for most macroscopic physical collisions is actually electromagnetic, but it is very common to simplify the interaction by ignoring the mechanism of collision and just calculate the beginning and end result.
- There are several sign conventions for this equation. Here, the signs in this equation follow the IUPAC convention.
References
- "Earth's energy flow". Energy Education. Retrieved 2024-08-28.
- Bobrowsky, Matt (2021). "SCIENCE 101: Q: What Is Energy?". Science and Children. 59 (1): 61–65. doi:10.1080/19434812.2021.12291716. ISSN 0036-8148. JSTOR 27133353. S2CID 266084433. Retrieved February 5, 2024.
- "Nuclear Energy | Definition, Formula & Examples | nuclear-power.com". Nuclear Power. Archived from the original on 2022-07-06. Retrieved 2022-07-06.
- Harper, Douglas. "Energy". Online Etymology Dictionary. Archived from the original on October 11, 2007. Retrieved May 1, 2007.
- Smith, Crosbie (1998). The Science of Energy – a Cultural History of Energy Physics in Victorian Britain. The University of Chicago Press. ISBN 978-0-226-76420-7.
- Lofts, G.; O'Keeffe, D.; et al. (2004). "11 – Mechanical Interactions". Jacaranda Physics 1 (2 ed.). Milton, Queensland, Australia: John Wiley & Sons Australia Limited. p. 286. ISBN 978-0-7016-3777-4.
- The Hamiltonian MIT OpenCourseWare website 18.013A Chapter 16.3 Accessed February 2007
- "Retrieved on May-29-09". Uic.edu. Archived from the original on 2010-06-04. Retrieved 2010-12-12.
- Bicycle calculator – speed, weight, wattage etc. "Bike Calculator". Archived from the original on 2009-05-13. Retrieved 2009-05-29..
- Ito, Akihito; Oikawa, Takehisa (2004). "Global Mapping of Terrestrial Primary Productivity and Light-Use Efficiency with a Process-Based Model. Archived 2006-10-02 at the Wayback Machine" in Shiyomi, M. et al. (Eds.) Global Environmental Change in the Ocean and on Land. pp. 343–58.
- "Earth's Energy Budget". Okfirst.ocs.ou.edu. Archived from the original on 2008-08-27. Retrieved 2010-12-12.
- ^ Misner, Charles W.; Thorne, Kip S.; Wheeler, John Archibald (1973). Gravitation. San Francisco: W.H. Freeman. ISBN 978-0-7167-0344-0.
- Charles Kittel, Walter D. Knight and Malvin A. Ruderman. Berkeley Physics Course, Vol. 1.
- ^ The Laws of Thermodynamics. Archived 2006-12-15 at the Wayback Machine including careful definitions of energy, free energy, et cetera.
- ^ Feynman, Richard (1964). "Ch. 4: Conservation of Energy". The Feynman Lectures on Physics; Volume 1. US: Addison Wesley. ISBN 978-0-201-02115-8. Archived from the original on 2022-07-30. Retrieved 2022-05-04.
- Byers, Nina (December 1996). "E. Noether's Discovery of the Deep Connection Between Symmetries and Conservation Laws". UCLA Physics & Astronomy. Archived from the original on 2011-05-14. Retrieved 2010-12-12.
- "Time Invariance". EECS20N. Ptolemy Project. Archived from the original on 2011-07-17. Retrieved 2010-12-12.
- Jaffe, Robert L.; Taylor, Washington (2018). The Physics of Energy. Cambridge University Press. p. 611. ISBN 9781107016651. Archived from the original on 2022-07-30. Retrieved 2022-05-22.
- I. Klotz, R. Rosenberg, Chemical Thermodynamics – Basic Concepts and Methods, 7th ed., Wiley (2008), p. 39
- Kittel and Kroemer (1980). Thermal Physics. New York: W.H. Freeman. ISBN 978-0-7167-1088-2.
- Onsager, L. (1931). "Reciprocal relations in irreversible processes". Phys. Rev. 37 (4): 405–26. Bibcode:1931PhRv...37..405O. doi:10.1103/PhysRev.37.405.
- Martyushev, L. M.; Seleznev, V. D. (2006). "Maximum entropy production principle in physics, chemistry and biology". Physics Reports. 426 (1): 1–45. Bibcode:2006PhR...426....1M. doi:10.1016/j.physrep.2005.12.001.
- Belkin, A.; et., al. (2015). "Self-Assembled Wiggling Nano-Structures and the Principle of Maximum Entropy Production". Sci. Rep. 5 (1): 8323. Bibcode:2015NatSR...5.8323B. doi:10.1038/srep08323. PMC 4321171. PMID 25662746.
Further reading
- Alekseev, G. N. (1986). Energy and Entropy. Moscow, Russia: Mir Publishers.
- The Biosphere (A Scientific American Book), San Francisco, California, W. H. Freeman and Company, 1970.ISBN 0-7167-0945-7. This book, originally a 1970 Scientific American issue, covers virtually every major concern and concept since debated regarding materials and energy resources, population trends, and environmental degradation.
- Crowell, Benjamin (2011). "ch. 11". Light and Matter. Fullerton, California: Light and Matter. Archived from the original on 2011-05-19. Retrieved 2017-04-12.
- Energy and Power (A Scientific American Book), San Francisco, California, W. H. Freeman and Company, 1971.ISBN 0-7167-0938-4.
- Ross, John S. (23 April 2002). "Work, Power, Kinetic Energy" (PDF). Project PHYSNET. Michigan State University. Archived (PDF) from the original on 26 April 2011. Retrieved 10 April 2009.
- Santos, Gildo M. "Energy in Brazil: a historical overview," The Journal of Energy History (2018), online.Archived 2019-02-09 at the Wayback Machine
- Smil, Vaclav (2008). Energy in nature and society: general energetics of complex systems. Cambridge, Massachusetts: MIT Press. ISBN 978-0-262-19565-2.
- Walding, Richard; Rapkins, Greg; Rossiter, Glenn (1999). New Century Senior Physics. Melbourne, Australia: Oxford University Press. ISBN 978-0-19-551084-3.
Journals
External links
- Differences between Heat and Thermal energy (Archived 2016-08-27 at the Wayback Machine) – BioCab
| Elements of nature | |||||
|---|---|---|---|---|---|
| Universe | |||||
| Earth | |||||
| Weather | |||||
| Natural environment | |||||
| Life | |||||
| See also | |||||
| Natural resources | |||||||
|---|---|---|---|---|---|---|---|
| Air |
| ||||||
| Energy | |||||||
| Land | |||||||
| Life | |||||||
| Water |
| ||||||
| Related |
| ||||||

















 ) is equal to the
) is equal to the 
 and some of the energy is used to convert
and some of the energy is used to convert  (where
(where  is the
is the  the frequency). In the case of an electromagnetic wave these energy states are called quanta of light or
the frequency). In the case of an electromagnetic wave these energy states are called quanta of light or  where
where
 is the rest energy.
is the rest energy. ) to kinetic energy (
) to kinetic energy ( ) and then back to potential energy constantly. This is referred to as conservation of energy. In this
) and then back to potential energy constantly. This is referred to as conservation of energy. In this 
 (mass times acceleration due to gravity times the height) and
(mass times acceleration due to gravity times the height) and  (half mass times velocity squared). Then the total amount of energy can be found by adding
(half mass times velocity squared). Then the total amount of energy can be found by adding  .
.
 is extremely large relative to ordinary human scales, the conversion of an everyday amount of rest mass (for example, 1 kg) from rest energy to other forms of energy (such as kinetic energy, thermal energy, or the radiant energy carried by light and other radiation) can liberate tremendous amounts of energy (~ 9×10 joules, equivalent to 21 megatons of TNT), as can be seen in
is extremely large relative to ordinary human scales, the conversion of an everyday amount of rest mass (for example, 1 kg) from rest energy to other forms of energy (such as kinetic energy, thermal energy, or the radiant energy carried by light and other radiation) can liberate tremendous amounts of energy (~ 9×10 joules, equivalent to 21 megatons of TNT), as can be seen in 

 is the amount of energy transferred,
is the amount of energy transferred,  represents the heat flow into or out of the system. As a simplification, the heat term,
represents the heat flow into or out of the system. As a simplification, the heat term, 
 , one may write
, one may write



 is the heat supplied to the system and
is the heat supplied to the system and  is the work applied to the system.
is the work applied to the system.