| Revision as of 17:14, 28 November 2006 editJance (talk | contribs)3,137 edits DO NOT DELETE THIS. I left Oliver's all-optimistic statements, and added context for an NPOV approach← Previous edit | Latest revision as of 16:40, 8 November 2024 edit undoPek (talk | contribs)Extended confirmed users772 edits Made internal link. | ||
| Line 1: | Line 1: | ||
| {{short description|Prosthesis used to change the size, shape, and contour of a person's breast}} | |||
| A '''breast implant''' is a ] used to enlarge the size of a woman's ]s (known as '''breast augmentation''') for ], to ] (e.g. after a ] or to correct genetic deformities), or as an aspect of ]. | |||
| {{Infobox medical intervention | |||
| According to the ], breast augmentation is the third most commonly performed cosmetic surgical procedure in the United States. In ], 291,000 breast augmentation procedures were performed.<ref>. American Society of Plastic Surgeons. 15 March 2006. Retrieved 17 April 2006.</ref> | |||
| | synonym = | |||
| | image = Breast Implant Markings.ogg | |||
| | other_codes = | |||
| | MedlinePlus = | |||
| | eMedicine = | |||
| }} | |||
| <!-- Definition and medical uses --> | |||
| A '''breast implant''' is a ] used to change the size, shape, and contour of a person's ]. In reconstructive ], breast implants can be placed to restore a natural looking breast following a ], to correct ]s and ] of the chest wall or, cosmetically, to enlarge the appearance of the breast through ]. | |||
| <!-- Side effects --> | |||
| There are two primary types of breast implants: saline filled and silicone gel filled implants. ''Saline implants'' have a ] elastomer shell filled with sterile ] liquid. ''Silicone gel implants'' have a silicone shell filled with a viscous ] gel. | |||
| Complications of implants may include ], rashes, skin changes, infection, rupture, cosmetic changes to the breasts such as asymmetry and hardness, and a fluid collection around the breast.<ref>{{cite web |title=Risks and Complications of Breast Implants |url=https://www.fda.gov/medical-devices/breast-implants/risks-and-complications-breast-implants |website=FDA |access-date=30 October 2019 |language=en |date=21 October 2019}}</ref> | |||
| A rare complication associated with textured surfaced implants and polyurethane foam-covered implants is a type of lymphoma (cancer of the immune system) known as breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL).<ref>{{Cite journal |last=Loch-Wilkinson |first=Anna |last2=Beath |first2=Kenneth J |last3=Magnusson |first3=Mark R |last4=Cooter |first4=Rodney |last5=Shaw |first5=Karen |last6=French |first6=James |last7=Vickery |first7=Karen |last8=Prince |first8=H Miles |last9=Deva |first9=Anand K |date=2020-07-13 |title=Breast Implant-Associated Anaplastic Large Cell Lymphoma in Australia: A Longitudinal Study of Implant and Other Related Risk Factors |url=https://academic.oup.com/asj/article/40/8/838/5628192 |journal=Aesthetic Surgery Journal |language=en |volume=40 |issue=8 |pages=838–846 |doi=10.1093/asj/sjz333 |issn=1090-820X}}</ref> | |||
| ==History== | |||
| Implants have been used since ] to augment the size or shape of women's ]s. The earliest known implant was attempted by Czerny, using a woman's own ] (from a ], a benign growth, on her back).<ref>Czerny V. Plastischer Ersatz der Brusthus durch ein Lipoma. ''Zentralbl Chir'' 1895;27:72. </ref> Gersuny tried paraffin injections in 1889, with disastrous results. Subsequently, in the early to mid-1900s, a number of other substances were tried, including ivory, glass balls, ground rubber, ox cartilage, Terylene wool, gutta percha, Dicora, polyethylene chips, polyvinyl alcohol-formaldehyde polymer sponge (Ivalon), Ivalon in a polyethylene sac, polyether foam sponge (Etheron), polyethylene tape (Polystan) or strips wound into a ball, polyester (polyurethane foam sponge) Silastic rubber, and teflon-silicone prostheses.In recent history, various creams and medicaments have been used in attempts to increase bust size, and Berson in 1945 and Maliniac in 1950 performed a flap-based augmentation by rotating the patients chest wall tissue into the breast to add volume. Various synthetics were used throughout the 1950s and 1960s, including silicone injections, which an estimated 50,000 women received.<ref>Anderson N. Lawsuit Science: Lessons from the Silicone Breast Implant Controversy. "New York Law School Law Review" 1997; 41 (2), 401-407.</ref> Development of silicone granulomas and hardening of the breasts were in some cases so severe that women needed to have ] for treatment. Women sometimes seek medical treatment for complications up to 30 years after receiving this type of injection. | |||
| <!-- Types --> | |||
| ==Indications== | |||
| There are four general types of breast implants, defined by their filler material: saline solution, silicone gel, structured and composite filler. The saline implant has an ] ] shell filled with sterile ] during surgery; the silicone implant has an elastomer silicone shell pre-filled with viscous ] gel; structured implants use nested elastomer silicone shells and two saline-filled lumen; and the alternative composition implants featured miscellaneous fillers, such as hydrogel, ] or ].{{cn|date=May 2024}} | |||
| Clinical indications for the use of breast implants are for ], ], and for abnormalities that affect the shape and size of the breast. In some countries ] will reimburse insertion of breast implants only for these indications{{fact}}. Non-clinical indications (the most common reasons) are cosmetic. | |||
| <!-- Techniques --> | |||
| ==Patient Characteristics== | |||
| In surgical practice, for the reconstruction of a breast, the ] device is a temporary breast prosthesis used to form and establish an implant pocket for the future permanent breast implant. For the correction of male breast defects and deformities, the pectoral implant is the breast prosthesis used for the reconstruction and the aesthetic repair of a man's chest wall (see: ] and ]).{{cn|date=May 2024}} | |||
| Patients seeking breast augmentation are usually younger, healthier, and from higher socio-economic status than the population at large.<ref name="Brinton2000">{{cite journal | author = Brinton L, Brown S, Colton T, Burich M, Lubin J | title = Characteristics of a population of women with breast implants compared with women seeking other types of plastic surgery. | journal = Plast Reconstr Surg | volume = 105 | issue = 3 | pages = 919-27; discussion 928-9 | year = 2000 | id = PMID 10724251}}</ref> Many of these patients have greater distress about their appearance in a variety of situations, and have endured more frequent teasing about their appearance. Studies have identified a pattern (shared by many cosmetic surgery procedures) that suggest women who undergo breast implantation are slightly more likely to have undergone psychotherapy, have low levels of self-esteem, and have higher prevalences of depression, suicide attempts and mental illness as compared to the general population.<ref name="Sarwer2003">{{cite journal | author=Sarwer DB,, et al. | title=Body image concerns of breast augmentation patients. | journal=Plast Reconstr Surg.| year=2003 | issue=July| pages=83-90| id=PMID 12832880 }}</ref> </p> | |||
| {{TOC limit}} | |||
| ==Uses== | |||
| Post-operative surveys on mental health and quality of life issues have shown improvement on a number of dimensions including: physical health, physical appearance, social life and self confidence.<ref name="Young1994">{{cite journal | author=Young VL, et al. | title=The efficacy of breast augmentation: breast size increase, patient satisfaction, and psychological effects. | journal=Plast Reconstr Surg.| year=1994 | issue=Dec| pages=958-69| id=PMID 7972484 | |||
| }}</ref><ref name="chahraoui2006">{{cite journal | author=Chahraoui K,, et al. | title=Aesthetic surgery and quality of life before and four months postoperatively | journal=J Long-Term Effects Medical Implants | year=2006 | pages=207-210 | id=PMID 16181718 }}</ref><ref name="Cash2002">{{cite journal | author=Cash TF, et al. | title=Women's psychosocial outcomes of breast augmentation with silicone gel-filled implants: a 2-year prospective study. | journal=Plast Reconstr Surg.| year=2002 | issue=May| pages=2112-21| id=PMID 11994621 }}</ref> The large majority of patients reports being satisfied long-term with their implants even when they have required reopertion for complications or aesthetic reasons.<ref name="HandelN">{{cite journal | author=HandelN, et al. | title=A long-term study of outcomes, complications, and patient satisfaction with breast implants. | journal=Plast Reconstr Surg.| year=2006 | issue=Mar| pages=757-67| id=PMID 16525261 }}</ref> </p> | |||
| ] of a woman with breast implants]] | |||
| <ref name="Young1994">{{cite journal | author=Young VL, et al. | title=The efficacy of breast augmentation: breast size increase, patient satisfaction, and psychological effects. | journal=Plast Reconstr Surg.| year=1994 | issue=Dec| pages=958-69| id=PMID 7972484 | |||
| }}</ref> </p> | |||
| A ] procedure for the placement of breast implant devices has three purposes: | |||
| ==Types of implants== | |||
| ===Saline Implants=== | |||
| Saline-filled breast implants were first manufactured in France in 1964, introduced by Arion<ref name="Arion1965">{{cite journal | author=Arion HG| title=Retromammary prosthesis| journal=C R Soc Fr Gynecol | year=1965| volume=5}}</ref> with the goal of being surgically placed via smaller incisions. These original devices had a high failure rate and were discontinued in the early 1970s {{fact}}. The current devices are manufactured with thicker, room temperature vulcanized (RTV) shells. These shells are made of silicone elastomer and the implants are filled with salt water after the implant is placed in the body. Since the implants are empty when they are surgically inserted, the scar is smaller than is necessary for silicone gel breast implants (which are filled with silicone before the surgery is performed). | |||
| # primary reconstruction: the replacement of breast tissues damaged by trauma (], ], ]), disease (]), and failed anatomic development (]). | |||
| Saline-filled implants are the most common implant used in the United States due to restrictions on silicone implants, but are rarely used in other countries. Good to excellent results may be obtained, but as compared to silicone gel implants, saline implants are more likely to cause cosmetic problems such as rippling, wrinkling, and be noticeable to the eye or the touch. Particularly for women with very little breast tissue, or for post-mastectomy reconstruction, plastic surgeons believe that silicone gel implants are the superior device. In patients with more breast tissue, however, saline implants can look very similar to silicone gel.<p> | |||
| # revision and reconstruction: to revise (correct) the outcome of a previous breast reconstruction surgery. | |||
| # primary augmentation: to aesthetically ] the size, form, and feel of the breasts. | |||
| The ] time of post–] ], and of ] surgery is determined by the procedure employed, the type of incisions, the breast implant (type and materials), and the pectoral locale of the implant pocket. | |||
| ===Silicone gel implants=== | |||
| ] and ], two ], ], ], developed the first silicone breast prosthesis with the ] in ]. The first woman was implanted in ]. | |||
| Recent research has indicated that mammograms should not be done with any greater frequency than that used in normal procedure in patients undergoing breast surgery, including breast implant, augmentation, mastopexy, and breast reduction.<ref>{{Citation|author1=American Society of Plastic Surgeons |author1-link=American Society of Plastic Surgeons |date=24 April 2014 |title=Five Things Physicians and Patients Should Question |publisher=American Society of Plastic Surgeons |work=]: an initiative of the ] |url=http://www.choosingwisely.org/doctor-patient-lists/american-society-of-plastic-surgeons/ |access-date=25 July 2014 |url-status=dead |archive-url=https://web.archive.org/web/20140719103909/http://www.choosingwisely.org/doctor-patient-lists/american-society-of-plastic-surgeons/ |archive-date=19 July 2014 }}</ref> | |||
| ====Silicone implant generations==== | |||
| Silicone implants are generally described in terms of five generations which segregates common characteristics of manufacturing techniques. | |||
| ===Psychology=== | |||
| * '''First generation''' | |||
| {{further|Body dysmorphic disorder|Body image|Beauty}} | |||
| The Cronin-Gerow implants were made of a silicone rubber envelope (or sac), filled with a thick, viscous silicone gel with a Dacron patch on the posterior shell.<ref>Cronin TD, Gerow FJ. Augmentation mammaplasty: a new "natural feel" prosthesis. Excerpta Medica International Congress Series 1963;66:41.</ref> They were firm and had a "teardrop" anatomic shape. | |||
| * '''Second generation''' | |||
| In response to surgeons' requests for softer and more lifelike implants, breast implants were redesigned in the 1970s with thinner gel and thinner shells. These implants had a greater tendency to rupture and leak, or "bleed" silicone through the porous shell, and complications such as capsular contracture were also quite common. It was predominantly implants of this generation that were involved in the class action-lawsuits against Dow-Corning in the early 1990s. | |||
| Another development in the ] was a '''''polyurethane foam coating''''' on the implant shell which was effective in diminishing capsular contracture by causing an inflammatory reaction that discouraged formation of fibrous tissue around the capsule. These implants were later discontinued due to concern of potential carcinogenic breakdown products from the polyurethane.<!-- --><ref name="Luu11998">{{cite journal | author=Luu HM, Hutter JC, Bushar HF | title=A physiologically based pharmacokinetic model for 2,4-toluenediamine leached from polyurethane foam-covered breast implants | journal=Environ Health Perspect | year=1998 | pages=393-400 | volume=106 | issue=7 | id=PMID 9637796 }}</ref> A review of the risk for cancer from TDA by the FDA later concluded that the risk was so small so as not to justify removal of the devices. Polyurethane implants are still used in Europe and South America, but no manufacturer has sought FDA approval for sale in the United States.<!-- --><ref name=”Hester2001”>{{cite journal | author=Hester TR Jr, Tebbetts JB, Maxwell GP | title=The polyurethane-covered mammary prosthesis: facts and fiction (II): a look back and a "peek" ahead | journal=Clin Plast Surg | year=2001 | pages=579-86 | volume=28 | issue=3 | id=PMID 11471963}}</ref> | |||
| Second-generation implants also included various '''"double lumen" designs'''. These implants were essentially a silicone implant inside a saline implant. The double lumen was an attempt to provide the cosmetic benefits of gel in the inside lumen, while the outside lumen contained saline and its volume could be adjusted after placement. The failure rate of these implants is higher than for single lumen implants due to their more complex design. The contemporary versions of these devices ("Becker Implants") are used primarily for breast reconstruction. | |||
| * '''Third & Fourth generation''' | |||
| ] | |||
| Third & fourth generation implants, represented sequential advances in manufacturing principles and were elastomer-coated to decrease gel bleed, and a filled with thicker, more cohesive gel. These implants are sold under restricted conditions in the U.S. and Canada, and are widely used in other countries. The increased cohesion of the gel filler reduces leakage of the gel compared to earlier devices. A variety of both round and tapered anatomic shapes are available. Anatomic shaped implants are uniformly textured to reduce rotation, while round devices are available in smooth or textured surfaces. ] | |||
| * '''Fifth generation''' | |||
| Evaluation of "]" or high-cohesive, form-stable implants is in preliminary stages in the United States but enjoys wide use in the rest of the world. It is believed that the high degree of gel cohesion in these implants is likely to eliminate or significantly reduce the possibility of silicone migration. Early reports of these devices have shown excellent safety and efficacy. <!-- --><ref name="Brown2005">{{cite journal | author=Brown MH, Shenker R, Silver SA | title=Cohesive silicone gel breast implants in aesthetic and reconstructive breast surgery | journal=Plast Reconstr Surg | year=2005 | pages=768-79; discussion 780-1 | volume=116 | issue=3 | id=PMID 16141814}}</ref><!-- --><ref name="Fruhstorfer2004">{{cite journal | author=Fruhstorfer BH, Hodgson EL, Malata CM | title=Early experience with an anatomical soft cohesive silicone gel prosthesis in cosmetic and reconstructive breast implant surgery | journal=Ann Plast Surg | year=2004 | pages=536-42 | volume=53 | issue=6 | id=PMID 15602249}}</ref><!-- --><ref name="Heden2001">{{cite journal | author=Heden P, Jernbeck J, Hober M | title=Breast augmentation with anatomical cohesive gel implants: the world's largest current experience | journal=Clin Plast Surg | year=2001 | pages=531-52 | volume=28 | issue=3 | id=PMID 11471959}}</ref> | |||
| The ] patient usually is a young woman whose personality profile indicates psychological distress about her personal appearance and her bodily ], and a history of having endured criticism (teasing) about the ] of her person.<ref name="Brinton2000">{{cite journal |vauthors=Brinton LA, Brown SL, Colton T, Burich MC, Lubin J | s2cid = 32599107 | title = Characteristics of a Population of Women with Breast Implants Compared with Women Seeking other Types of Plastic Surgery | journal = Plastic and Reconstructive Surgery | volume = 105 | issue = 3 | pages = 919–927 | year = 2000 | pmid = 10724251 | doi = 10.1097/00006534-200003000-00014 | url = https://zenodo.org/record/1234820 }}</ref> The studies ''Body Image Concerns of Breast Augmentation Patients'' (2003){{fcn|date=June 2022}} and ''Body Dysmorphic Disorder and Cosmetic Surgery'' (2006){{fcn|date=June 2022}} reported that the woman who underwent breast augmentation surgery also had undergone ], suffered low ], presented frequent occurrences of ], had attempted ], and had ], a type of mental illness. | |||
| ====Silicone Implant Crisis of early 1990s==== | |||
| Although silicone gel-filled breast implants were introduced into the US market decades prior, the FDA did not have a statutory basis to regulate most medical devices until the late 1970s. After a number of anecdotal reports surfaced alleging problems in patients with gel implants, the FDA asked for and reviewed hastily assembled data submitted by several implant manufacturers. They later concluded that the amount of data presented was not sufficient to prove safety but did not necessarily reflect any evidence of an associated risk between the devices and disease. In 1992, amid intense political pressure and media publicity, the FDA reclassified the devices as experimental and restricted silicone gel-filled breast implants to clinical trials, primarily for women needing reconstruction after mastectomy or breast deformity, or for women with implants that need to be replaced. Prior to the FDA’s restrictions on silicone gel-filled implants in 1992, approximately 97% of women who underwent breast implant surgery chose to have silicone gel-filled implants. In November 2006, the FDA reapproved silicone implants for cosmetic and reconstructive indications. | |||
| Post-operative patient surveys about mental health and quality-of-life, reported improved physical health, physical appearance, social life, self-confidence, self-esteem, and satisfactory ]. Furthermore, the women reported long-term satisfaction with their breast implant outcomes; some despite having medical complications that required surgical revision, either corrective or aesthetic. Likewise, in Denmark, 8% of breast augmentation patients had a pre-operative history of psychiatric hospitalization.<ref name="Jacobsen PH 2004">{{cite journal |vauthors=Jacobsen PH, Hölmich LR, McLaughlin JK, Johansen C, Olsen JH, Kjøller K, Friis S | title = Mortality and suicide among Danish women with cosmetic breast implants | journal = Arch. Intern. Med. | volume = 164 | issue = 22 | pages = 2450–5 | year = 2004 | pmid = 15596635 | doi = 10.1001/archinte.164.22.2450 | doi-access = }}</ref><ref name="Young1994">{{cite journal |vauthors=Young VL, Nemecek JR, Nemecek DA | s2cid = 753343 | title = The Efficacy of Breast Augmentation: Breast Size Increase, Patient Satisfaction, and Psychological Effects | journal = Plastic and Reconstructive Surgery | volume = 94 | issue = Dec | pages = 958–969 | year = 1994 | pmid = 7972484 | doi = 10.1097/00006534-199412000-00009 }}</ref><ref name="Crerand 2006">{{cite journal |vauthors=Crerand CE, Franklin ME, Sarwer DB | s2cid = 8925060 | title = Body Dysmorphic Disorder and Cosmetic Surgery | journal = Plastic and Reconstructive Surgery | volume = 118 | issue = July | pages = 167e–180e | year = 2006 | pmid = 17102719 | doi = 10.1097/01.prs.0000242500.28431.24 }}</ref><ref name="Sarwer2003">{{cite journal |vauthors=Sarwer DB, LaRossa D, Bartlett SP, Low DW, Bucky LP, Whitaker LA | s2cid = 45574374 | title = Body Image Concerns of Breast Augmentation Patients | journal = Plastic and Reconstructive Surgery | volume = 112 | issue = July | pages = 83–90 | year = 2003 | pmid = 12832880 | doi = 10.1097/01.PRS.0000066005.07796.51 }}</ref><ref name="chahraoui2006">{{cite journal |vauthors=Chahraoui K, Danino A, Frachebois C, Clerc AS, Malka G | title = Aesthetic Surgery and Quality of Life Before and Four Months Postoperatively | journal = Journal of Long-Term Effects of Medical Implants | volume = 51 | issue = 3 | pages = 207–210 | year = 2006 | pmid = 16181718 | doi = 10.1016/j.anplas.2005.07.010 }}</ref><ref name="Cash2002">{{cite journal |vauthors=Cash TF, Duel LA, Perkins LL | title = Women's Psychosocial Outcomes of Breast Augmentation with Silicone gel-filled implants: a 2-year Prospective Study | journal = Plastic and Reconstructive Surgery | volume = 109 | issue = May | pages = 2112–2121 | year = 2002 | pmid = 11994621 | doi = 10.1097/00006534-200205000-00049 }}</ref><ref name="Haas2007">{{cite journal | author = Figueroa-Haas CL | s2cid = 23169107 | title = Effect of Breast Augmentation Mammoplasty on Self-esteem and Sexuality: A Quantitative Analysis | journal = Plastic Surgery Nursing | volume = 27 | issue = Mar | pages = 16–36 | year = 2007 | pmid = 17356451 | doi = 10.1097/01.PSN.0000264159.30505.c9 }}</ref><ref name="Inamed2006">{{cite web |title=Important Information for Women About Breast Augmentation with Inamed Silicone Gel-Filled Implants |website=] |year=2006 |url=https://www.fda.gov/cdrh/pdf2/P020056d.pdf |url-status=dead |archive-url=https://web.archive.org/web/20070103050703/https://www.fda.gov/cdrh/pdf2/P020056d.pdf |archive-date=2007-01-03}}</ref><ref>{{Cite web |last=Dolan |first=Eric W. |date=2023-10-06 |title=Breast implants have a positive impact on female sexuality, according to new research |url=https://www.psypost.org/2023/10/breast-implants-have-a-positive-impact-on-female-sexuality-according-to-new-research-213917 |access-date=2023-10-09 |website=PsyPost |language=en-US}}</ref><ref name="HandelN">{{cite journal |vauthors=Handel N, Cordray T, Gutierrez J, Jensen JA | s2cid = 15228702 | title = A Long-term Study of Outcomes, Complications, and Patient Satisfaction with Breast Implants | journal = Plastic and Reconstructive Surgery | volume = 117 | issue = Mar | pages = 757–767 | year = 2006 | pmid = 16525261 | doi = 10.1097/01.prs.0000201457.00772.1d }}</ref> | |||
| In ], a similar series of events occurred where silicone gel-filled prostheses were restricted by Health Canada in ] prior to a limited reintroduction in ]. A 2005 Canadian panel of experts reviewed the data and research again, and found no compelling reason to restrict access to the devices<!-- --><ref name="HC-Implants">{{cite web | author= Health Canada | title=It's Your Health: Breast Implants | url=http://www.hc-sc.gc.ca/iyh-vsv/alt_formats/cmcd-dcmc/pdf/implants_e.pdf | publisher=Health Canada}}</ref>, and in October 2006, Health Canada removed the restrictions on the use of these implants. | |||
| In 2008, the ] ''Excess Mortality from Suicide and other External Causes of Death Among Women with Cosmetic Breast Implants'' (2007), reported that women who sought breast implants are almost 3 times as likely to commit ] as are women who have not sought breast implants. Compared to the standard suicide-rate for women of the general populace, the suicide-rate for women with augmented breasts remained constant until 10-years post-implantation, yet, it increased to 4.5 times greater at the 11-year mark, and so remained until the 19-year mark, when it increased to 6 times greater at 20-years post-implantation. Moreover, additional to the suicide-risk, women with breast implants also faced a trebled death-risk from ] and the abuse of prescription and recreational drugs.<ref name="reuters.com">{{cite news | url=https://www.reuters.com/article/healthNews/idUSN0836919020070808?rpc=22&sp=true | work=Reuters | title=Breast Implants Linked with Suicide in Study | date=2007-08-08 | url-status=live | archive-url=https://web.archive.org/web/20081221140806/http://www.reuters.com/article/healthNews/idUSN0836919020070808?feedType=RSS&rpc=22&sp=true | archive-date=2008-12-21 }}</ref><ref name="usatoday.com">{{cite news | url=https://www.usatoday.com/news/health/2007-08-06-breast-implants_N.htm | work=USA Today | title=Breast Implants Linked to Higher Suicide Rates | first=Anita | last=Manning | name-list-style = vanc | date=2007-08-06 | access-date=2010-04-26 | url-status=live | archive-url=https://web.archive.org/web/20110318150357/http://www.usatoday.com/news/health/2007-08-06-breast-implants_N.htm | archive-date=2011-03-18 }}</ref> Although seven studies have statistically connected a woman's breast augmentation to a greater suicide-rate, the research indicates that breast augmentation surgery does not increase the death rate; and that, in the first instance, it is the ]-inclined woman who is more likely to undergo a breast augmentation procedure.<ref name="pmid11306343">{{cite journal |vauthors=Brinton LA, Lubin JH, Burich MC, Colton T, Brown SL, Hoover RN | title = Cancer risk at sites other than the breast following augmentation mammoplasty | journal = Ann Epidemiol | volume = 11 | issue = 4 | pages = 248–256 | year = 2001 | pmid = 11306343 | doi = 10.1016/s1047-2797(00)00223-4| url = https://zenodo.org/record/1260190}}</ref><ref name="pmid12623911">{{cite journal |vauthors=Koot VC, Peeters PH, Granath F, Grobbee DE, Nyren O | title = Total and cause specific mortality among Swedish women with cosmetic breast implants: prospective study | journal = BMJ | volume = 326 | issue = 7388 | pages = 527–8 | year = 2003 | pmid = 12623911 | pmc = 150462 | doi = 10.1136/bmj.326.7388.527 }}</ref><ref name="pmid14520056">{{cite journal |vauthors=Pukkala E, Kulmala I, Hovi SL, Hemminki E, Keskimäki I, Pakkanen M, Lipworth L, Boice JD, McLaughlin JK | s2cid = 34929987 | title = Causes of death among Finnish women with cosmetic breast implants, 1971-2001 | journal = Ann Plast Surg | volume = 51 | issue = 4 | pages = 339–42; discussion 343–4 | year = 2003 | pmid = 14520056 | doi = 10.1097/01.sap.0000080407.97677.A5 }}</ref><ref name="pmid16777929">{{cite journal |vauthors=Villeneuve PJ, Holowaty EJ, Brisson J, Xie L, Ugnat AM, Latulippe L, Mao Y | title = Mortality among Canadian women with cosmetic breast implants | journal = Am. J. Epidemiol. | volume = 164 | issue = 4 | pages = 334–41 | year = 2006 | pmid = 16777929 | doi = 10.1093/aje/kwj214 | doi-access = }}</ref><ref name="pmid16477256">{{cite journal |vauthors=Brinton LA, Lubin JH, Murray MC, Colton T, Hoover RN | s2cid = 22285852 | title = Mortality rates among augmentation mammoplasty patients: an update | journal = Epidemiology | volume = 17 | issue = 2 | pages = 162–9 | year = 2006 | pmid = 16477256 | doi = 10.1097/01.ede.0000197056.84629.19 | doi-access = free }}</ref><ref>National Plastic Surgery Procedural Statistics, 2006. Arlington Heights, Illinois, American Society of Plastic Surgeons, 2007</ref> | |||
| No other countries in the world have any significant restrictions on access to silicone breast implants. | |||
| The study ''Effect of Breast Augmentation Mammoplasty on Self-Esteem and Sexuality: A Quantitative Analysis'' (2007), reported that the women attributed their improved ], ], and increased, satisfactory sexual functioning to having undergone breast augmentation; the cohort, aged 21–57 years, averaged post-operative self-esteem increases that ranged from 20.7 to 24.9 points on the 30-point ], which data supported the 78.6 per cent increase in the woman's ], relative to her pre-operative level of libido.<ref>{{cite web|url=http://psychcentral.com/news/2007/03/23/plastic-surgery-helps-self-esteem/703.html|title=Plastic Surgery Helps Self-Esteem|work=Psych Central.com|url-status=live|archive-url=https://web.archive.org/web/20100619034928/http://psychcentral.com/news/2007/03/23/plastic-surgery-helps-self-esteem/703.html|archive-date=2010-06-19}}</ref> Therefore, before agreeing to any surgery, the plastic surgeon evaluates and considers the woman's ] to determine if breast implants can positively affect her self-esteem and ]. | |||
| == Implant Placement Techniques == | |||
| ===Incision Types=== | |||
| Breast implants for augmentation may be placed via various types of incisions: | |||
| * '''Inframammary''' - an incision is placed below the breast in the infra-mammary fold (IMF). This incision is the most commona approach and affords maximum access for disection and placement of an implant. It is often the preferred technique for silicone gel implants due to the longer incisions required. | |||
| * '''Periareolar''' - an incision is placed along the areolar border. This incision provides an optimal approach when adjustments to the IMF position or mastopexy (breast lift) procedures are planned. The incision is generally placed around the inferior half, or the medial half of the areola's circumference. Larger silicone gel implants are difficult to place via this incision. | |||
| * '''Transaxillary''' - an incision is placed in the armpit and the dissection tunnels medially. This approach allows implants to be placed with no visible scars on the breast. Transaxillary procedures can be performed with or without an endoscope (tiny lighted camera). | |||
| * '''Transumbilical (TUBA)''' - a less common technique where an incision is placed in the navel and dissection tunnels superiorly. This approach enables implants to be placed with no visible scars on the breast, but makes appropriate disection and implant placement more difficult. Transumbilical procedures may be performed bluntly or with an endoscope (tiny lighted camera) to assist dissection. This technique is not appropriate for placing silicone gel implants due to potential damage of the implant shell during blunt insertion. | |||
| * '''Transabdominoplasty (TABA)''' - procedure similar to TUBA, where the implants are tunneled up from the abdomen into bluntly dissected pockets while a patient is simultaneously undergoing an abdominoplasty procedure. | |||
| ===Implant Pocket Placement=== | |||
| The placement of implants is described in relation to the pectoralis major muscle. | |||
| * '''Subglandular'''- implant between the breast tissue and the pectoralis muscle. This position closely resembles the plane of normal breast tissue and is felt by many to achieve the most aesthetic results. The subglandular position in patients with thin soft-tissue coverage is most likely to show ripples or wrinkles of the underlying implant. Capsular contracture rates are also slightly higher with this approach | |||
| * '''Subfascial'''- the implant is placed in the subglandular position, but underneath the fascia of the pectoralis muscle. The benefits of this technique are debated, but proponents believe the thin vascularized fascia may help with coverage and sustaining positioning of the implant. | |||
| * '''Subpectoral''' ("dual plane")- the implant is placed underneath the pectoralis major muscle after releasing the inferior muscular attachments. As a result, the implant is partially beneath the pectoralis in the upper pole, while the lower half of the implant is in the subglandular plane. This is the most common technique in North America and achieves maximal upper implant coverage while allowing expansion of the lower pole. Capsular contracture rates have been lower after widespread adoption of this technique. | |||
| * '''Submuscular'''- the implant is placed below the pectoralis without release of the inferior origin of the muscle. Total muscular coverage may be achieved by releasing the lateral chest wall muscles (seratus and/or pectoralis minor) and sewn to the pectoralis major. This technique is most commonly used for maximal coverage of implants used in breast reconstruction. | |||
| ==Complications== | ==Complications== | ||
| The ] emplacement of breast implant devices, either for ] or for ], presents the same health risks common to ], such as adverse reaction to ], ] (post-operative bleeding), late hematoma (post-operative bleeding after 6 months or more),<ref name="Grippaudo2013">{{cite journal |vauthors=Grippaudo FR, Renzi L, Costantino B, Longo B, Santanelli F | title = Late unilateral hematoma after breast reconstruction with implants: case report and literature review | journal = Aesthetic Surgical Journal | volume = 33 | issue = 6 | pages = 830–834 | year = 2013 | pmid = 23864111 | doi = 10.1177/1090820X13496249 | doi-access = free }}</ref> ] (fluid accumulation), incision-site breakdown (wound infection). Complications specific to breast augmentation include breast pain, altered sensation, impeded breast-feeding function, visible wrinkling, asymmetry, thinning of the breast tissue, and ], the "bread loafing" of the bust that interrupts the natural plane between the breasts. Specific treatments for the complications of indwelling breast implants—] and capsular rupture—are periodic ] monitoring and physical examinations. Furthermore, ] and re-operations related to the implantation surgery, and to ] (implant place-holders during surgery) can cause unfavorable ]ring in approximately 6–7 percent of the patients. | |||
| Local complications that can occur with breast implants include post-operative bleeding (]), fluid collections (]), surgical site infection, breast pain, alterations in nipple sensation, interference with breast feeding, visible wrinkling, asymmetric appearance, wound dehiscence (with potential implant exposure), thinning of the breast tissue, and synmastia (disruption of the natural plane between breasts). | |||
| <ref name = AUG_2006 /><ref name=MMG_2006>{{cite web | title = Important Information for Augmentation Patients About Mentor MemoryGel Silicone Gel-Filled Breast Implants | url = http://theplasticsurgeon.org/p030053d.pdf | date = 2006-11-03 | access-date = 11 October 2014 | url-status = dead | archive-url = https://web.archive.org/web/20141016082842/http://theplasticsurgeon.org/p030053d.pdf | archive-date = 16 October 2014 }}</ref><ref name=FDA_2004d>{{cite web|title=Saline-Filled Breast Implant Surgery: Making An Informed Decision (Mentor Corporation) |work=FDA Breast Implant Consumer Handbook - 2004 |url=https://www.fda.gov/cdrh/breastimplants/labeling/mentor_patient_labeling_5900.html |date=2004-01-13 |access-date=2007-05-04 |url-status=dead |archive-url=https://web.archive.org/web/20061126155432/https://www.fda.gov/cdrh/breastimplants/labeling/mentor_patient_labeling_5900.html |archive-date=2006-11-26 }}</ref> ], 20 percent of women who underwent cosmetic implantation, and 50 percent of women who underwent breast reconstruction implantation, required their explantation (surgical removal) at the 10-year mark.<ref>{{cite web |url=https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm260235.htm |title=FDA NEWS RELEASE |website=] |access-date=2011-11-09 |url-status=live |archive-url=https://web.archive.org/web/20111103032451/https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm260235.htm |archive-date=2011-11-03 }}</ref> | |||
| ===Safety=== | |||
| The most common reasons cited for reoperation after breast augmentation are ] and disatification with size or appearance. | |||
| In the 1990s, several reports reviewed the few studies evaluating any increased risk of systemic and auto-immune diseases among women with breast implants. The conclusion at that time was that there was no evidence establishing a causal connection between the implantation of silicone breast implants and either type of disease.<ref name=":2">Diamond BA; Hulka BS; Kerkvliet NI; Tugwell P. "Silicone Breast Implants in Relation to Connective Tissue Diseases and Immunologic Dysfunction: A Report by a National Science Panel to the Honorable Sam C. Pointer Jr. Coordinating Judge for the Federal Breast Implant Multi-District Litigation". 1998.</ref><ref>{{Cite journal |last1=Janowsky |first1=E. C. |last2=Kupper |first2=L. L. |last3=Hulka |first3=B. S. |date=2000-03-16 |title=Meta-analyses of the relation between silicone breast implants and the risk of connective-tissue diseases |journal=The New England Journal of Medicine |volume=342 |issue=11 |pages=781–790 |doi=10.1056/NEJM200003163421105 |issn=0028-4793 |pmid=10717013|url=https://cdr.lib.unc.edu/downloads/g445cp12p |doi-access=free }}</ref> However, the Institute of Medicine report pointed out that these earlier studies included too few women to conclusively evaluate the impact on these rare diseases.<ref name=":2" /> In addition, many of the studies included women who had breast implants for just a few months, which would be too early to develop a diagnosed autoimmune disease. In recent years, large epidemiological studies have reported clinically and statistically significant increases in some of these diseases. A study by Watad and colleagues that was published in 2018 compared and examined the medical records of more than 24,000 women with breast implants to more than 98,000 "matched controls" who did not have breast implants but shared very similar demographic traits.<ref name=":3" /> The study found a statistically significant 22% overall increase in diagnosed autoimmune or rheumatic disorders. The greatest increases in diagnoses for women with breast implants was for ], Multiple Sclerosis (MS), and sarcoidosis, each of which were 58%-98% higher in women with breast implants. That analysis was based on Israeli women with breast implants as confirmed by medical records, and the analyses of diseases were based on diagnoses made after the women got breast implants that were included in medical records during up to 20 years of follow-up.<ref name=":3" /> | |||
| A published study of U.S. women with similar results was published in 2019 by Coroneos and his colleagues at MD Anderson Medical Center.<ref>{{Cite journal |last1=Coroneos |first1=Christopher J. |last2=Selber |first2=Jesse C. |last3=Offodile |first3=Anaeze C. |last4=Butler |first4=Charles E. |last5=Clemens |first5=Mark W. |date=January 2019 |title=US FDA Breast Implant Postapproval Studies: Long-term Outcomes in 99,993 Patients |journal=Annals of Surgery |volume=269 |issue=1 |pages=30–36 |doi=10.1097/SLA.0000000000002990 |issn=1528-1140 |pmid=30222598 |s2cid=52284936}}</ref> The data were based on two studies with a combined total of almost 100,000 women with breast implants, but many dropped out of the study within a few years of their breast implant surgery. However, of the women in the study for at least two years, the researchers reported an 800% increase in Sjögren syndrome, 700% increase in scleroderma, and almost 600% increase in rheumatoid arthritis among women with breast implants compared to the general population of women of the same age and demographics. | |||
| ===Rupture=== | |||
| ====Saline Implant Rupture==== | |||
| When saline breast implants break, they often deflate quickly and can be easily removed. Prospective studies of saline-filled breast implants approved by FDA in May 2000 showed rupture/deflation rates of 3-5% at 3 years and 7-10% at 5 years for augmentation patients. | |||
| Recent research on women who reported autoimmune and other system symptoms but were not diagnosed with an autoimmune disease evaluated whether the women's symptoms changed after their implants were removed. A 2020 study on the effectiveness of explant surgery on women with ] found that nearly all of 750 women who underwent explant surgery reported a significant improvement in their health within a month after their surgery. Researchers focused on the following symptoms: hair loss, memory loss, dry eyes and/or blurred vision, numbness or tingling in the extremities, chronic fatigue, joint pain, rashes, breast pain, food intolerance, flu-like symptoms, and difficulty breathing. The same authors also published a study on the impact of breast implant removal on breathing difficulties and found a statistically significant improvement in well-established objective measures of pulmonary function following explant surgery.<ref name=":4" /> | |||
| ====Silicone Implant Rupture==== | |||
| {| class="wikitable sortable" | |||
| ]<p>] | |||
| |- | |||
| Breast implants do not last a lifetime. When saline breast implants break, they often deflate quickly and can be easily removed. Prospective studies of saline-filled breast implants approved by FDA in May 2000 showed rupture/deflation rates of 3-5% at 3 years and 7-10% at 5 years for augmentation patients.<p> | |||
| ! Year | |||
| ! Country | |||
| ! Systemic Review Group | |||
| ! Conclusions | |||
| |- | |||
| | 1991–93 | |||
| | United Kingdom | |||
| | Independent Expert Advisory Group (IEAG) | |||
| | There was no evidence of an increased risk of connective-tissue disease in patients who had undergone silicone-gel breast implant emplacement, and no cause for changing either breast implant practice or policy in the U.K. | |||
| |- | |||
| | 1996 | |||
| | United States | |||
| | U.S. Institute of Medicine (IOM)<!-- | |||
| --><ref name="Brinton1996">{{cite journal |vauthors=Brinton LA, Malone KE, Coates RJ, Schoenberg JB, Swanson CA, Daling JR, Stanford JL | s2cid = 29456173 | title = Breast Enlargement and Reduction: Results from a Breast Cancer Case-control Study | journal = Plastic and Reconstructive Surgery | volume = 97 | issue = 2 | pages = 269–275 | year = 1996 | pmid = 8559808 | doi = 10.1097/00006534-199602000-00001 | url = https://zenodo.org/record/1234818 }}</ref> | |||
| | There was "insufficient evidence for an association of silicone gel- or saline-filled breast implants with defined connective tissue disease." | |||
| |- | |||
| | 1996 | |||
| | France | |||
| | Agence Nationale pour le Developpement de l'Evaluation Medicale (ANDEM) <ref>{{cite news|title=Histoire des protheses mammaires |last1=Benadiba |first1=Laurent | name-list-style = vanc |date=2004 |language=fr |url=http://laurent.benadiba.free.fr/SITE%20These/Analyse%20resultats.htm|access-date=12 October 2015|archive-url=https://web.archive.org/web/20150129035409/http://laurent.benadiba.free.fr/SITE%20These/Analyse%20resultats.htm|archive-date=29 January 2015}}</ref> | |||
| | French original: "Nous n'avons pas observé de connectivité ni d'autre pathologie auto-immune susceptible d'être directement ou indirectement induite par la présence d'un implant mammaire en particulier en gel de silicone...." | |||
| English translation: "We did not observe connective tissue diseases to be directly or indirectly associated by the presence of a breast implant, in particular one of silicone gel...." | |||
| |- | |||
| | 1997 | |||
| | Australia | |||
| | Therapeutic Devices Evaluation Committee (TDEC) | |||
| | The "current, high-quality literature suggest that there is no association between breast implants and connective tissue disease-like syndromes (atypical connective tissue diseases)."<ref>{{cite book|url=http://www.tga.gov.au/docs/pdf/breasti4.pdf|title=Breast Implant Information Booklet|date=2001|publisher=Commonwealth of Australia|location=Canberra|isbn=0642735794|archive-url=https://web.archive.org/web/20070101081442/http://www.tga.gov.au/docs/pdf/breasti4.pdf|archive-date=2007-01-01|url-status=dead|access-date=2006-12-29|edition=4th}}</ref> | |||
| |- | |||
| | 1998 | |||
| | Germany | |||
| | Federal Institute for Medicine and Medical Products | |||
| | Reported that "silicone breast implants neither cause auto-immune diseases nor rheumatic diseases and have no disadvantageous effects on pregnancy, breast-feeding capability, or the health of children who are breast-fed. There is no scientific evidence for the existence of silicone allergy, silicone poisoning, atypical silicone diseases or a new silicone disease."<ref name="Germany1998">{{Cite news |title=German Society for Senology, Declaration of Consensus for the Security of Silicone Breast Implants |date=24 September 1998}}</ref> | |||
| |- | |||
| | 2000 | |||
| | United States | |||
| | Federal court-ordered review<!-- | |||
| --><ref name="NEJM2000-Janowsky">{{cite journal |vauthors=Janowsky EC, Kupper LL, Hulka BS | title = Meta-analyses of the Relation between Silicone Breast Implants and the Risk of Connective-tissue Diseases | journal = New England Journal of Medicine | volume = 342 | issue = 11 | pages = 781–790 | year = 2000 | pmid = 10717013 | doi = 10.1056/NEJM200003163421105 | url = https://cdr.lib.unc.edu/downloads/g445cp12p | doi-access = free }}</ref> | |||
| | "No evidence of an association between... silicone-gel-filled breast implants specifically, and any of the individual CTDs, all definite CTDs combined, or other auto-immune or rheumatic conditions." | |||
| |- | |||
| | 2000 | |||
| | European Union | |||
| | European Committee on Quality Assurance & Medical Devices in Plastic Surgery (EQUAM) | |||
| | "Additional medical studies have not demonstrated any association between silicone-gel filled breast implants and traditional auto-immune or connective tissue diseases, ], nor any other malignant disease. . . . EQUAM continues to believe that there is no scientific evidence that silicone allergy, silicone intoxication, atypical disease or a 'new silicone disease' exists."<ref> {{webarchive|url=https://web.archive.org/web/20051227102900/http://www.secpre.org/pdf/equam.pdf|date=December 27, 2005}}</ref> | |||
| |- | |||
| | 2001 | |||
| | United Kingdom | |||
| | UK Independent Review Group (UK-IRG) | |||
| | "There is no evidence of an association with an abnormal immune response or typical or atypical connective tissue diseases or syndromes."<ref> {{webarchive|url=https://web.archive.org/web/20060623053747/http://www.silicone-review.gov.uk/press_notice.htm|date=June 23, 2006}}</ref> | |||
| |- | |||
| | 2001 | |||
| | United States | |||
| | Court-appointed National Science Panel review<!-- | |||
| --><ref name="ArthritisRheum2001-Tugwell">{{cite journal |vauthors=Tugwell P, Wells G, Peterson J, Welch V, Page J, Davison C, McGowan J, Ramroth D, Shea B | title = Do silicone Breast Implants Cause Rheumatologic Disorders? A Systematic Review for a Court-appointed National Science Panel | journal = Arthritis Rheum | volume = 44 | issue = 11 | pages = 2477–84 | year = 2001 | pmid = 11710703 | doi = 10.1002/1529-0131(200111)44:11<2477::AID-ART427>3.0.CO;2-Q | doi-access = }}</ref> | |||
| | The panel evaluated established and undifferentiated connective tissue diseases (CTD), and concluded there was no causal evidence between breast implants and these CTDs. | |||
| |- | |||
| | 2003 | |||
| | Spain | |||
| | Science and Technology Options Assessment (STOA) | |||
| | The STOA report to the European Parliament Petitions Committee reported that the current scientific evidence demonstrates no solid, causal evidence linking SBI to severe diseases, e.g. ], connective tissue diseases.<ref>{{cite report |vauthors=Gorgojo L, Gonzalez J, Wisbaum W, Martin-Moreno J |date=30 May 2003 |title=Health risks posed by silicone implants in general, with a special attention to breast implants |url=http://www.eucomed.be/docs/STOA-SILICONE%20BREAST%20IMPLANT%20Study%20update-30May03.pdf |access-date=2019-01-28 |url-status=dead |archive-url=https://web.archive.org/web/20030829114951/http://www.eucomed.be/docs/STOA-SILICONE%20BREAST%20IMPLANT%20Study%20update-30May03.pdf |archive-date=2003-08-29}}</ref> | |||
| |- | |||
| | 2009 | |||
| | European Union | |||
| | International Committee for Quality Assurance, Medical Technologies & Devices in Plastic Surgery panel (IQUAM) | |||
| | The consensus statement of the Transatlantic Innovations conference (April 2009) indicated that additional medical studies demonstrated no association between silicone gel-filled breast implants and carcinoma, or any metabolic, immune, or allergic disorder.<ref name="iquam2009">{{cite journal |vauthors=Neuhann-Lorenz C, Fedeles J, Eisenman-Klein M, Kinney B, Cunningham BL |s2cid=29112694 |title=Eighth IQUAM Consensus Position Statement: Transatlantic Innovations, April 2009 |journal=Plastic and Reconstructive Surgery |volume=127 |issue=3 |pages=1368–1375 |year=2001 |pmid=21364439 |doi=10.1097/PRS.0b013e318206312e}}</ref> | |||
| |} | |||
| ===Implant rupture=== | |||
| The recent FDA approval of silicone implants stipulates that the manufacturers inform women that the implants "are not lifetime devices" and that most recipients will need at least one additional surgery to replace or remove their implants. Rupture is one reason for reoperation. Among the causes of rupture are damage during implantation or other procedures, trauma to the chest, and the pressure of mammograms. | |||
| ] | |||
| Since the research indicates that most ruptures of silicone gel implants are "silent," with no symptoms, the FDA recommends an MRI at three years after implantation and then every two years thereafter for screening purposes. <p> | |||
| Because a breast implant is a ] of limited product-life, the principal rupture-rate factors are its age and design; nonetheless, a breast implant device can retain its mechanical integrity for decades in a woman's body.<ref name="Brown2000">{{cite journal |vauthors=Brown SL, Middleton MS, Berg WA, Soo MS, Pennello G |title=Prevalence of Rupture of Silicone gel Breast Implants Revealed on MR Imaging in a Population of Women in Birmingham, Alabama |journal=American Journal of Roentgenology |volume=175 |issue=4 |pages=1057–1064 |year=2000 |pmid=11000165 |doi=10.2214/ajr.175.4.1751057}}</ref> When a saline breast implant ruptures, leaks, and empties, it quickly deflates, and thus can be readily explanted (surgically removed). In some cases, saline implant rupture can result in an infection due to bacteria or mold that had been within the implant, though this is uncommon.<ref>{{Cite web|publisher=U.S. Food and Drug Administration|title=Risks and Complications of Breast Implants|date=28 September 2020 |url=https://www.fda.gov/medical-devices/breast-implants/risks-and-complications-breast-implants|access-date=14 October 2021}}</ref> The follow-up report, ''Natrelle Saline-filled Breast Implants: a Prospective 10-year Study'' (2009) indicated rupture-deflation rates of 3–5 per cent at 3-years post-implantation, and 7–10 per cent rupture-deflation rates at 10-years post-implantation.<ref name="Walker2009">{{cite journal |vauthors=Walker PS, Walls B, Murphy DK |title= Natrelle Saline-filled Breast Implants: a Prospective 10-year Study |journal=Aesthetic Surgery Journal |volume=29 |issue=1 |pages=19–25 |year=2009 |pmid=19233001 |doi=10.1016/j.asj.2008.10.001 |doi-access=}}</ref> In a study of his 4761 augmentation mammaplasty patients, Eisenberg reported that overfilling saline breast implants 10-13% significantly reduced the rupture-deflation rate to 1.83% at 8-years post-implantation.<ref name="Eisenberg 2021">{{cite journal |author=Eisenberg, TS| title=Does Overfilling Smooth Inflatable Saline-Filled Breast Implants Decrease the Deflation Rate? Experience with 4761 Augmentation Mammaplasty Patients |journal=Aesthetic Plastic Surgery |year=2021| volume=45 |issue=5 |pages=1991–1999 |doi=10.1007/s00266-021-02198-3 |pmid=33712871 |pmc=8481168 |doi-access=free}}</ref> | |||
| ] | |||
| The age and design of the implant are the most important factors in rupture, but estimating ruptures rates of contemporary devices has been difficult, because rutpure rate is not linear with time, follow-up has been limited, and most previous reports<!-- --><ref name="Brown2000"> {{cite journal | author=Brown SL, Middleton MS, Berg WA, Soo MS, Pennello G | title=Prevalence of rupture of silicone gel breast implants revealed on MR imaging in a population of women in Birmingham, Alabama | journal=AJR Am J Roentgenol | year=2000 | pages=1057-64 | volume=175 | issue=4 | id=PMID 11000165}}</ref> mixed heterogeneous groups of devices in non-randomized populations. Data from the MRI cohorts of the US-FDA proscribed "core" studies of contemporary implants has demonstrated low rupture rates within the first 3-4 years after implantation and will continue to be followed longitudinally.] The only available literature with longer term MRI data on single lumen 3rd/4th generation silicone implants has estimated between 6% to a minimum of 15% device failure rates between three and ten years. <!-- --><ref name="Holmich2003"> {{cite journal | author=Holmich LR, et al | title=Incidence of silicone breast implant rupture.| journal=Arch Surg. | year=2003 | pages=801-6| volume=138 | issue=7 | id=PMID 12860765}}</ref> <!-- --><ref name="Heden2006"> {{cite journal | author=Heden P, et al | title=Prevalence of rupture in inamed silicone breast implants.| journal=Plast Reconstr Surg. | year=2006 | pages=303-8| volume=118 | issue=2 | id=PMID 16874191}}</ref>. An additional unpublished retrospective series from Collis and Sharpe reporting a 10% failure rate at a decade in breast augmentation patients was presented in 2005 FDA hearings.<!-- --><ref name="Collis2005"> {{cite web | www.fda.gov/ohrms/dockets/ac/05/briefing/ 2005-4101b1_Mentor-Briefing%20doc-supplement.DOC}}</ref>Evaluation of highly-cohesive (5th generation) gel implants suggests better performance, with a rupture rate on MRI-screened patients reported at 0.3% at six years.<!-- --><ref name="Heden"> {{cite journal | author=Heden P, et al | title=Style 410 cohesive silicone breast implants: safety and effectiveness at 5 to 9 years after implantation.| journal=Plast Reconstr Surg. | year=2006 | pages=1281-7| volume=118 | issue=6 | id=PMID 17051096}}</ref></p> | |||
| When a silicone breast implant ruptures it usually does not deflate, yet the filler gel does leak from it, which can migrate to the implant pocket; therefore, an intracapsular rupture (in-capsule leak) can become an extracapsular rupture (out-of-capsule leak), and each occurrence is resolved by explantation. Although the leaked silicone filler-gel can migrate from the chest tissues to elsewhere in the woman's body, most clinical ] are limited to the breast and armpit areas, usually manifested as ]s (inflammatory nodules) and axillary ] (enlarged ] in the armpit area).<ref name="Holmich2004">{{cite journal |vauthors=Hölmich LR, Vejborg IM, Conrad C, Sletting S, Høier-Madsen M, Fryzek JP, McLaughlin JK, Kjøller K, Wiik A, Friis S | s2cid = 25947224 | title = Untreated Silicone Breast Implant Rupture | journal = Plastic and Reconstructive Surgery | volume = 114 | issue = 1 | pages = 204–214 | year = 2004 | pmid = 15220594 | doi = 10.1097/01.PRS.0000128821.87939.B5 }}</ref><ref name="Katzin 2005">{{cite journal|vauthors=Katzin WE, Centeno JA, Feng LJ, Kiley M, Mullick FG |s2cid=31982669 |title=Pathology of Lymph Nodes From Patients With Breast Implants: A Histologic and Spectroscopic Evaluation |journal=American Journal of Surgical Pathology |volume=29 |issue=4 |pages=506–11 |year=2001 |pmid=15767806 |doi=10.1097/01.pas.0000155145.60670.e4 |url=http://www.ajsp.com/pt/re/ajsp/abstract.00000478-200504000-00013.htm;jsessionid=G3QMCQKJ6hM5VsXLk60GQByjJfkSq7rzMMVZwKbyvpwxmmZXrQpK!-1734750035!-949856144!8091!-1 |archive-url=https://web.archive.org/web/20090524051708/http://www.ajsp.com/pt/re/ajsp/abstract.00000478-200504000-00013.htm%3Bjsessionid%3DG3QMCQKJ6hM5VsXLk60GQByjJfkSq7rzMMVZwKbyvpwxmmZXrQpK%21-1734750035%21-949856144%218091%21-1 |archive-date=May 24, 2009 |url-status=dead|url-access=subscription }}</ref><ref name=FDA_2004c>{{cite web |title=Study of Rupture of Silicone Gel-filled Breast Implants (MRI Component) |work=FDA Breast Implant Consumer Handbook - 2004 |url=https://www.fda.gov/cdrh/breastimplants/handbook2004/diseases.html |date=2000-05-22 |access-date=2007-05-04 |url-status=live |archive-url=https://web.archive.org/web/20070609134559/https://www.fda.gov/cdrh/breastimplants/handbook2004/diseases.html |archive-date=2007-06-09}}</ref> | |||
| Studies have concluded that clinical exams alone are inadequate to identify suspected rupture.<!-- --><ref name="Holmich2005">{{cite journal | author=Holmich LR, Fryzek JP, Kjoller K, Breiting VB, Jorgensen A, Krag C, McLaughlin JK | title= The diagnosis of silicone breast-implant rupture: clinical findings compared with findings at magnetic resonance imaging | journal=Ann Plast Surg | year=2005 | pages=583-9 | volume=54 | issue=6 | id=PMID 15900139}}</ref> Since the research indicates that most ruptures of silicone gel implants are "silent," with no symptoms, the FDA has endorsed MRIs as the gold standard for evaluating potential rupture. | |||
| When silicone implants break they rarely deflate, and the silicone from the implant can leak out into the intracapsular space around the implant. An intracapsular rupture can progress to outside of the capsule (extracapsular rupture), and when recognized, both conditions are generally agreed to indicate the need for removal of the implant. Extracapsular silicone has the potential to migrate, but most clinical complications have appeared to be limited to the breast and axillae <!-- --><ref name="Holmich2004">{{cite journal| author=Holmich LR , et. al |title= Untreated silicone breast implant rupture | journal=Plast Reconstr Surg.| year=2004|pages=204-14|volume=114 | issue=1 |id= PMID 15220594 }}</ref> in the form of ] (inflammatory nodules) and ]ry ] <!-- --><ref name="Katzin 2005">{{cite journal | author=Katzin, William E, Ceneno, Jose A, Feng, Lu-Jean et al | title=Pathology of Lymph Nodes From Patients With Breast Implants: A Histologic and Spectroscopic Evaluation. | journal=American Journal of Surgical Pathology | year=2001 | pages=506-511 | volume=29 | issue=4 | id = }}</ref>(enlarged lymph glands in the armpit area). | |||
| The suspected mechanisms of breast implant rupture are: | |||
| The specific risk and treatment of extracapsular silicone gel is still controversial. A 2001 study on silicone gel breast implants reported an increase in ] among women with extracapsular leakage, compared to women whose implants were not broken or leaking outside the capsule. <!-- --><ref name="Brown2001">{{cite journal | author=Brown SL, Pennello G, Berg WA, Soo MS, Middleton MS | title=Silicone gel breast implant rupture, extracapsular silicone, and health status in a population of women | journal=J Rheumatol | year=2001 | pages=996-1003 | volume=28 | issue=5 | id=PMID 11361228 }}</ref>. This association has not been reproduced in a number of other studies, and the FDA has since concluded "the weight of the epidemiological evidence published in the literature does not support an association between fibromyalgia and breast implants." <p> | |||
| * damage during implantation | |||
| * damage during (other) surgical procedures | |||
| * chemical degradation of the breast implant shell | |||
| * trauma (], ], ]) | |||
| * mechanical pressure of traditional ] breast examination<ref name=FDA_2004b>{{cite web|title=Local Complications |work=FDA Breast Implant Consumer Handbook - 2004 |url=https://www.fda.gov/cdrh/breastimplants/handbook2004/localcomplications.html |date=2004-06-08 |access-date=2007-05-04 |url-status=dead |archive-url=https://web.archive.org/web/20070513003957/https://www.fda.gov/cdrh/breastimplants/handbook2004/localcomplications.html |archive-date=2007-05-13 }}</ref> | |||
| Silicone implant rupture can be evaluated using magnetic resonance imaging; from the long-term ] data for single-lumen breast implants, the European literature about second generation silicone-gel breast implants (1970s design), reported silent device-rupture rates of 8–15 per cent at 10-years post-implantation (15–30% of the patients).<ref> {{webarchive|url=https://web.archive.org/web/20130926005715/http://www.claripacs.com/case/CL0335 |date=2013-09-26}} 2013-04-05</ref><ref name="Holmich2003">{{cite journal |vauthors=Hölmich LR, Friis S, Fryzek JP, Vejborg IM, Conrad C, Sletting S, Kjøller K, McLaughlin JK, Olsen JH |title=Incidence of Silicone Breast Implant Rupture |journal=Arch. Surg. |volume=138 |issue=7 |pages=801–806 |year=2003 |pmid=12860765 |doi=10.1001/archsurg.138.7.801 |doi-access=}}</ref><ref name="Heden2006">{{cite journal |vauthors=Hedén P, Nava MB, van Tetering JP, Magalon G, Fourie le R, Brenner RJ, Lindsey LE, Murphy DK, Walker PS | s2cid = 30442865 |title=Prevalence of Rupture in Inamed Silicone Breast Implants |journal=Plastic and Reconstructive Surgery |volume=118 |issue=2 |pages=303–308 |year=2006 |pmid=16874191 |doi=10.1097/01.prs.0000233471.58039.30}}</ref><ref name="Collis2005">{{cite web |url=https://www.fda.gov/ohrms/dockets/ac/05/briefing/2005-4101b1_Mentor-Briefing%20doc-supplement.DOC |title=FDA summary of clinical issues (MS Word document) |website=] |url-status=live |archive-url=https://web.archive.org/web/20080308085809/https://www.fda.gov/ohrms/dockets/ac/05/briefing/2005-4101b1_Mentor-Briefing%20doc-supplement.DOC |archive-date=2008-03-08}}</ref> | |||
| The study ''Safety and Effectiveness of Mentor's MemoryGel Implants at 6 Years'' (2009), which was a branch study of the U.S. FDA's core ]s for primary ] surgery patients, reported low device-rupture rates of 1.1 per cent at 6-years post-implantation.<ref name="cunningham2009">{{cite journal |vauthors=Cunningham B, McCue J | s2cid = 25722841 | title = Safety and effectiveness of Mentor's MemoryGel implants at 6 years | journal = Plastic and Reconstructive Surgery | volume = 33 | issue = 3 | pages = 440–444 | year = 2009 | pmid = 19437068 | doi = 10.1007/s00266-009-9364-6 }}</ref> The first series of ] evaluations of the silicone breast implants with thick filler-gel reported a device-rupture rate of 1 percent, or less, at the median 6-year device-age.<ref name="Heden">{{cite journal |vauthors=Hedén P, Boné B, Murphy DK, Slicton A, Walker PS | s2cid = 34380204 | title = Style 410 Cohesive Silicone Breast Implants: Safety and Effectiveness at 5 to 9 years after Implantation | journal = Plastic and Reconstructive Surgery | volume = 118 | issue = 6 | pages = 1281–1287 | year = 2006 | pmid = 17051096 | doi = 10.1097/01.prs.0000239457.17721.5d }}</ref> Statistically, the manual examination (palpation) of the woman is inadequate for accurately evaluating if a breast implant has ruptured. The study, ''The Diagnosis of Silicone Breast implant Rupture: Clinical Findings Compared with Findings at Magnetic Resonance Imaging'' (2005), reported that, in asymptomatic patients, only 30 per cent of the ruptured breast implants are accurately palpated and detected by an experienced plastic surgeon, whereas MRI examinations accurately detected 86 per cent of breast implant ruptures.<ref name="Holmich2005">{{cite journal |vauthors=Hölmich LR, Fryzek JP, Kjøller K, Breiting VB, Jørgensen A, Krag C, McLaughlin JK | s2cid = 39525474 | title = The Diagnosis of Silicone Breast implant Rupture: Clinical Findings Compared with Findings at Magnetic Resonance Imaging | journal = Annals of Plastic Surgery | volume = 54 | issue = 6 | pages = 583–589 | year = 2005 | pmid = 15900139 | doi = 10.1097/01.sap.0000164470.76432.4f }}</ref> Therefore, the U.S. FDA recommended scheduled MRI examinations, as silent-rupture screenings, beginning at the 3-year-mark post-implantation, and then every two years, thereafter.<ref name=AUG_2006>{{cite web|title=Important Information for Women About Breast Augmentation with INAMED Silicone-Filled Breast Implants |website=] |url=https://www.fda.gov/cdrh/pdf2/P020056d.pdf |date=2006-11-03 |access-date=2007-05-04 |url-status=dead |archive-url=https://web.archive.org/web/20070103050703/https://www.fda.gov/cdrh/pdf2/P020056d.pdf |archive-date=2007-01-03 }}</ref> Nonetheless, beyond the U.S., the medical establishments of other nations have not endorsed routine MRI screening, and, in its stead, proposed that such a ] examination be reserved for two purposes: (i) for the woman with a suspected breast implant rupture; and (ii) for the confirmation of mammographic and ] studies that indicate the presence of a ruptured breast implant.<ref name=Canada_2005>{{cite web|title=Expert Advisory Panel on Breast Implants: Record of Proceedings |publisher=HealthCanada |url=http://www.hc-sc.gc.ca/dhp-mps/md-im/activit/sci-consult/implant-breast-mammaire/eapbi_rop_gceim_crd_2005-09-29_e.html |date=2005-09-29 |access-date=2007-05-04 |url-status=dead |archive-url=https://web.archive.org/web/20071107040623/http://www.hc-sc.gc.ca/dhp-mps/md-im/activit/sci-consult/implant-breast-mammaire/eapbi_rop_gceim_crd_2005-09-29_e.html |archive-date=2007-11-07 }}</ref> | |||
| Furthermore, ''The Effect of Study design Biases on the Diagnostic Accuracy of Magnetic Resonance Imaging for Detecting Silicone Breast Implant Ruptures: a Meta-analysis'' (2011) reported that the breast-screening MRIs of asymptomatic women might overestimate the incidence of breast implant rupture.<ref name="song2011">{{cite journal |vauthors=Song JW, Kim HM, Bellfi LT, Chung KC | title = The Effect of Study design Biases on the Diagnostic Accuracy of Magnetic Resonance Imaging for Detecting Silicone Breast Implant Ruptures: a Meta-analysis | journal = Plastic and Reconstructive Surgery | volume = 127 | issue = 3 | pages = 1029–1044 | year = 2011 | pmid = 21364405 | pmc = 3080104 | doi = 10.1097/PRS.0b013e3182043630 }}</ref> In the event, the U.S. Food and Drug Administration emphasised that "breast implants are not lifetime devices. The longer a woman has silicone gel-filled breast implants, the more likely she is to experience complications."<ref>{{cite news|url=https://www.independent.co.uk/life-style/health-and-families/breast-implants-safe-but-not-for-life-us-experts-2303085.html|title=Breast implants safe, but not for life: US experts|author=AFP|date=18 September 2011|work=The Independent|url-status=live|archive-url=https://web.archive.org/web/20160803074016/http://www.independent.co.uk/life-style/health-and-families/breast-implants-safe-but-not-for-life-us-experts-2303085.html|archive-date=3 August 2016}}</ref> | |||
| ===Capsular contracture=== | ===Capsular contracture=== | ||
| {{main|Capsular contracture}} | |||
| Capsules of tightly-woven collagen fibers form as an ] around a foreign body (eg. breast implants, pacemakers, orthopedic joint prosthetics), tending to wall it off. ] occurs when the capsule tightens and squeezes the implant. This contracture is a complication that can be very painful and distort the appearance of the implanted breast. The exact cause of contracture is not known. However, some factors include bacterial contamination, silicone rupture or leakage, and hematoma.] may happen again after this additional surgery.<p> | |||
| The human body's ] to a surgically installed foreign object—breast implant, cardiac ], ] ]—is to encapsulate it with ] capsules of tightly woven ] fibers, in order to maintain the integrity of the body by isolating the foreign object, and so tolerate its presence. ]—which should be distinguished from normal capsular tissue—occurs when the collagen-fiber capsule thickens and compresses the breast implant; it is a painful ] that might distort either the breast implant, or the breast, or both. Capsular contracture is diagnosed through a visual and physical examiniation according to level of increasing severity based on the Baker Grade scale: Baker Grade I, Baker Grade II, Baker Grade III, and Baker Grade IV. | |||
| Methods which have been successful for reducing capsular contracture rates include submuscular implant placement, using textured <!-- --><ref name="Barnsley2006">{{cite journal | author=Barnsley GP| title= Textured surface breast implants in the prevention of capsular contracture among breast augmentation patients: a meta-analysis of randomized controlled trials. | journal=Plast Reconstr Surg. | year=2006 | pages=2182-90| volume=117 | issue=7 | id=PMID 16772915}}</ref>or polyurethane-coated implants <!-- --><ref name="Handel2006">{{cite journal | author=Handel N, et al| title= Long-term safety and efficacy of polyurethane foam-covered breast implants. | journal=Aesth. Surg Journal | year=2006 | month=may | pages=265-74| volume=26 | issue=3 |}}</ref> , limiting handling of the implants and skin contact prior to insertion <!-- --><ref name="Mladick1993">{{cite journal | author=Mladick RA| title= "No-touch" submuscular saline breast augmentation technique. | journal=Aesth. Surg Journal | year=1993 | pages=183-92| volume=17 | issue=3 | id= PMID 8213311 }}</ref> , and irrigation with triple-antibiotic solutions as described by Adams, et al.<!-- --><ref name="Adams2006">{{cite journal | author=Adams WP jr., et al| title= Enhancing patient outcomes in aesthetic and reconstructive breast surgery using triple antibiotic breast irrigation: six-year prospective clinical study. | journal=Plast Reconstr Surg. | year=2006 | pages=30-36 | volume=117 | issue=1 | id=PMID 16404244}}</ref> <p> <br> | |||
| Correction of ] may require surgical removal or release of the capsule or removal (and possible replacement) of the implant itself. Closed capsulotomy (disrupting the capsule via external manipulation), a once common maneuver for treating hard capsules, has been discouraged as it can facilitate implant rupture. Nonsurgical methods of treating capsules include external ultrasound <!-- --><ref name="Planas2001">{{cite journal | author=Planas J| title= Five-year experience on ultrasonic treatment of breast contractures. | journal=Aesthetic Plast Surg. | year=2001| pages=89-93 | volume=25 | issue=2 | id=PMID 11349308}}</ref>,treatment with leukotriene pathway inhibitors (Accolate, Singulair) <!-- --><ref name="Schlesinger2002">{{cite journal | author=Schlesinger SL, wt al| title= Zafirlukast (Accolate): A new treatment for capsular contracture. | journal=Aesthetic Plast Surg. | year=2002| pages=329-336| volume=22 | issue=4}}</ref>, and pulsed electromagnetic field therapy. <!-- --><ref name="Silver1982">{{cite journal | author=Silver H| title= Reduction of capsular contracture with two-stage augmentation mammaplasty and pulsed electromagnetic energy (Diapulse therapy). | journal=Plast Reconstr Surg. | year=1982| pages=802-8 | volume=69 | issue=5 | id=PMID 7071225}}</ref> <P> | |||
| ] | |||
| ===Systematic reviews on claims of systemic illness=== | |||
| Since the early 1990s, nearly a dozen comprehensive systemic reviews have been commissioned by various governments' health ministries to examine the alleged links between silicone gel breast implants and systemic diseases. A clear consensus has emerged from these independent scientific reviews that there is no clear evidence of a causal link between the implantation of silicones and connective tissue disease. The conclusions of these reviews are summarized:<p> | |||
| The cause of capsular contracture is unknown, but the common incidence factors include bacterial contamination, device-shell rupture, filler leakage, and ]. The surgical implantation procedures that have reduced the incidence of capsular contracture include submuscular emplacement, the use of breast implants with a textured surface (polyurethane-coated);<ref name="Barnsley2006">{{cite journal |vauthors=Barnsley GP, Sigurdson LJ, Barnsley SE | s2cid = 35420582 | title = Textured surface Breast Implants in the Prevention of Capsular Contracture among Breast Augmentation Patients: a Meta-analysis of Randomized Controlled Trials | journal = Plastic and Reconstructive Surgery | volume = 117 | issue = 7 | pages = 2182–2190 | year = 2006 | pmid = 16772915 | doi = 10.1097/01.prs.0000218184.47372.d5 }}</ref><ref name="Wong2006">{{cite journal |vauthors=Wong CH, Samuel M, Tan BK, Song C | s2cid = 29643167 | title = Capsular Contracture in Subglandular Breast Augmentation with Textured versus Smooth Breast Implants: a Systematic Review | journal = Plastic and Reconstructive Surgery | volume = 118 | issue = 5 | pages = 1224–1236 | year = 2006 | pmid = 17016195 | doi = 10.1097/01.prs.0000237013.50283.d2 }}</ref><ref name="HandelGut2006">{{cite journal |vauthors=Handel N, Gutierrez J | title = Long-term safety and efficacy of polyurethane foam-covered breast implants | journal = Journal of Aesthetic Surgery | volume = 26 | issue = 3 | pages = 265–274 | date = May 2006 | pmid = 19338905 | doi = 10.1016/j.asj.2006.04.001 | doi-access = free }}</ref> limited pre-operative handling of the implants, limited contact with the chest skin of the implant pocket before the emplacement of the breast implant, and irrigation of the recipient site with triple-antibiotic solutions.<ref name="Mladick1993">{{cite journal | author = Mladick RA | s2cid = 39767802 | title = "No-touch" submuscular saline breast augmentation technique | journal = Journal of Aesthetic Surgery | volume = 17 | issue = 3 | pages = 183–192 | year = 1993 | pmid = 8213311 | doi = 10.1007/BF00636260 }}</ref><ref name="Adams2006">{{cite journal |vauthors=Adams WP, Rios JL, Smith SJ | s2cid = 35238465 | title = Enhancing Patient Outcomes in Aesthetic and Reconstructive Breast Surgery using Triple Antibiotic Breast Irrigation: Six-year Prospective Clinical Study | journal = Plastic and Reconstructive Surgery | volume = 117 | issue = 1 | pages = 30–6 | year = 2006 | pmid = 16404244 | doi = 10.1097/01.prs.0000185671.51993.7e }}</ref> | |||
| {| border=1 | |||
| ! Year | |||
| ! Country | |||
| ! Systematic Review Group | |||
| ! Conclusions | |||
| |- | |||
| | 1991-1993 | |||
| | United Kingdom | |||
| | Independent Expert Advisory Group (IEAG) | |||
| | The IEAG concluded that there was no evidence of an increased risk of connective tissue disease in patients who had undergone silicone gel breast implantation and that there was no scientific case for changing practice or policy in the UK in respect of breast implantation | |||
| |- | |||
| | 1996 | |||
| | USA | |||
| | US Institute of Medicine ('''IOM''')<!-- | |||
| --> <ref name="Brinton1996">{{cite journal | author=Brinton LA, Malone KE, Coates RJ, Schoenberg JB, Swanson CA, Daling JR, Stanford JL | title=Breast enlargement and reduction: results from a breast cancer case-control study | journal=Plast Reconstr Surg | year=1996 | pages=269-75 | volume=97 | issue=2 | id=PMID 8559808}}</ref> | |||
| | Not "sufficient evidence for an association of silicone gel- or saline-filled breast implants with defined connective tissue disease". | |||
| |- | |||
| | 1996 | |||
| | France | |||
| | Agence Nationale pour le Developpement de l'Evaluation Medicale ('''ANDEM''') | |||
| | "Nous n’avons pas observé de connectivite ni d’autre pathologie auto-immune susceptible d’être directement ou indirectement induite par la présence d’un implant mammaire en particulier en gel de silicone..." (We did not observe connective tissue or other diseases to be directly or indirectly associated with (in particular) silicone gel breast implants) | |||
| |- | |||
| | 1997 | |||
| | Australia | |||
| | Australia’s Therapeutic Devices Evaluation Committee review | |||
| | "current high quality literature suggest that there is no association between breast implants and connective tissue disease-like syndromes (atypical connective tissue diseases)" | |||
| |- | |||
| | 1998 | |||
| | Germany | |||
| | Germany’s Federal Institute for Medicine and Medical Products | |||
| | concluded that "silicone breast implants neither cause auto-immune diseases nor rheumatic diseases and have no disadvantageous effects on pregnancy, breast feeding capability or the health of children who are breast fed. There is no scientific evidence for the existence of silicone allergy, silicone poisoning, atypical silicone diseases or a new silicone disease"<!-- --> <ref name="Germany1998">{{cite ref | title=German Society for Senology, Declaration of Consensus for the Security of Silicone Breast Implants-September 24, 1998 | year=1998}}</ref> | |||
| |- | |||
| | 2000 | |||
| | USA | |||
| | Review request of the United States Federal Judiciary<!-- | |||
| --><ref name="NEJM2000-Janowsky">{{cite journal | author=Janowsky EC, Kupper LL, Hulka BS | title=Meta-analyses of the relation between silicone breast implants and the risk of connective-tissue diseases | journal=N Engl J Med | year=2000 | pages=781-90 | volume=342 | issue=11 | id=PMID 10717013}}</ref> | |||
| | "no evidence of an association between...silicone-gel-filled breast implants specifically, and any of the individual CTDs, all definite CTDs combined, or other autoimmune or rheumatic conditions." | |||
| |- | |||
| | 2000 | |||
| | European Union | |||
| | European Committee on Quality Assurance & Medical Devices in Plastic Surgery ('''EQUAM''') | |||
| | "Additional medical studies have not demonstrated any association between silicone-gel filled breast implants and traditional auto-immune or connective tissue diseases, cancer, nor any other malignant disease....EQUAM continues to believe that there is no scientific evidence that silicone allergy, silicone intoxication, atypical disease or a 'new silicone disease' exists." | |||
| |- | |||
| | 2001 | |||
| | Great Britain | |||
| | UK Independent Review Group ('''UK-IRG''') | |||
| | "there is no evidence of an association with an abnormal immune response or typical or atypical connective tissue diseases or syndromes" | |||
| |- | |||
| | 2001 | |||
| | USA | |||
| | Review for court appointed ] <!-- | |||
| --><ref name="ArthritisRheum2001-Tugwell">{{cite journal | author=Tugwell P, Wells G, Peterson J, Welch V, Page J, Davison C, McGowan J, Ramroth D, Shea B | title=Do silicone breast implants cause rheumatologic disorders? A systematic review for a court-appointed national science panel | journal=Arthritis Rheum | year=2001 | pages=2477-84 | volume=44 | issue=11 | id=PMID 11710703}}</ref> | |||
| | The panel evaluated both established and undifferentiated connective tissue diseases and concluded that there was no evidence of an association between breast implants and these CTDs. | |||
| |- | |||
| | 2003 | |||
| | Spain | |||
| | '''STOA''' Report to the European Parliament Petitions Committee | |||
| | Regarding new scientific evidence, the currently available information shows that there is not solid evidence linking SBI to severe diseases (such as breast cancer or connective tissue diseases). | |||
| |- | |||
| |} | |||
| The correction of capsular contracture might require an open capsulotomy (surgical release) of the collagen-fiber capsule, or the removal, and possible replacement, of the breast implant. Furthermore, in treating capsular contracture, the closed capsulotomy (disruption via external manipulation) once was a common maneuver for treating hard capsules, but now is a discouraged technique, because it can rupture the breast implant. Non-surgical treatments for collagen-fiber capsules include massage, external ] therapy, ] such as ] (Accolate) or ] (Singulair), and ] (PEMFT).<ref name="Planas2001">{{cite journal |vauthors=Planas J, Cervelli V, Planas G | s2cid = 2784003 | title = Five-year experience on ultrasonic treatment of breast contractures | journal = Aesthetic Plastic Surgery | volume = 25 | issue = 2 | pages = 89–93 | year = 2001 | pmid = 11349308 | doi = 10.1007/s002660010102 }}</ref><ref name="Schlesinger2002">{{cite journal |vauthors=Schlesinger SL, Ellenbogen R, Desvigne MN, Svehlak S, Heck R | title = Zafirlukast (Accolate): A new treatment for capsular contracture | journal = Aesthetic Plast. Surg. | volume = 22 | issue = 4 | pages = 329–36 | year = 2002 | pmid = 19331987 | doi = 10.1067/maj.2002.126753 | doi-access = free }}</ref><ref name="Scuderi">{{cite journal |vauthors=Scuderi N, Mazzocchi M, Fioramonti P, Bistoni G | s2cid = 251008 | title = The effects of zafirlukast on capsular contracture: preliminary report | journal = Aesthetic Plast. Surg. | volume = 30 | issue = 5 | pages = 513–520 | year = 2006 | pmid = 16977359 | doi = 10.1007/s00266-006-0038-3 }}</ref><ref name="Silver1982">{{cite journal | author = Silver H | s2cid = 8451166 | title = Reduction of capsular contracture with two-stage augmentation mammaplasty and pulsed electromagnetic energy (Diapulse therapy) | journal = Plastic and Reconstructive Surgery | volume = 69 | issue = 5 | pages = 802–805 | year = 1982 | pmid = 7071225 | doi = 10.1097/00006534-198205000-00013 }}</ref> | |||
| Thousands of women have still claimed that they have become ill from their implants. Complaints include systemic fungal infections, ] and ] problems. | |||
| ===Repair and revision surgeries=== | |||
| When the patient is unsatisfied with the outcome of the augmentation mammoplasty; or when technical or medical complications occur; or because of the breast implants' limited product life, it is likely she might require replacing the breast implants. Common revision surgery indications include major and minor medical complications, ], shell rupture, and device deflation.<ref name = FDA_2004b /> Revision incidence rates were greater for breast reconstruction patients, because of the post-mastectomy changes to the soft-tissues and to the skin envelope of the breast, and to the ] borders of the breast, especially in women who received adjuvant external ].<ref name = FDA_2004b /> Moreover, besides breast reconstruction, ] patients usually undergo revision surgery of the nipple-areola complex (NAC), and symmetry procedures upon the opposite breast, to create a bust of natural appearance, size, form, and feel. Carefully matching the type and size of the breast implants to the patient's pectoral soft-tissue characteristics reduces the incidence of revision surgery. Appropriate tissue matching, implant selection, and proper implantation technique, the re-operation rate was 3 percent at the 7-year-mark, compared with the re-operation rate of 20 per cent at the 3-year-mark, as reported by the U.S. Food and Drug Administration.<ref name="Tebbets2006">{{cite journal | author = Tebbetts JB | title = Out Points Criteria for Breast Implant Removal without Replacement and Criteria to Minimize Reoperations following Breast Augmentation | journal = Plastic and Reconstructive Surgery | volume = 114 | issue = 5 | pages = 1258–1262 | date = October 2006 | pmid = 15457046 | doi = 10.1097/01.prs.0000136802.91357.cf }}</ref><ref name="Tebbets2">{{cite journal | author = Tebbetts JB | s2cid = 27630646 | title = Achieving a Zero Percent Reoperation Rate at 3 years in a 50-consecutive-case Augmentation Mammaplasty Premarket Approval Study | journal = Plastic and Reconstructive Surgery | volume = 118 | issue = 6 | pages = 1453–7 | date = December 2006 | pmid = 17051118 | doi = 10.1097/01.prs.0000239602.99867.07 }}</ref> | |||
| ===Systemic disease=== | |||
| ] | |||
| In the 1990s, the national health ministries of the listed countries reviewed the pertinent studies for causal links among silicone-gel breast implants and systemic and diagnosed autoimmune diseases and breast cancer.<ref name="Breiting2004">{{cite journal |vauthors=Breiting VB, Hölmich LR, Brandt B, Fryzek JP, Wolthers MS, Kjøller K, McLaughlin JK, Wiik A, Friis S |year=2004 |title=Long-term Health Status of Danish Women with Silicone Breast Implants |journal=Plastic and Reconstructive Surgery |volume=114 |issue=1 |pages=217–226 |doi=10.1097/01.PRS.0000128823.77637.8A |pmid=15220596 |s2cid=20584928}}</ref><ref name="NEJM2000-Janowsky" /><ref>{{Cite book |last1=Grigg|first1=Martha |last2=Bondurant|first2=Stuart|date=2000|title=Information for Women About the Safety of Silicone Breast Implants|chapter=A Report of a Study by the Institute of Medicine |chapter-url=https://www.ncbi.nlm.nih.gov/books/NBK44775/|journal=Institute of Medicine |via=NCBI}}</ref> A study by researchers at the U.S. National Institute of Health reported an increase in four types of cancer among women with breast implants compared to other plastic surgery patients of the same age and similar demographic traits and health habits.<ref name="pmid11306343"/> This increase in cancer was attenuated but did not disappear when the researchers followed the women for 5 more years, with a 43% increase in brain cancer and a 63% increase in respiratory cancer compared to other plastic surgery patients.<ref>{{Cite journal|last=Brinton|first=LA|date=2006|title=Mortality among augmentation mammoplasty patients: An update |journal=Epidemiology|volume=17|issue=2|pages=162–169|doi=10.1097/01.ede.0000197056.84629.19|pmid=16477256|s2cid=22285852|doi-access=free}}</ref> A study by Watad et al. evaluated the medical records of more than 24,000 women with breast implants compared to more than 98,000 women with the same demographic traits who did not have breast implants. The researchers found a statistically significant 22 percent increase in several diagnosed diseases, increasing to more than 60% for ], ], and ]<ref name=":3">{{Cite journal |date=2018|title=Silicone breast implants and the risk of autoimmune/rheumatic disorders: a real-world analysis|journal=Int J Epidemiol|pmid=30329056|last1=Watad|first1=A.|last2=Rosenberg|first2=V. |last3=Tiosano|first3=S.|last4=Cohen Tervaert|first4=J. W.|last5=Yavne |first5=Y.|last6=Shoenfeld |first6=Y.|last7=Shalev |first7=V.|last8=Chodick|first8=G.|last9=Amital|first9=H.|volume=47|issue=6 |pages=1846–1854|doi=10.1093/ije/dyy217|doi-access=free}}</ref> After investigating this issue, in 2021 the U.S. FDA revised its "black box warnings" on breast implants to acknowledge the association between breast implants and systemic autoimmune, rheumatologic, and neurological symptoms to state: "Patients receiving breast implants have reported a variety of systemic symptoms, such as joint pain, muscle aches, confusion, chronic fatigue, autoimmune diseases, and others. Individual patient risk for developing these symptoms has not been well established. Some patients report complete resolution of symptoms when the implants are removed without replacement".<ref>{{cite web|date=3 December 2021|title=Labeling for Approved Breast Implants |url=https://www.fda.gov/medical-devices/breast-implants/labeling-approved-breast-implants|url-status=live|access-date=19 January 2022|website=U.S. Food and Drug Administration |archive-url=https://web.archive.org/web/20190911143950/https://www.fda.gov/medical-devices/breast-implants/labeling-approved-breast-implants |archive-date=2019-09-11}}</ref> | |||
| A 2021 study by Cleveland Clinic physicians investigated the outcome of breast implant removal on women with breast implants who had reported difficulty breathing or tightness in their chest; this is one of the symptoms associated with '''breast implant illness'''.<ref name=":4">{{Cite journal |last=Wee |first=Corinne E |date=June 2021 |title=The Objective Effect of Breast Implant Removal and Capsulectomy on Pulmonary Function |url=https://journals.lww.com/prsgo/fulltext/2021/06000/the_objective_effect_of_breast_implant_removal_and.57.aspx |journal=Plastic and Reconstructive Surgery - Global Open |volume=9 |issue=6 |pages=e3636 |doi=10.1097/GOX.0000000000003636 |s2cid=235801794 |doi-access=free }}</ref> The study examined breast implant patients who reported problems breathing, comparing their scores on 6 ] before and after their implants and scar capsules were removed. The researchers reported that 74% of the patients reported significant improvements on at least 3 of the pulmonary function tests. In self-reports, all study participants reported that their breathing improved after their implant surgery. | |||
| ===Platinum toxicity=== | |||
| ] is a catalyst used in the making of silicone implant polymer shells and other silicone devices used in medicine. The literature indicates that small amounts of platinum leaches (leaks) from these implants and is present in the surrounding tissue. The FDA reviewed the available studies from the medical literature on platinum and breast implants in 2002 and concluded there was little evidence suggesting toxicity from platinum in implant patients.<ref name="pmid12627791">{{cite journal | vauthors = Arepalli SR, Bezabeh S, Brown SL | title = Allergic reaction to platinum in silicone breast implants | journal = Journal of Long-Term Effects of Medical Implants | volume = 12 | issue = 4 | pages = 299–306 | date = 2002 | pmid = 12627791 | doi = 10.1615/jlongtermeffmedimplants.v12.i4.80 }}</ref> The FDA revisited this study and additional literature several years later, reaffirming prior conclusions that platinum catalysts used in implants is likely not ionized and therefore would not represent a risk to women.<ref name="FDA Backgrounder on Platinum in breast implants">{{ cite web | title = FDA Backgrounder on Platinum in Silicone Breast Implants | url = https://www.fda.gov/cdrh/breastimplants/platinum.html | publisher = U.S. Food and Drug Administration | date = 16 June 2006 | archive-url = https://web.archive.org/web/20060715180729/https://www.fda.gov/cdrh/breastimplants/platinum.html | archive-date = 2006-07-15 }}</ref> | |||
| ===Anaplastic large-cell lymphoma=== | |||
| {{main|Anaplastic large-cell lymphoma#Breast implant-associated anaplastic large-cell lymphoma}} | |||
| The FDA has identified that breast implants may be associated with a rare form of cancer called ] (ALCL), which some experts believe is believed to be associated with chronic bacterial inflammation.<ref>{{cite web|title=Safety Alerts for Human Medical Products - Breast Implants: Update - Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL)|url= https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm547622.htm |author=Office of the Commissioner|website=www.fda.gov|access-date=28 April 2018|url-status=live|archive-url=https://web.archive.org/web/20180428170514/https://www.fda.gov/Safety/MedWatch/SafetyInformation/SafetyAlertsforHumanMedicalProducts/ucm547622.htm|archive-date=28 April 2018}}</ref> Similar ALCL phenomena have been seen with other types of medical implants including vascular access ports, orthopedic hip implants, and jaw (TMJ) implants. The causal association between breast implants and ALCL was conclusively established in December 2013, when researchers at MD Anderson Cancer Center published a study of 60 women with breast implants who were diagnosed with ALCL in the breast. In 2015, plastic surgeons published an article reviewing 37 articles in the literature on 79 patients and collected another 94 unreported cases, resulting in 173 women with breast implants who had developed ALCL of the breast. They concluded that "Breast implant-associated ALCL is a novel manifestation of site- and material-specific lymphoma originating in a specific scar location, presenting a wide array of diverse characteristics and suggesting a multifactorial cause." They stated that "There was no preference for saline or silicone fill or for cosmetic or reconstructive indications." Where implant history was known, the patient had received at least one textured-surface device. In 2016, the World Health Organization (WHO) officially recognized BIA-ALCL.<ref>{{Cite journal|last1=Swerdlow|first1=Steven H.|last2=Campo |first2=Elias|last3=Pileri|first3=Stefano A.|last4=Harris|first4=Nancy Lee|last5=Stein|first5=Harald |last6=Siebert |first6=Reiner|last7=Advani|first7=Ranjana|last8=Ghielmini|first8=Michele|last9=Salles |first9=Gilles A.|last10=Zelenetz|first10=Andrew D.|last11=Jaffe|first11=Elaine S.|date=2016-05-19 |title=The 2016 revision of the World Health Organization classification of lymphoid neoplasms |journal=Blood|language=en|volume=127|issue=20|pages=2375–2390|doi=10.1182/blood-2016-01-643569 |pmid=26980727|pmc=4874220|issn=0006-4971}}</ref> | |||
| As of April 2022, the FDA has received 1,130 global medical device reports (MDRs) of BIA-ALCL, including 59 deaths.<ref name=":0">{{cite web|date=20 August 2020|title=Breast Implants - Medical Device Reports of Breast Implant-Associated Anaplastic Large Cell Lymphoma|url=https://www.fda.gov/medical-devices/breast-implants/medical-device-reports-breast-implant-associated-anaplastic-large-cell-lymphoma|access-date=18 August 2021|website=www.fda.gov}}</ref> Since ALCL was thought to be diagnosed in only 1 woman in half a million, 60 women was a much higher number than would be expected. The researchers pointed out that BIA-ALCL could be fatal.<ref>{{cite journal | vauthors = Miranda RN, Aladily TN, Prince HM, Kanagal-Shamanna R, de Jong D, Fayad LE, Amin MB, Haideri N, Bhagat G, Brooks GS, Shifrin DA, O'Malley DP, Cheah CY, Bacchi CE, Gualco G, Li S, Keech JA, Hochberg EP, Carty MJ, Hanson SE, Mustafa E, Sanchez S, Manning JT, Xu-Monette ZY, Miranda AR, Fox P, Bassett RL, Castillo JJ, Beltran BE, de Boer JP, Chakhachiro Z, Ye D, Clark D, Young KH, Medeiros LJ | display-authors = 6 | title = Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients | journal = Journal of Clinical Oncology | volume = 32 | issue = 2 | pages = 114–20 | date = January 2014 | pmid = 24323027 | pmc = 4062709 | doi = 10.1200/JCO.2013.52.7911 }}</ref> If women with implants present with delayed swelling or fluid collection, cytologic studies and a test for the marker ] are suggested. The American Society of Plastic Surgery (ASPS) states, "CD30 is the main diagnostic test that must be performed on the seroma fluid as routine pathology or ] staining can frequently miss the diagnosis."<ref name="plasticsurgery.org">Clemens, Mark. " {{webarchive|url=https://web.archive.org/web/20170326231741/https://www.plasticsurgery.org/for-medical-professionals/quality-and-registries/bia-alcl-by-the-numbers |date=2017-03-26 }}" (2017).</ref> Diagnosis and treatment of breast implant-associated ALCL now follows standardized guidelines established by the National Comprehensive Cancer Network.<ref>{{cite journal |vauthors=Clemens MW, Horwitz SM |title=NCCN Consensus Guidelines for the Diagnosis and Management of Breast Implant-Associated Anaplastic Large Cell Lymphoma |journal=Aesthetic Surgery Journal |volume=37 |issue=3 |pages=285–289 |date=March 2017 |pmid=28184418 |doi=10.1093/asj/sjw259 |doi-access=free}}</ref> | |||
| The current lifetime risk of BIA-ALCL in the U.S. is unknown, but estimates have ranged between one in 70,000 and one in 500,000 women with breast implants, according to the ].<ref>{{cite web|url=https://www.mdanderson.org/cancer-types/implant-associated-anaplastic-large-cell-lymphoma/implant-associated-anaplastic-large-cell-lymphoma-facts.html|title=Implant-associated ALCL Facts {{!}} The MD Anderson Foundation|website=www.mdanderson.org|access-date=2017-12-08|url-status=live|archive-url=https://web.archive.org/web/20171209152413/https://www.mdanderson.org/cancer-types/implant-associated-anaplastic-large-cell-lymphoma/implant-associated-anaplastic-large-cell-lymphoma-facts.html|archive-date=2017-12-09}}</ref> Countries with breast implant registries have the best data on the risks of BIA-ALCL. For example, as of October 2020, the Therapeutic Goods Administration of Australia and New Zealand reported a "1:3,345 risk with Allergan Biocell and a 1:86,029 risk with Mentor Siltex."<ref name="plasticsurgery.org"/> AIn the U.S, estimates of the risk of BIA-ALCL in textured implants ranges from 1.79 per 1,000 (1 woman with BIA-ALCL per 559 implants) to 2.82 per 1,000 (1 woman per 355 implants). As of April 2022, the FDA reported 1,130 medical device reports (MDRs) of BIA-ALCL. Of those MDRs, 59 of the women died. FDA states that 798 of the 1,130 MDRs for BIA-ALCL involved textured breast implants, 37 MDRs involved smooth-surfaced implants, and 295 MDRs did not specify whether the implants were textured or smooth.<ref name=":0" /> In some cases, the women who developed BIA-ALCL after smooth breast implants had textured expanders prior to their implants. Most patients had BIA-ALCL affect only one breast with only 8 patients diagnosed with bilateral BIA-ALCL. The most common reported symptom of BIA-ALCL according to the FDA was seroma, followed by breast swelling and pain, capsular contracture, and peri-implant mass or lump. Furthermore, 84 percent of the reported implants were made by Allergan, the largest breast implant manufacturer at the time.<ref name=":0" /> | |||
| A study conducted at Memorial Sloan Kettering cancer center of women undergoing prophylactic mastectomies indicated that 1 in 355 women were subsequently diagnosed with BIA-ALCL. <ref>{{Cite journal |last=Cordeiro |first=Peter |date=20 Jan 2020 |title=Risk of breast implant associated anaplastic large cell lymphoma (BIA-ALCL) in a cohort of 3546 women prospectively followed long term after reconstruction with textured breast implants. |journal=J Plast Reconstr Aesthet Surg |volume=73 |issue=5 |pages=841–846 |doi=10.1016/j.bjps.2019.11.064 |pmid=32008941 |pmc=7247945 }}</ref> Almost all those women had Allergan biocell implants and expanders. These reports strongly suggest that BIA-ALCL develops primarily in patients with textured implants or expanders. In all cases, however, many researchers suspect that BIA-ALCL is an under-recognized, misdiagnosed, and under-reported complication of breast implants. | |||
| The ASPS and the Plastic Surgery Foundation (PSF) have partnered with the FDA to study this condition and in doing so created the Patient Registry and Outcomes for Breast Implants and Anaplastic Large Cell Lymphoma Etiology and Epidemiology (PROFILE). The United States FDA strongly encourages all physicians to report cases to PROFILE in an effort to better understand the role of breast implants in ALCL and the management of this disease.<ref>{{cite web|url=http://www.thepsf.org/research/clinical-impact/profile.htm|title=Breast Implant Associated ALCL: PROFILE Project {{!}} The Plastic Surgery Foundation|website=www.thepsf.org|access-date=2017-04-25|url-status=live|archive-url=https://web.archive.org/web/20170507235532/http://www.thepsf.org/research/clinical-impact/profile.htm|archive-date=2017-05-07}}</ref> | |||
| === Other lymphomas and squamous-cell carcinoma === | |||
| In September 2022, the FDA announced new information regarding other types of cancers related to breast implants, based on medical device reports (MDRs) of cases of patients with ] or various lymphomas found in the scar tissue around breast implants. The agency reported 10 MDRs about squamous-sell carcinoma found in the capsule scar tissue and 12 MDRs about various lymphomas also in the scar tissue around the breast implants. These new cases do not overlap with cases of BIA-ALCL. SCC and other lymphomas have been found in smooth-surface and textured implants as well as silicone-gel-filled and saline-filled implants. Patients who reported symptoms specified swelling, pain, lumps, and changes to their skin. As of 2022, neither the incidence rate nor prevalence is known.<ref>{{Cite web |date=September 8, 2022 |title=Breast Implants: Reports of Squamous Cell Carcinoma and Various Lymphomas in Capsule Around Implants: FDA Safety Communication |url=https://www.fda.gov/medical-devices/safety-communications/breast-implants-reports-squamous-cell-carcinoma-and-various-lymphomas-capsule-around-implants-fda |website=U.S. Food and Drug Administration}}</ref> | |||
| ==Surgical procedures== | |||
| ===Incision types=== | |||
| Breast implant emplacement is performed with five types of surgical incisions: | |||
| # Inframammary: an incision made to the ] (natural crease under the breast), which affords maximal access for precise dissection of the tissues and emplacement of the breast implants. It is the preferred surgical technique for emplacing silicone-gel implants, because it better exposes the ]–] interface; yet, IMF implantation can produce thicker, slightly more visible surgical ]s. | |||
| # Periareolar: a border-line incision along the periphery of the ], which provides an optimal approach when adjustments to the IMF position are required, or when a ] (breast lift) is included to the primary ] procedure. In periareolar emplacement, the incision is around the medial-half (inferior half) of the areola's circumference. Silicone gel implants can be difficult to emplace via periareolar incision, because of the short, five-centimetre length (~ 5.0 cm) of the required access-incision. Aesthetically, because the scars are at the areola's border (periphery), they usually are less visible than the IMF-incision scars of women with light-pigment areolae; when compared to cutaneous-incision scars, the modified ] of the areolae are less prone to (raised) hypertrophic scars. | |||
| # Transaxillary: an incision made to the ] (armpit), from which the dissection tunnels medially, to emplace the implants, either bluntly or with an ] (illuminated video microcamera), without producing visible scars on the breast proper; yet, it is likelier to produce inferior asymmetry of the implant-device position. Therefore, surgical revision of transaxillary emplaced breast implants usually requires either an IMF incision or a periareolar incision. | |||
| # Transumbilical: a trans-umbilical breast augmentation (]) is a less common implant-device emplacement technique wherein the incision is at the umbilicus (]), and the dissection tunnels superiorly, up towards the bust. The TUBA approach allows emplacing the breast implants without producing visible scars upon the breast proper; but makes appropriate dissection and device-emplacement more technically difficult. A TUBA procedure is performed bluntly—without the endoscope's visual assistance—and is not appropriate for emplacing (pre-filled) silicone-gel implants, because of the great potential for damaging the ] ] shell of the breast implant during its manual insertion through the short (~2.0 cm) incision at the navel, and because pre-filled silicone gel implants are incompressible, and cannot be inserted through so small an incision.<ref name="johnson">{{cite journal |vauthors=Johnson GW, Christ JE | title = The Endoscopic Breast augmentation: The Transumbilical Insertion of Saline-filled Breast Implants | journal = Plastic and Reconstructive Surgery | volume = 92 | issue = 5 | pages = 801–8 | year = 1993 | pmid = 8415961 | doi = 10.1097/00006534-199392050-00004 }}</ref> | |||
| # Transabdominal: as in the TUBA procedure, in the transabdominoplasty breast augmentation (TABA), the breast implants are tunneled superiorly from the abdominal incision into bluntly dissected implant pockets, whilst the patient simultaneously undergoes an ].<ref name="TABA">{{cite journal | author = Wallach SG | s2cid = 44430032 | title = Maximizing the Use of the Abdominoplasty Incision | journal = Plastic and Reconstructive Surgery | volume = 113 | issue = 1 | pages = 411–417 | year = 2004 | pmid = 14707667 | doi = 10.1097/01.PRS.0000091422.11191.1A }}</ref> | |||
| ===Implant pocket placement=== | |||
| {{multiple image|caption_align=center|header_align=center | |||
| | align = right | |||
| | direction = horizontal | |||
| | total_width = 450 | |||
| | header = Implant placement comparison | |||
| | image1 = Subglandular_breast_implants.png | |||
| | alt1 = Subglandular breast implant diagram | |||
| | width1 = 155 | height1 = 192 | |||
| | caption1 = Subglandular implant | |||
| | image2 = Subpectoral_breast_implants.png | |||
| | alt2 = Subpectoral breast implant diagram | |||
| | width2 = 147 | height2 = 196 | |||
| | caption2 = Subpectoral implant | |||
| | image3 = Submuscular_breast_implants.png | |||
| | alt3 = Submuscular breast implant diagram | |||
| | caption3 = Submuscular implant | |||
| | width3 = 147 | height3 = 196 | |||
| }} | |||
| The five ] approaches to emplacing a breast implant to the implant pocket are often described in ] relation to the ]. | |||
| # Subglandular: the breast implant is emplaced to the ], between the ] (the mammary gland) and the pectoralis major muscle (major muscle of the chest), which most approximates the plane of normal breast tissue, and affords the most aesthetic results. Yet, in women with thin pectoral soft-tissue, the subglandular position is likelier to show the ripples and wrinkles of the underlying implant. Moreover, the ] incidence rate is slightly greater with subglandular implantation. | |||
| # Subfascial: the breast implant is emplaced beneath the ] of the pectoralis major muscle; the subfascial position is a variant of the subglandular position for the breast implant.<ref name="Graf">{{cite journal |vauthors=Graf RM, Bernardes A, Rippel R, Araujo LR, Damasio RC, Auersvald A | title = Subfascial Breast Implant: A New Procedure | journal = Plastic and Reconstructive Surgery | volume = 111 | issue = 2 | pages = 904–908 | year = 2003 | pmid = 12560720 | doi = 10.1097/01.PRS.0000041601.59651.15 }}</ref> The technical advantages of the subfascial implant-pocket technique are debated; proponent surgeons report that the layer of ] provides greater implant coverage and better sustains its position.<ref name="subfascial">{{cite journal | author = Tebbetts JB | title = Does Fascia Provide Additional, Meaningful Coverage over a Breast Implant? | journal = Plastic and Reconstructive Surgery | volume = 113 | issue = 2 | pages = 777–779 | year = 2004 | pmid = 14758271 | doi = 10.1097/01.PRS.0000104516.13465.96 }}</ref> | |||
| # Subpectoral (dual plane): the breast implant is emplaced beneath the pectoralis major muscle, after the surgeon releases the inferior muscular attachments, with or without partial dissection of the subglandular plane. Resultantly, the upper pole of the implant is partially beneath the pectoralis major muscle, while the lower pole of the implant is in the subglandular plane. This implantation technique achieves maximal coverage of the upper pole of the implant, whilst allowing the expansion of the implant's lower pole; however, "animation deformity", the movement of the implants in the subpectoral plane can be excessive for some patients.<ref name="Tebbettsdual">{{cite journal | author = Tebbetts JB | title = A System for Breast Implant Selection Based on Patient Tissue Characteristics and Implant-soft tissue Dynamics | journal = Plastic and Reconstructive Surgery | volume = 109 | issue = 4 | pages = 1396–1409 | year = 2002 | pmid = 11964998 | doi = 10.1097/00006534-200204010-00030 }}</ref> | |||
| # Submuscular: the breast implant is emplaced beneath the pectoralis major muscle, without releasing the inferior origin of the muscle proper. Total muscular coverage of the implant can be achieved by releasing the lateral muscles of the chest wall—either the ] or the ], or both—and ] it, or them, to the pectoralis major muscle. In ] surgery, the submuscular implantation approach effects maximal coverage of the breast implants. This technique is rarely used in cosmetic surgery due to high risk of animation deformities. | |||
| # Prepectoral or subcutaneous: in a breast reconstruction following a skin-sparing or skin- and ], the implant is placed above the ''pectoralis major'' muscle without dissecting it so that the implant fills directly the volume of the mammary gland that has been removed. To avoid the issue of capsular contracture, the implant is often covered frontally or completely with a mesh in ], either biological or synthetic. | |||
| ===Post-surgical recovery=== | |||
| The ] scars of a ] mammoplasty develop approximately at 6-weeks post-operative, and fade within months. Depending upon the daily-life physical activities required of the woman, the breast augmentation patient usually resumes her normal life at 1-week post-operative. Moreover, women whose breast implants were emplaced beneath the chest muscles (submuscular placement) usually have a longer, slightly more painful convalescence, because of the healing of the incisions to the chest muscles. Usually, she does not exercise or engage in strenuous physical activities for approximately 6 weeks. During the initial post-operative recovery, the woman is encouraged to regularly exercise (flex and move) her arm to alleviate pain and discomfort; if required, ] indwelling medication catheters can alleviate pain<ref name="pmid19083538">{{cite journal |vauthors=Pacik PT, Nelson CE, Werner C | title = Pain control in augmentation mammaplasty: safety and efficacy of indwelling catheters in 644 consecutive patients | journal = Aesthet Surg J | volume = 28 | issue = 3 | pages = 279–84 | year = 2008 | pmid = 19083538 | doi = 10.1016/j.asj.2008.02.001 | doi-access = free }}</ref><ref name="pmid19083591">{{cite journal |vauthors=Pacik PT, Nelson CE, Werner C | title = Pain control in augmentation mammaplasty using indwelling catheters in 687 consecutive patients: data analysis | journal = Aesthet Surg J | volume = 28 | issue = 6 | pages = 631–41 | year = 2008 | pmid = 19083591 | doi = 10.1016/j.asj.2008.09.001 | doi-access = | s2cid = 13441800 }}</ref> Moreover, significantly improved patient recovery has resulted from refined breast-device implantation techniques (submuscular, subglandular) that allow 95 per cent of women to resume their normal lives at 24-hours post-procedure, without bandages, fluid drains, pain pumps, catheters, medical support brassières, or ] pain medication.<ref name="pmid11964998">{{cite journal | author = Tebbetts JB | title = A system for breast implant selection based on patient tissue characteristics and implant-soft tissue dynamics | journal = Plast. Reconstr. Surg. | volume = 109 | issue = 4 | pages = 1396–409; discussion 1410–5 | year = 2002 | pmid = 11964998 | doi = 10.1097/00006534-200204010-00030}}</ref><ref name="pmid16327616">{{cite journal |vauthors=Tebbetts JB, Adams WP | s2cid = 11180810 | title = Five critical decisions in breast augmentation using five measurements in 5 minutes: the high five decision support process | journal = Plast. Reconstr. Surg. | volume = 116 | issue = 7 | pages = 2005–16 | year = 2005 | pmid = 16327616 | doi = 10.1097/01.prs.0000191163.19379.63}}</ref><ref name="pmid11786826">{{cite journal | author = Tebbetts JB | s2cid = 26419990 | title = Achieving a predictable 24-hour return to normal activities after breast augmentation: part I. Refining practices by using motion and time study principles | journal = Plast. Reconstr. Surg. | volume = 109 | issue = 1 | pages = 273–90; discussion 291–2 | year = 2002 | pmid = 11786826 | doi = 10.1097/00006534-200201000-00044}}</ref><ref name="pmid11786828">{{cite journal | author = Tebbetts JB | s2cid = 21392313 | title = Achieving a predictable 24-hour return to normal activities after breast augmentation: Part II. Patient preparation, refined surgical techniques, and instrumentation | journal = Plast. Reconstr. Surg. | volume = 109 | issue = 1 | pages = 293–305; discussion 306–7 | year = 2002 | pmid = 11786828 | doi = 10.1097/00006534-200201000-00046}}</ref> | |||
| ===Types=== | |||
| ]-solution-filled breast implant device models.]] | |||
| ] gel-filled prostheses.]] | |||
| Today, there are three types of breast implants commonly used for ], ], and breast augmentation procedures:<ref>{{cite web |url=http://mpsmn.com/breast-procedures/choosing-breast-implants/ |title=Choosing Your Breast Implants |publisher=Minneapolis Plastic Surgery, LTD |format=Web |access-date=23 November 2016 |url-status=live |archive-url=https://web.archive.org/web/20161124092228/http://mpsmn.com/breast-procedures/choosing-breast-implants/ |archive-date=24 November 2016 }}</ref> | |||
| # saline implant filled with sterile ]. | |||
| # silicone implant filled with viscous ] gel. | |||
| # structured implants using nested elastomer silicone shells and two saline filled lumen. | |||
| A fourth type of implant, composite (or alternative-composite) implants, have largely been discontinued. These types featured fillers such as soy oil and polypropylene string. Other discontinued materials include ox cartilage, Terylene wool, ground rubber, silastic rubber, and teflon-silicone prostheses.<ref name="TalesForTaglicozzi">{{cite book |last1=Zannis |first1=John | name-list-style = vanc |title=Tales for Tagliacozzi: An Inside Look at Modern-Day Plastic Surgery |date=2017 |publisher=AuthorHouse |isbn=9781524659073 |url=https://books.google.com/books?id=z9YADgAAQBAJ&q=Alternative-composition+implants+soy&pg=PT19 |access-date=7 June 2019}}</ref> | |||
| ====Saline implants==== | |||
| The saline breast implant—filled with saline solution (biological-concentration salt water 0.90% ] of ], ca. 300 ]/L.)—was first manufactured by the Laboratoires Arion company, in France, and was introduced for use as a prosthetic ] in 1964. The contemporary models of saline breast implant are manufactured with thicker, room-temperature ] (RTV) shells made of a ] ]. The study ''In vitro Deflation of Pre-filled Saline Breast Implants'' (2006) reported that the rates of deflation (filler leakage) of the pre-filled saline breast implant made it a second-choice prosthesis for corrective breast surgery.<ref name="Stevens" /> Nonetheless, in the 1990s, the saline breast implant was the ] most common device used for breast augmentation surgery in the United States, because of the U.S. FDA's restriction against the implantation of silicone-filled breast implants outside of clinical studies. Saline breast implants have enjoyed little popularity in the rest of the world, possessing negligible market share. | |||
| The technical goal of saline-implant technology was a physically less invasive surgical technique for emplacing an empty breast implant device through a smaller surgical incision.<ref name="Arion1965">{{cite journal |vauthors=Arion HG|title=Retromammary Prosthesis |journal=Comptes Rendus de la Société Française de Gynécologie | year=1965|volume=5}}</ref> In surgical praxis, after having emplaced the empty breast implants to the implant pockets, the plastic surgeon then filled each device with ], and, because the required insertion-incisions are short and small, the resultant incision-scars will be smaller and shorter than the surgical scars usual to the long incisions required for inserting pre-filled, silicone-gel implants. | |||
| When compared to the results achieved with a silicone-gel breast implant, the saline implant can yield acceptable results, of increased breast-size, smoother hemisphere-contour, and realistic texture; yet, it is likelier to cause cosmetic problems, such as the rippling and the wrinkling of the breast-envelope skin, accelerated lower breast pole stretch, and technical problems, such as the presence of the implant being noticeable to the eye and to the touch. The occurrence of such cosmetic problems is likelier in the case of the woman with very little breast tissue, and in the case of the woman who requires ] breast reconstruction; thus, the silicone-gel implant is the technically superior ] device for breast augmentation, and for breast reconstruction. In the case of the woman with much breast tissue, for whom sub-muscular emplacement is the recommended surgical approach, saline breast implants can produce an aesthetic result much like that afforded by silicone breast implants.<ref>{{cite journal | author = Eisenberg TS | s2cid = 136191732 | title = Silicone Gel Implants Are Back — So What? | journal = American Journal of Cosmetic Surgery | year = 2009 | volume = 26 | pages = 5–7| doi = 10.1177/074880680902600103 }}</ref><ref>{{cite journal | author = Eisenberg TS | title = The Underappreciated Saline Breast Implant | journal = Aesthetic Plastic Surgery | year = 2022 | volume = 47 | issue = 2 | pages = 897–900 | doi = 10.1007/s00266-022-03106-z | pmid = 36131136 | s2cid = 252437143 }}</ref> Ultrasound examination and outcome studies have revealed that saline and silicone breast implants look and feel similar.<ref>{{cite journal | author = Swanson E | title = Prospective Study of Saline versus Silicone Gel Implants for Subpectoral Breast Augmentation | journal = Plastic and Reconstructive Surgery | year = 2020 | volume = 8 | issue = 6 | pages = e2882 | doi = 10.1097/GOX.0000000000002882 | pmid = 32766047 | pmc = 7339341 }}</ref> | |||
| ====Silicone gel implants==== | |||
| As a ] ], there are five generations of silicone breast implant, each defined by common model-manufacturing techniques. | |||
| {{Citation needed |date=October 2014}} | |||
| The modern prosthetic breast was invented in 1961 by the American ] Thomas Cronin and Frank Gerow, and manufactured by the ]; in due course, the first augmentation mammoplasty was performed in 1962. | |||
| =====First generation===== | |||
| The Cronin–Gerow Implant, prosthesis model 1963, was a silicone rubber envelope-sac, shaped like a teardrop, which was filled with viscous silicone-gel. To reduce the rotation of the emplaced breast implant upon the chest wall, the model 1963 prosthesis was affixed to the implant pocket with a fastener-patch, made of Dacron material (]), which was attached to the rear of the breast implant shell.<ref name=Cronin_1963>{{cite journal |vauthors=Cronin TD, Gerow FJ | title = Augmentation Mammaplasty: A New "natural feel" Prosthesis | journal = Excerpta Medica International Congress Series | year = 1963 | volume = 66 | page = 41}}</ref> | |||
| =====Second generation===== | |||
| In the 1970s, manufacturers presented the second generation of breast implant prostheses that featured functional developments and aesthetic improvements to the technology: | |||
| * the first technological developments were a thinner-gauge device-shell, and a filler gel of low-cohesion silicone, which improved the functionality and the verisimilitude (size, appearance, and texture) of the silicone-gel breast implant. Yet, in clinical practice, second-generation breast implants proved fragile and saw greater instances of shell rupture, and of filler leakage ("silicone-gel bleed") through the intact device shell. The consequent, increased incidence-rates of medical complications (e.g. ]) precipitated faulty-product, ], by the U.S. government, against the Dow Corning Corporation, and other manufacturers of breast prostheses. | |||
| * the second technological development was a ] foam coating for the shell of the breast implant; the coating reduced the incidence of ], by causing an ] that impeded the formation of a capsule of fibrous ] tissue around the breast implant. Nevertheless, despite that prophylactic measure, the medical use of polyurethane-coated breast implants was briefly discontinued, because of the potential health-risk posed by 2,4-toluenediamine (TDA), a ]ic by-product of the chemical breakdown of the polyurethane foam coating of the breast implant proven to cause liver and skin cancers in animal-model studies.<ref name="Luu11998">{{cite journal |vauthors=Luu HM, Hutter JC, Bushar HF | title = A Physiologically based Pharmacokinetic Model for 2,4-toluenediamine Leached from Polyurethane foam-covered Breast Implants | journal = Environ Health Perspect | volume = 106 | issue = 7 | pages = 393–400 | year = 1998 | pmid = 9637796 | pmc = 1533137 | doi = 10.2307/3434066 | jstor = 3434066 }}</ref> | |||
| :After reviewing the medical data, the U.S. ] concluded that TDA-induced ] was an infinitesimal health-risk to women with breast implants, and did not justify legally requiring physicians to explain the matter to their patients. In the event, polyurethane-coated breast implants remain in plastic surgery practice in Europe and in South America; and no manufacturer has sought FDA approval for medical sales of such breast implants in the U.S.<ref>{{cite journal |vauthors=Hester TR, Tebbetts JB, Maxwell GP | title = The Polyurethane-covered Mammary Prosthesis: Facts and Fiction (II): A Look Back and a "peek" Ahead | journal = Clinical Plastic Surgery | volume = 28 | issue = 3 | pages = 579–86 | year = 2001 | doi = 10.1016/S0094-1298(20)32397-X | pmid = 11471963 }}</ref> | |||
| * the third technological development was the double lumen breast implant device, a double-cavity prosthesis composed of a silicone breast implant contained within a saline breast implant. The two-fold, technical goal was: (i) the cosmetic benefits of silicone-gel (the inner lumen) enclosed in saline solution (the outer lumen); (ii) a breast implant device the volume of which is post-operatively adjustable. Nevertheless, the more complex design of the double-lumen breast implant suffered a device-failure rate greater than that of single-lumen breast implants. The contemporary versions of second-generation breast implant devices (presented in 1984) are the "Becker Expandable" models of breast implant, which are primarily used for ]. | |||
| =====Third and fourth generations===== | |||
| In the 1980s, the models of the third and of the fourth generations of breast implant devices were sequential advances in manufacturing technology, such as ]-coated shells that decreased gel-bleed (filler leakage), and a thicker (increased-cohesion) filler gel. ], the manufacturers of prosthetic breasts then designed and made anatomic models (natural breast) and shaped models (round, tapered) that realistically corresponded with the breast- and body- types of women. The tapered models of breast implant have a uniformly textured surface, which reduces the rotation of the prosthesis within the implant pocket; the round models of breast implant are available in smooth-surface- and textured-surface- types. | |||
| =====Fifth generation===== | |||
| Since the mid-1990s, the fifth generation of silicone-gel breast implant is made of a high-strength, highly cohesive silicone gel that mostly eliminates the occurrences of filler leakage ("silicone gel bleed") and of the migration of the silicone filler from the implant pocket to elsewhere in the woman's body. These implants are commonly referred to as "gummy bear breast implants" for their firm, pliant consistency, which is similar to gummy candies. The studies ''Experience with Anatomical Soft Cohesive Silicone gel Prosthesis in Cosmetic and Reconstructive Breast Implant Surgery'' (2004) and ''Cohesive Silicone gel Breast Implants in Aesthetic and Reconstructive Breast Surgery'' (2005) reported low incidence-rates of ] and of device-shell rupture; and greater rates of improved medical-safety and technical-efficacy than that of early generation breast implant devices.<ref>{{cite journal |vauthors=Brown MH, Shenker R, Silver SA | s2cid = 35392851 | title = Cohesive silicone gel breast implants in aesthetic and reconstructive breast surgery | journal = Plastic and Reconstructive Surgery | volume = 116 | issue = 3 | pages = 768–779; discussion 779–1 | year = 2005 | pmid = 16141814 | doi = 10.1097/01.prs.0000176259.66948.e7 }}</ref><ref>{{cite journal |vauthors=Fruhstorfer BH, Hodgson EL, Malata CM | s2cid = 24661896 | title = Early experience with an anatomical soft cohesive silicone gel prosthesis in cosmetic and reconstructive breast implant surgery | journal = Annals of Plastic Surgery | volume = 53 | issue = 6 | pages = 536–542 | year = 2004 | pmid = 15602249 | doi = 10.1097/01.sap.0000134508.43550.6f }}</ref><ref>{{cite journal |vauthors=Hedén P, Jernbeck J, Hober M | title = Breast augmentation with anatomical cohesive gel implants: The world's largest current experience | journal = Clinics in Plastic Surgery | volume = 28 | issue = 3 | pages = 531–552 | year = 2001 | doi = 10.1016/S0094-1298(20)32393-2 | pmid = 11471959 }}</ref> | |||
| ==Breastfeeding== | |||
| ]. {{ordered list |1=] |2=]s |3=] |4=] |5=] |6=] |7=] |8=]}}]] | |||
| The presence of breast implants currently presents no contraindication to breast feeding, and no evidence to support that the practice may present health issues to a ] infant is recognized by the USFDA. | |||
| Women with breast implants may have functional breast-feeding difficulties; mammoplasty procedures that feature periareolar incisions are especially likely to cause breastfeeding difficulties. Surgery may also damage the lactiferous ducts and the nerves in the nipple-areola area.<ref> {{webarchive|url=https://web.archive.org/web/20101230173437/http://www.llli.org/NB/NBsurgery.html |date=2010-12-30 }}, La Leche League, contains references.</ref><ref> {{webarchive|url=https://web.archive.org/web/20101231121338/http://www.llli.org/cbi/bibimplant.html |date=2010-12-31 }}, Selected Bibliography April 2003, LLLI Center for Breastfeeding Information</ref><ref name="slate"> {{webarchive|url=https://web.archive.org/web/20100125010657/http://www.slate.com/id/2238096 |date=2010-01-25 }}, Christopher Beam, ], 11 December 2009</ref> | |||
| Functional breastfeeding difficulties arise if the surgeon cut the milk ducts or the major nerves innervating the breast, or if the milk glands were otherwise damaged. Milk duct and nerve damage are more common if the incisions cut tissue near the nipple. The milk glands are most likely to be affected by subglandular implants (under the gland), and by large-sized breast implants, which pinch the lactiferous ducts and impede milk flow. Small-sized breast implants, and submuscular implantation, cause fewer breast-function problems; however, it is impossible to predict whether a woman who undergoes breast augmentation will be able to successfully breast feed since some women are able to breast-feed after periareolar incisions and subglandular placement and some are not able to after augmentation using submuscular and other types of surgical incisions.<ref name="slate"/> | |||
| ==Mammography== | |||
| ] | |||
| The presence of ] opaque breast implants (either saline or silicone) might interfere with the radiographic sensitivity of the ], that is, the image might not show any tumor(s) present. In this case, an Eklund view mammogram is required to ascertain either the presence or the absence of a cancerous tumor, wherein the breast implant is manually displaced against the chest wall and the breast is pulled forward, so that the mammograph can visualize a greater volume of the internal tissues; nonetheless, approximately one-third of the breast tissue remains inadequately visualized, resulting in an increased incidence of mammograms with false-negative results.<ref name="Handel1992">{{cite journal |vauthors=Handel N, Silverstein MJ, Gamagami P, Jensen JA, Collins A | title = Factors Affecting Mammographic Visualization of the Breast after Augmentation Mammaplasty | journal = JAMA | volume = 268 | issue = 14 | pages = 1913–1917 | year = 1992 | pmid = 1404718 | doi = 10.1001/jama.268.14.1913 }}</ref><ref>{{cite journal | vauthors = O'Keefe JR, Wilkinson JM, Spuur KM | title = Current practice in mammographic imaging of the augmented breast in Australia | journal = Journal of Medical Radiation Sciences | volume = 67 | issue = 2 | pages = 102–110 | date = June 2020 | pmid = 31981297 | pmc = 7276184 | doi = 10.1002/jmrs.374 }}</ref> | |||
| The ] studies '' Cancer in the Augmented Breast: Diagnosis and Prognosis'' (1993) and ''Breast Cancer after Augmentation Mammoplasty'' (2001) of women with breast implant prostheses reported no significant differences in disease-stage at the time of the diagnosis of cancer; prognoses are similar in both groups of women, with augmented patients at a lower risk for subsequent cancer recurrence or death.<ref name="Clark1993">{{cite journal |vauthors=Clark CP, Peters GN, O'Brien KM | title = Cancer in the Augmented Breast: Diagnosis and Prognosis | journal = Cancer | volume = 72 | issue = 7 | pages = 2170–4 | year = 1993 | pmid = 8374874 | doi = 10.1002/1097-0142(19931001)72:7<2170::AID-CNCR2820720717>3.0.CO;2-1 | doi-access = free }}</ref><ref name="Skinner2001">{{cite journal |vauthors=Skinner KA, Silberman H, Dougherty W, Gamagami P, Waisman J, Sposto R, Silverstein MJ | s2cid = 26010159 | title = Breast cancer after augmentation mammoplasty | journal = Ann Surg Oncol | volume = 8 | issue = 2 | pages = 138–44 | year = 2001 | pmid = 11258778 | doi = 10.1007/s10434-001-0138-x }}</ref> Conversely, the use of implants for ] ''after'' breast cancer ] appears to have no negative effect upon the incidence of cancer-related death.<ref name="Lee2005">{{cite journal |vauthors=Le GM, O'Malley CD, Glaser SL, Lynch CF, Stanford JL, Keegan TH, West DW | title = Breast implants following mastectomy in women with early-stage breast cancer: prevalence and impact on survival | journal = Breast Cancer Res. | volume = 7 | issue = 2 | pages = R184–93 | year = 2005 | pmid = 15743498 | pmc = 1064128 | doi = 10.1186/bcr974 | doi-access = free }}</ref> That patients with breast implants are more often diagnosed with palpable—but not larger—tumors indicates that equal-sized tumors might be more readily palpated in augmented patients, which might compensate for the impaired mammogram images.<ref name="HandelSilver2006">{{cite journal |vauthors=Handel N, Silverstein MJ | s2cid = 36277679 | title = Breast cancer diagnosis and prognosis in augmented women | journal = Plastic and Reconstructive Surgery | volume = 118 | issue = 3 | pages = 587–93 | year = 2006 | pmid = 16932162 | doi = 10.1097/01.prs.0000233038.47009.04 }}</ref> The ready palpability of the breast-cancer tumor(s) is consequent to breast tissue thinning by compression, innately in smaller breasts ''a priori'' (because they have lesser tissue volumes), and that the implant serves as a radio-opaque base against which a cancerous tumor can be differentiated.<ref name="Cunningham2006">{{cite journal | author = Cunningham B | title = Breast cancer diagnosis and prognosis in augmented women- Discussion | journal = Plastic and Reconstructive Surgery | volume = 118 | issue = 3 | pages = 594–595 | year = 2006 | doi = 10.1097/01.prs.0000233047.87102.8e | s2cid = 220562548 }}</ref> | |||
| ] | |||
| The breast implant has no clinical bearing upon ] breast-conservation surgery for women who developed breast cancer after the implantation procedure, nor does the breast implant interfere with external beam radiation treatments (XRT); moreover, the post-treatment incidence of breast-tissue fibrosis is common, and thus a consequent increased rate of capsular ].<ref name="Scwartz2006">{{cite journal |vauthors=Schwartz GF, Veronesi U, Clough KB, Dixon JM, Fentiman IS, Heywang-Köbrunner SH, Holland R, Hughes KS, Mansel RE, Margolese R, Mendelson EB, Olivotto IA, Palazzo JP, Solin LJ | title = Consensus Conference on Breast Conservation | journal = Journal of the American College of Surgeons| volume = 203 | issue = 2 | pages = 198–207 | year = 2006 | pmid = 16864033 | doi = 10.1016/j.jamcollsurg.2006.04.009 }}</ref> There is tentative evidence that women who have had breast augmentation, have worse breast cancer prognosis.<ref>{{cite journal | vauthors = Lavigne E, Holowaty EJ, Pan SY, Villeneuve PJ, Johnson KC, Fergusson DA, Morrison H, Brisson J | display-authors = 6 | title = Breast cancer detection and survival among women with cosmetic breast implants: systematic review and meta-analysis of observational studies | journal = BMJ | volume = 346 | issue = apr29 1 | pages = f2399 | date = April 2013 | pmid = 23637132 | doi = 10.1136/bmj.f2399 | doi-access = free }}</ref> The use of implants for breast reconstruction ''after'' breast cancer mastectomy appears to have no negative effect upon cancer-related death.<ref name="Lee2005" /><ref>{{cite journal | vauthors = Hwang ES, Lichtensztajn DY, Gomez SL, Fowble B, Clarke CA | title = Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status | journal = Cancer | volume = 119 | issue = 7 | pages = 1402–11 | date = April 2013 | pmid = 23359049 | pmc = 3604076 | doi = 10.1002/cncr.27795 }}</ref> | |||
| There have been multiple reported cases of other adverse effects of mammography of women with breast implants; ruptures resulting from pressure exerted on the breast implant make up a majority of these cases.<ref name=":1" /> Compression may also lead to pain or exacerbate already existing pain in the breasts.<ref name=":1">{{cite web|date=18 January 2018|title=Breast Implant Adverse Events During Mammography|url=https://www.fda.gov/medical-devices/breast-implants/breast-implant-adverse-events-during-mammography|url-status=live|website=Federal Drug Administration|archive-url=https://web.archive.org/web/20200919214328/https://www.fda.gov/medical-devices/breast-implants/breast-implant-adverse-events-during-mammography |archive-date=2020-09-19 }}</ref> | |||
| ==History== | |||
| ] (1842–1916), a pioneer in ]]] | |||
| ===19th century=== | |||
| As studies have followed women with implants for a longer period of time, more information has been made available to assess these issues. A 2004 Danish study, reported that women who had breast implants for an average of 19 years were no more likely to report an excess number of rheumatic symptoms then control groups.<ref name="Breiting2004">{{cite journal | author=Breiting VB, Holmich LR, Brandt B, Fryzek JP, Wolthers MS, Kjoller K, McLaughlin JK, Wiik A, Friis S | title=Long-term health status of Danish women with silicone breast implants | journal= Plastic and Reconstructive Surgery| year=2004 | pages=217-226 | volume=114 | id= PMID 15220596}}</ref> | |||
| A large study of plastic surgery patients found a decreased ] in both breast implant and other plastic surgery patients, but a relatively increased risk of ] deaths in breast implant recipients compared to other forms of plastic surgery. The authors attributed this to differences in smoking rates.<ref name=Brinton2006>{{cite journal | author=Brinton LA, Lubin JH, Murray MC, Colton T, Hoover RN | title=Mortality rates among augmentation mammoplasty patients: an update | journal=Epidemiology | year=2006 | pages=162-9 | volume=17 | issue=2 | id=PMID 16477256}}</ref> Another large study of nearly 25,000 Canadian women with implants recently reported a 43 percent lower rate of breast cancer compared with the general population and a lower-than-average risk of developing cancer of any kind.<ref name="Villenueve2006">{{cite journal | author=Villenueve PJ, et. al | title=Mortality among Canadian Women with Cosmetic Breast Implants. | journal=Am J Epidemiol| year=2006 | month=June| id=PMID 16777929 }}</ref> | |||
| Since the late nineteenth century, breast implants have been used to ] augment the size (volume), modify the shape (contour), and enhance the feel (tact) of a woman's breasts. In 1895, surgeon ] effected the earliest breast implant emplacement when he used the patient's autologous ], harvested from a benign ] ], to repair the asymmetry of the breast from which he had removed a tumor.<ref>{{cite journal | vauthors = Czerny V | title = Plastischer Ersatz der Brusthus durch ein Lipoma | journal = Zentralblatt für Chirurgie | year = 1895 | volume = 27 | page = 72 }}</ref> In 1889, surgeon ] experimented with ]<!--?--> injections, with disastrous results arising from the breakup of the paraffin into smaller bodies following the procedure.<ref>{{cite journal | vauthors = Glicenstein J | title = | journal = Annales de Chirurgie Plastique et Esthétique | volume = 52 | issue = 2 | pages = 157–61 | date = April 2007 | pmid = 16860452 | doi = 10.1016/j.anplas.2006.05.003 }}</ref> | |||
| ===Platinum=== | |||
| ] is a ] used in the making of silicone implant polymer shells and other silicone devices used in medicine. The literature indicates that small amounts of platinum leaches (leaks) from these implants and is present in the surrounding tissue. The FDA reviewed the available studies from the medical literature on platinum and breast implants in 2002 and concluded there was little evidence suggesting toxicity from platinum in implant patients. <ref name="arepelli2003">{{cite journal | author=Arepelli S, et al. | title=Allergic reactions to platinum in silicone breast implants. | journal=J Long-Term Effects Medical Implants | year=2002 | pages=299-306 | id=PMID 12627791 }}</ref> </p> | |||
| In 2006, researchers published a controversial study that claimed to identify the previously undocumented presence of toxic platinum oxidative states in vivo. <ref name="AnalChem2006-Lykissa">{{cite journal | author = Lykissa E.D. and Maharaj S.V.M. | year = 2006 | month = April | title = Total Platinum Concentration and Platinum Oxidation States in Body Fluids, Tissue, and Explants from Women Exposed to Silicone and Saline Breast Implants by IC-ICPMS | journal = Anal. Chem. | volume = | issue = | pages = | id =(due publication May 2006) | url =http://pubs.acs.org/cgi-bin/abstract.cgi/ancham/asap/abs/ac0514016.html | accessdate =2006-04-06 (Web)}}</ref>. A letter from the editors of the publishing journal, ''Analytical Chemistry'', subsequently expressed concern over the research's experimental design and urged the journal's readers to "use caution in evaluating the conclusions drawn in the paper."The FDA reviewed this study and the existing literature, concluding that the body of existing research did not support their findings, and that the platinum in new implants is likely not ionized and therefore would not represent a significant risk to women. | |||
| ===20th century=== | |||
| ==Concerns with breast cancer screening and treatment in patients with breast implants== | |||
| From the first half of the twentieth century, physicians used other substances as breast implant fillers—], glass balls, ground rubber, ox ], ], ], Dicora, ] chips, Ivalon (]—formaldehyde polymer sponge), a polyethylene sac with Ivalon, polyether foam sponge (Etheron), polyethylene tape (Polystan) strips wound into a ball, ] (polyurethane foam sponge) Silastic rubber, and teflon-silicone prostheses.<ref>{{cite book | veditors = Bondurant S, Ernster V, Herdman R | collaboration = Committee on the Safety of Silicone Breast Implants | title = Safety of Silicone Breast Implants | publisher = Institute of Medicine | year = 1999 | url = http://www.nap.edu/books/0309065321/html/21.html | isbn = 0-309-06532-1 | page = 21 | url-status = live | archive-url = https://web.archive.org/web/20070313091328/http://www.nap.edu/books/0309065321/html/21.html | archive-date = 2007-03-13 | doi = 10.17226/9602 | pmid = 20669503 | vauthors = ((Institute of Medicine (US) Committee on the Safety of Silicone Breast Implants)), Bondurant S, Ernster V, Herdman R }}</ref> | |||
| The presence of radio-opaque breast implants may interfere with the sensitivity of screening mammography. Specialized radiographic techniques where the implant is manually displaced ("'''Eklund views'''") may improve this somewhat, but approximately 1/3 of the breast is still not adequately visualized with a resultant increase in false-negative mammograms.<ref name="Handel1992">{{cite journal | author=Handel, N., Silverstein, M. J., Gamagami, P., Jensen, J. A., and Collins, A. | title=actors affecting mammographic visualization of the breast after augmentation mammaplasty.| journal=JAMA| year=1992| pages=1913-17 | id=PMID 1404718}}</ref>A number of studies looking at breast cancers in women with implants have found no significant difference in stage of disease at time of diagnosis, and prognosis appears to be similar in both groups with augmented patients not a higher risk for subsequent cancer recurrence or death.<ref name="Clark1993">{{cite journal | author=Clark CP 3rd, Peters GN, O'Brien KM. | title=Cancer in the augmented breast. Diagnosis and prognosis.| journal=Cancer | year=1993| pages=2170-4 | id=PMID 8374874 }}</ref><ref name="Skinner2001">{{cite journal | author=Skinner KA, Silberman H, Dougherty W, Gamagami P, Waisman J, Sposto R, Silverstein MJ. | title=Breast cancer after augmentation mammoplasty.| journal=Ann Surg Oncol. | year=2001| pages=138-44 | id=PMID 11258778 }}</ref> Conversely, the use of implants for reconstruction ''after'' mastectomy for breast cancer also appears not to have a negative affect on cancer-related mortality.<ref name="Lee2005">{{cite journal | author=Le GM, O'Malley CD, Glaser SL, Lynch CF, Stanford JL, Keegan TH, West DW. | title=Breast implants following mastectomy in women with early-stage breast cancer: prevalence and impact on survival.| journal=Breast Cancer Res.| year=2005| pages=R184-93 | id=PMID 15743498}}</ref></p> | |||
| In the mid-twentieth century, Morton I. Berson, in 1945, and Jacques Maliniac, in 1950, each performed flap-based breast augmentations by rotating the patient's chest wall tissue into the breast to increase its volume. Furthermore, throughout the 1950s and the 1960s, plastic surgeons used synthetic fillers—including ] injections received by some 50,000 women, from which developed silicone ]s and breast hardening that required treatment by ].<ref>{{cite journal | author = Anderson N| title = Lawsuit Science: Lessons from the Silicone Breast Implant Controversy | journal = New York Law School Law Review | year = 1997 | volume = 41 | issue = 2 | pages = 401–07 }}</ref> In 1961, the American plastic surgeons Thomas Cronin and Frank Gerow, and the ], developed the first silicone breast prosthesis, filled with silicone gel; in due course, the first augmentation mammoplasty was performed in 1962 using the Cronin–Gerow Implant, prosthesis model 1963. In 1964, the French company Laboratoires Arion developed and manufactured the saline breast implant, filled with ], and then introduced for use as a medical device in 1964.<ref name="Stevens">{{cite journal |vauthors=Stevens WG, Hirsch EM, Stoker DA, Cohen R | s2cid = 41156555 | title = In vitro Deflation of Pre-filled Saline Breast Implants | journal = Plastic and Reconstructive Surgery | volume = 118 | issue = 2 | pages = 347–349 | year = 2006 | pmid = 16874200 | doi = 10.1097/01.prs.0000227674.65284.80 }}</ref> | |||
| An observation that patients with implants are more often diagnosed with palpable tumors (but not larger ones) suggest that tumors of equal size may be more easily palpated in augmented patients, and this may compensate somewhat for the potential impairment of mammography.<ref name="Handel2006">{{cite journal | author=Handel N, Silverstein MJ | title=Breast cancer diagnosis and prognosis in augmented women.| journal=Plast Reconstr Surg. | year=2006 | pages=587-93 | id=PMID 16932162 }}</ref> This palpability is due to thinning of the breast by compression, innately smaller breasts a priori, and that the implant serves as a base against which the mass may be differentiated.<ref name="Cunningham2006">{{cite journal | author=Cunningham B | title=Breast cancer diagnosis and prognosis in augmented women- Discussion| journal=Plast Reconstr Surg. | year=2006 | pages=594-5 | id=PMID 16932163 }}</ref> | |||
| ===FDA approval=== | |||
| The presence of a breast implant does not influence the ability for breast conservation (lumpectomy) surgery to be offered for women who subsequently develop breast cancer, and does not interfere with delivery of external beam radiation (XRT) treatments that may be required. <ref name="Scwartz2006">{{cite journal | author=SChwartz GF, et. al. | title=Consensus Conference on Breast Conservation| journal=JACAS | year=2006 | pages=198-207 | id=PMID 16864033 }}</ref>Fibrosis of breast tissue after XRT is common and an increase in capsular contracture rates would be expected. | |||
| In 1988, twenty-six years after the 1962 introduction of breast implants filled with silicone gel, the ] (FDA) investigated breast implant failures and the subsequent complications, and re-classified breast implant devices as Class III medical devices, and required from manufacturers the documentary data substantiating the safety and efficacy of their breast implant devices.<ref name="FDABICH">{{Cite web|url=https://www.fda.gov/cdrh/breastimplants/indexbip.html|archive-url=https://web.archive.org/web/20080917223436/https://www.fda.gov/cdrh/breastimplants/indexbip.html|url-status=dead|title=FDA Breast Implant Consumer Handbook - 2004|website=]|archive-date=September 17, 2008}}</ref> In 1992, the FDA placed silicone-gel breast implants in moratorium in the U.S., because there was "inadequate information to demonstrate that breast implants were safe and effective". Nonetheless, medical access to silicone-gel breast implant devices continued for clinical studies of post-mastectomy ], the correction of congenital deformities, and the replacement of ruptured silicone-gel implants. The FDA required from the manufacturers the clinical trial data, and permitted their providing breast implants to the ] patients for the statistical studies required by the U.S. Food and Drug Administration.<ref name="FDABICH" /> In mid-1992, the FDA approved an adjunct study protocol for silicone-gel filled implants for breast reconstruction patients, and for revision-surgery patients. Also in 1992, the ], a silicone products and breast implant manufacturer, announced the discontinuation of five implant-grade ]s, but would continue producing 45 other, medical-grade, silicone materials—three years later, in 1995, the Dow Corning Corporation went ] when it faced large class action lawsuits claiming a variety of illnesses.<ref name="FDABICH" /> | |||
| * In 1997, the ] (HHS) appointed the Institute of Medicine (IOM) of the ] (NAS) to investigate the potential risks of operative and post-operative ] from the emplacement of silicone breast implants. The IOM's review of the safety and efficacy of silicone gel-filled breast implants, reported that the "evidence suggests diseases or conditions, such as ] diseases, ], neurological diseases, or other systemic complaints or conditions are no more common in women with breast implants, than in women without implants" subsequent studies and systemic review found no causal link between silicone breast implants and disease.<ref name="FDABICH" /> | |||
| ] | |||
| * In 1998, the U.S. FDA approved adjunct study protocols for silicone-gel filled implants only for breast reconstruction patients and for revision-surgery patients; and also approved the Dow Corning Corporation's Investigational Device Exemption (IDE) study for silicone-gel breast implants for a limited number of breast augmentation-, reconstruction-, and revision-surgery patients.<ref name="FDABICH" /> | |||
| * In 1999, the Institute of Medicine published the ''Safety of Silicone Breast Implants'' (1999) study that reported no evidence that saline-filled and silicone-gel filled breast implant devices caused systemic health problems; that their use posed no new health or safety risks; and that local complications are "the primary safety issue with silicone breast implants", in distinguishing among routine and local medical complications and systemic health concerns."<ref name="FDABICH" /><ref>{{cite book|title=Safety of Silicone Breast Implants - The National Academies Press|work=nap.edu|doi=10.17226/9602|pmid=20669503|year=1999|isbn=978-0-309-15740-7| vauthors = ((Institute of Medicine (US) Committee on the Safety of Silicone Breast Implants)), Bondurant S, Ernster V, Herdman R }}</ref><ref>{{cite book | vauthors = Grigg M, Bondurant S, Ernster VL, Herdman R |title=Information for Women about the Safety of Silicone Breast Implants - The National Academies Press| veditors = Grigg M, Bondurant S, Ernster VL, Herdman R |work=nap.edu |doi=10.17226/9618|pmid=20669498|year=2000|isbn=978-0-309-06593-1 }}</ref> | |||
| * In 2000, the FDA approved saline breast implant Premarket Approval Applications (PMA) containing the type and rate data of the local medical complications experienced by the breast surgery patients.<ref>{{Cite web|url=https://www.fda.gov/bbs/topics/NEWS/NEW00727.html|archive-url=https://web.archive.org/web/20080113143409/https://www.fda.gov/bbs/topics/NEWS/NEW00727.html|url-status=dead|title=FDA study|website=]|archive-date=January 13, 2008}}</ref> "Despite complications experienced by some women, the majority of those women still in the ] and ] studies, after three years, reported being satisfied with their implants."<ref name="FDABICH" /> The premarket approvals were granted for breast augmentation, for women at least 18 years old, and for women requiring breast reconstruction.<ref>{{cite web|url=https://www.fda.gov/cdrh/pdf/p990075.html|title=FDA approval|website=fda.gov|access-date=28 April 2018|url-status=live|archive-url=https://web.archive.org/web/20090330052802/https://www.fda.gov/cdrh/pdf/p990075.html|archive-date=30 March 2009}}</ref><ref>{{cite web|url=https://www.fda.gov/cdrh/pdf/p990074.html|title=FDA approval|website=fda.gov|access-date=28 April 2018|url-status=live|archive-url=https://web.archive.org/web/20090526013245/https://www.fda.gov/cdrh/pdf/p990074.html|archive-date=26 May 2009}}</ref> | |||
| * In 2006, for the Inamed Corporation and for the Mentor Corporation, the U.S. Food and Drug Administration lifted its restrictions against using silicone-gel breast implants for breast reconstruction and for augmentation mammoplasty. Yet, the approval was conditional upon accepting FDA monitoring, the completion of 10-year-mark studies of the women who already had the breast implants, and the completion of a second, 10-year-mark study of the safety of the breast implants in 40,000 other women.<ref>{{cite web |url=https://www.fda.gov/bbs/topics/NEWS/2006/NEW01512.html |title=FDA Approves Silicone Gel-Filled Breast Implants |access-date=2008-07-01 |publisher=FDA |url-status=live |archive-url=https://web.archive.org/web/20080726061002/https://www.fda.gov/bbs/topics/NEWS/2006/NEW01512.html |archive-date=2008-07-26 }}</ref> The FDA warned the public that breast implants do carry medical risks, and recommended that women who undergo breast augmentation should periodically undergo ] examinations to screen for signs of either shell rupture or of filler leakage, or both conditions; and ordered that breast surgery patients be provided with detailed, informational brochures explaining the medical risks of using silicone-gel breast implants.<ref name="FDABICH" /> | |||
| * In March 2019, the FDA hosted a public meeting of their General and Plastic Surgery Devices Advisory Panel to discuss safety issues of silicone and saline breast implants, including BIA-ALCL and breast implant illness.<ref>{{Cite web |date=April 25, 2019 |title=March 25-26, 2019: General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee Meeting Announcement |url=https://www.fda.gov/advisory-committees/advisory-committee-calendar/march-25-26-2019-general-and-plastic-surgery-devices-panel-medical-devices-advisory-committee |website=U.S. Food and Drug Administration}}</ref> One of the major topics of that public meeting was the evidence that Allergan BIOCELL textured breast implants were the type of breast implants most likely to cause BIA-ALCL. Following the committee meeting, the FDA requested that Allergan recall their BIOCELL textured breast implants and tissue expanders, and in July 2019 Allergan took those BIOCELL textured implants and expanders off the market. Allergan subsequently announced they would contact all customers who had purchased their products to ensure they were aware of the recall.<ref>{{Cite web |date=June 1, 2020 |title=The FDA Requests Allergan Voluntarily Recall Natrelle BIOCELL Textured Breast Implants and Tissue Expanders from the Market to Protect Patients: FDA Safety Communication |url=https://www.fda.gov/medical-devices/safety-communications/fda-requests-allergan-voluntarily-recall-natrelle-biocell-textured-breast-implants-and-tissue |website=U.S. Food and Drug Administration}}</ref> | |||
| The U.S. Food and Drug Administration established the age ranges for women seeking breast implants; for breast reconstruction, silicone-gel filled implants and saline-filled implants were approved for women of all ages; for breast augmentation, saline implants were approved for women 18 years of age and older; silicone implants were approved for women 22 years of age and older.<ref name = "FDA_QA">{{cite web | title = Breast Implant Questions and Answers | url = https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/BreastImplants/ucm063719.htm#4 | archive-url = https://web.archive.org/web/20101112214319/https://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/ImplantsandProsthetics/BreastImplants/ucm063719.htm | archive-date=2010-11-12 | publisher = U.S. Food and Drug Administration | date = October 2010 }}</ref> Because each breast implant device entails different medical risks, the minimum age of the patient for saline breast implants is different from the minimum age of the patient for silicone breast implants—because of the filler leakage and silent shell-rupture risks; thus, periodic ] screening examinations are the recommended post-operative, follow-up therapy for the patient.<ref name = "FDA_QA" /> In other countries, in Europe and Oceania, the national health ministries' breast implant policies do not endorse periodic MRI screening of asymptomatic patients, but suggest palpation proper—with or without an ] screening—to be sufficient post-operative therapy for most patients. | |||
| ==Repair or revision surgery== | |||
| Regardless of the type of implant, it is likely that women with implants will need to have one or more additional surgeries (reoperations) over the course of their lives. The most common reasons for reoperations are cosmetic concerns and capsular contracture.{{fact}} Reoperation rates are more frequent in breast reconstruction cases, particularly when patients have received XRT. | |||
| ==See also== | |||
| It appears that reoperation rates can be improved dramatically in aesthetic surgery by more carefully matching individual patients' soft-tissue characteristics to the type and size of implants used. Using appropriate device selection and proper technique, reoperation rates at up to seven years follow up have been reported as low as 3% <ref name="Tebbets2006">{{cite journal | author= Tebbets JB | title=Out points" criteria for breast implant removal without replacement and criteria to minimize reoperations following breast augmentation. | year=2006 | journal=Plast Reconstr Surg. | month= Oct | volume=114 | issue=5 | pages=1258-62 |id= PMID 15457046}} </ref> <ref name="Tebbets2">{{cite journal | author= Tebbets JB | title=Achieving a zero percent reoperation rate at 3 years in a 50-consecutive-case augmentation mammaplasty premarket approval study.. | year=2006 | journal=Plast Reconstr Surg. | month= Dec | volume=118 | issue=6 | pages=1453-7 |id= PMID 17051118}} </ref> | |||
| {{colbegin|colwidth=27em}} | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] | |||
| * ] (breast lift) | |||
| * ] | |||
| * ] | |||
| * ] (TUBA) | |||
| {{colend}} | |||
| ==References== | ==References== | ||
| {{Reflist}} | |||
| <div class="references-small" style="-moz-column-count:2; column-count:2;"> | |||
| <references/> | |||
| </div> | |||
| ==External links== | ==External links== | ||
| {{Commons category|Breast implants}} | |||
| * | |||
| * at Medscape | |||
| * | |||
| * | |||
| * | |||
| {{Breast procedures}} | |||
| * | |||
| * | |||
| * | |||
| * | |||
| * | |||
| * | |||
| * | |||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
| ] | |||
Latest revision as of 16:40, 8 November 2024
Prosthesis used to change the size, shape, and contour of a person's breast Medical intervention| Breast implant | |
|---|---|
| [edit on Wikidata] |
A breast implant is a prosthesis used to change the size, shape, and contour of a person's breast. In reconstructive plastic surgery, breast implants can be placed to restore a natural looking breast following a mastectomy, to correct congenital defects and deformities of the chest wall or, cosmetically, to enlarge the appearance of the breast through breast augmentation surgery.
Complications of implants may include breast pain, rashes, skin changes, infection, rupture, cosmetic changes to the breasts such as asymmetry and hardness, and a fluid collection around the breast.
A rare complication associated with textured surfaced implants and polyurethane foam-covered implants is a type of lymphoma (cancer of the immune system) known as breast implant-associated anaplastic large-cell lymphoma (BIA-ALCL).
There are four general types of breast implants, defined by their filler material: saline solution, silicone gel, structured and composite filler. The saline implant has an elastomer silicone shell filled with sterile saline solution during surgery; the silicone implant has an elastomer silicone shell pre-filled with viscous silicone gel; structured implants use nested elastomer silicone shells and two saline-filled lumen; and the alternative composition implants featured miscellaneous fillers, such as hydrogel, soy oil or polypropylene string.
In surgical practice, for the reconstruction of a breast, the tissue expander device is a temporary breast prosthesis used to form and establish an implant pocket for the future permanent breast implant. For the correction of male breast defects and deformities, the pectoral implant is the breast prosthesis used for the reconstruction and the aesthetic repair of a man's chest wall (see: gynecomastia and mastopexy).
Uses

A mammoplasty procedure for the placement of breast implant devices has three purposes:
- primary reconstruction: the replacement of breast tissues damaged by trauma (blunt, penetrating, blast), disease (breast cancer), and failed anatomic development (tuberous breast deformity).
- revision and reconstruction: to revise (correct) the outcome of a previous breast reconstruction surgery.
- primary augmentation: to aesthetically augment the size, form, and feel of the breasts.
The operating room time of post–mastectomy breast reconstruction, and of breast augmentation surgery is determined by the procedure employed, the type of incisions, the breast implant (type and materials), and the pectoral locale of the implant pocket.
Recent research has indicated that mammograms should not be done with any greater frequency than that used in normal procedure in patients undergoing breast surgery, including breast implant, augmentation, mastopexy, and breast reduction.
Psychology
Further information: Body dysmorphic disorder, Body image, and BeautyThe breast augmentation patient usually is a young woman whose personality profile indicates psychological distress about her personal appearance and her bodily self image, and a history of having endured criticism (teasing) about the aesthetics of her person. The studies Body Image Concerns of Breast Augmentation Patients (2003) and Body Dysmorphic Disorder and Cosmetic Surgery (2006) reported that the woman who underwent breast augmentation surgery also had undergone psychotherapy, suffered low self-esteem, presented frequent occurrences of psychological depression, had attempted suicide, and had body dysmorphia, a type of mental illness.
Post-operative patient surveys about mental health and quality-of-life, reported improved physical health, physical appearance, social life, self-confidence, self-esteem, and satisfactory sexual functioning. Furthermore, the women reported long-term satisfaction with their breast implant outcomes; some despite having medical complications that required surgical revision, either corrective or aesthetic. Likewise, in Denmark, 8% of breast augmentation patients had a pre-operative history of psychiatric hospitalization.
In 2008, the longitudinal study Excess Mortality from Suicide and other External Causes of Death Among Women with Cosmetic Breast Implants (2007), reported that women who sought breast implants are almost 3 times as likely to commit suicide as are women who have not sought breast implants. Compared to the standard suicide-rate for women of the general populace, the suicide-rate for women with augmented breasts remained constant until 10-years post-implantation, yet, it increased to 4.5 times greater at the 11-year mark, and so remained until the 19-year mark, when it increased to 6 times greater at 20-years post-implantation. Moreover, additional to the suicide-risk, women with breast implants also faced a trebled death-risk from alcoholism and the abuse of prescription and recreational drugs. Although seven studies have statistically connected a woman's breast augmentation to a greater suicide-rate, the research indicates that breast augmentation surgery does not increase the death rate; and that, in the first instance, it is the psychopathologically-inclined woman who is more likely to undergo a breast augmentation procedure.
The study Effect of Breast Augmentation Mammoplasty on Self-Esteem and Sexuality: A Quantitative Analysis (2007), reported that the women attributed their improved self image, self-esteem, and increased, satisfactory sexual functioning to having undergone breast augmentation; the cohort, aged 21–57 years, averaged post-operative self-esteem increases that ranged from 20.7 to 24.9 points on the 30-point Rosenberg self-esteem scale, which data supported the 78.6 per cent increase in the woman's libido, relative to her pre-operative level of libido. Therefore, before agreeing to any surgery, the plastic surgeon evaluates and considers the woman's mental health to determine if breast implants can positively affect her self-esteem and sexual functioning.
Complications
The plastic surgical emplacement of breast implant devices, either for breast reconstruction or for aesthetic purpose, presents the same health risks common to surgery, such as adverse reaction to anesthesia, hematoma (post-operative bleeding), late hematoma (post-operative bleeding after 6 months or more), seroma (fluid accumulation), incision-site breakdown (wound infection). Complications specific to breast augmentation include breast pain, altered sensation, impeded breast-feeding function, visible wrinkling, asymmetry, thinning of the breast tissue, and symmastia, the "bread loafing" of the bust that interrupts the natural plane between the breasts. Specific treatments for the complications of indwelling breast implants—capsular contracture and capsular rupture—are periodic MRI monitoring and physical examinations. Furthermore, complications and re-operations related to the implantation surgery, and to tissue expanders (implant place-holders during surgery) can cause unfavorable scarring in approximately 6–7 percent of the patients. Statistically, 20 percent of women who underwent cosmetic implantation, and 50 percent of women who underwent breast reconstruction implantation, required their explantation (surgical removal) at the 10-year mark.
Safety
In the 1990s, several reports reviewed the few studies evaluating any increased risk of systemic and auto-immune diseases among women with breast implants. The conclusion at that time was that there was no evidence establishing a causal connection between the implantation of silicone breast implants and either type of disease. However, the Institute of Medicine report pointed out that these earlier studies included too few women to conclusively evaluate the impact on these rare diseases. In addition, many of the studies included women who had breast implants for just a few months, which would be too early to develop a diagnosed autoimmune disease. In recent years, large epidemiological studies have reported clinically and statistically significant increases in some of these diseases. A study by Watad and colleagues that was published in 2018 compared and examined the medical records of more than 24,000 women with breast implants to more than 98,000 "matched controls" who did not have breast implants but shared very similar demographic traits. The study found a statistically significant 22% overall increase in diagnosed autoimmune or rheumatic disorders. The greatest increases in diagnoses for women with breast implants was for Sjögren's syndrome, Multiple Sclerosis (MS), and sarcoidosis, each of which were 58%-98% higher in women with breast implants. That analysis was based on Israeli women with breast implants as confirmed by medical records, and the analyses of diseases were based on diagnoses made after the women got breast implants that were included in medical records during up to 20 years of follow-up.
A published study of U.S. women with similar results was published in 2019 by Coroneos and his colleagues at MD Anderson Medical Center. The data were based on two studies with a combined total of almost 100,000 women with breast implants, but many dropped out of the study within a few years of their breast implant surgery. However, of the women in the study for at least two years, the researchers reported an 800% increase in Sjögren syndrome, 700% increase in scleroderma, and almost 600% increase in rheumatoid arthritis among women with breast implants compared to the general population of women of the same age and demographics.
Recent research on women who reported autoimmune and other system symptoms but were not diagnosed with an autoimmune disease evaluated whether the women's symptoms changed after their implants were removed. A 2020 study on the effectiveness of explant surgery on women with breast implant illness found that nearly all of 750 women who underwent explant surgery reported a significant improvement in their health within a month after their surgery. Researchers focused on the following symptoms: hair loss, memory loss, dry eyes and/or blurred vision, numbness or tingling in the extremities, chronic fatigue, joint pain, rashes, breast pain, food intolerance, flu-like symptoms, and difficulty breathing. The same authors also published a study on the impact of breast implant removal on breathing difficulties and found a statistically significant improvement in well-established objective measures of pulmonary function following explant surgery.
| Year | Country | Systemic Review Group | Conclusions |
|---|---|---|---|
| 1991–93 | United Kingdom | Independent Expert Advisory Group (IEAG) | There was no evidence of an increased risk of connective-tissue disease in patients who had undergone silicone-gel breast implant emplacement, and no cause for changing either breast implant practice or policy in the U.K. |
| 1996 | United States | U.S. Institute of Medicine (IOM) | There was "insufficient evidence for an association of silicone gel- or saline-filled breast implants with defined connective tissue disease." |
| 1996 | France | Agence Nationale pour le Developpement de l'Evaluation Medicale (ANDEM) | French original: "Nous n'avons pas observé de connectivité ni d'autre pathologie auto-immune susceptible d'être directement ou indirectement induite par la présence d'un implant mammaire en particulier en gel de silicone...."
English translation: "We did not observe connective tissue diseases to be directly or indirectly associated by the presence of a breast implant, in particular one of silicone gel...." |
| 1997 | Australia | Therapeutic Devices Evaluation Committee (TDEC) | The "current, high-quality literature suggest that there is no association between breast implants and connective tissue disease-like syndromes (atypical connective tissue diseases)." |
| 1998 | Germany | Federal Institute for Medicine and Medical Products | Reported that "silicone breast implants neither cause auto-immune diseases nor rheumatic diseases and have no disadvantageous effects on pregnancy, breast-feeding capability, or the health of children who are breast-fed. There is no scientific evidence for the existence of silicone allergy, silicone poisoning, atypical silicone diseases or a new silicone disease." |
| 2000 | United States | Federal court-ordered review | "No evidence of an association between... silicone-gel-filled breast implants specifically, and any of the individual CTDs, all definite CTDs combined, or other auto-immune or rheumatic conditions." |
| 2000 | European Union | European Committee on Quality Assurance & Medical Devices in Plastic Surgery (EQUAM) | "Additional medical studies have not demonstrated any association between silicone-gel filled breast implants and traditional auto-immune or connective tissue diseases, cancer, nor any other malignant disease. . . . EQUAM continues to believe that there is no scientific evidence that silicone allergy, silicone intoxication, atypical disease or a 'new silicone disease' exists." |
| 2001 | United Kingdom | UK Independent Review Group (UK-IRG) | "There is no evidence of an association with an abnormal immune response or typical or atypical connective tissue diseases or syndromes." |
| 2001 | United States | Court-appointed National Science Panel review | The panel evaluated established and undifferentiated connective tissue diseases (CTD), and concluded there was no causal evidence between breast implants and these CTDs. |
| 2003 | Spain | Science and Technology Options Assessment (STOA) | The STOA report to the European Parliament Petitions Committee reported that the current scientific evidence demonstrates no solid, causal evidence linking SBI to severe diseases, e.g. breast cancer, connective tissue diseases. |
| 2009 | European Union | International Committee for Quality Assurance, Medical Technologies & Devices in Plastic Surgery panel (IQUAM) | The consensus statement of the Transatlantic Innovations conference (April 2009) indicated that additional medical studies demonstrated no association between silicone gel-filled breast implants and carcinoma, or any metabolic, immune, or allergic disorder. |
Implant rupture

Because a breast implant is a Class III medical device of limited product-life, the principal rupture-rate factors are its age and design; nonetheless, a breast implant device can retain its mechanical integrity for decades in a woman's body. When a saline breast implant ruptures, leaks, and empties, it quickly deflates, and thus can be readily explanted (surgically removed). In some cases, saline implant rupture can result in an infection due to bacteria or mold that had been within the implant, though this is uncommon. The follow-up report, Natrelle Saline-filled Breast Implants: a Prospective 10-year Study (2009) indicated rupture-deflation rates of 3–5 per cent at 3-years post-implantation, and 7–10 per cent rupture-deflation rates at 10-years post-implantation. In a study of his 4761 augmentation mammaplasty patients, Eisenberg reported that overfilling saline breast implants 10-13% significantly reduced the rupture-deflation rate to 1.83% at 8-years post-implantation.

When a silicone breast implant ruptures it usually does not deflate, yet the filler gel does leak from it, which can migrate to the implant pocket; therefore, an intracapsular rupture (in-capsule leak) can become an extracapsular rupture (out-of-capsule leak), and each occurrence is resolved by explantation. Although the leaked silicone filler-gel can migrate from the chest tissues to elsewhere in the woman's body, most clinical complications are limited to the breast and armpit areas, usually manifested as granulomas (inflammatory nodules) and axillary lymphadenopathy (enlarged lymph glands in the armpit area).
The suspected mechanisms of breast implant rupture are:
- damage during implantation
- damage during (other) surgical procedures
- chemical degradation of the breast implant shell
- trauma (blunt trauma, penetrating trauma, blast trauma)
- mechanical pressure of traditional mammographic breast examination
Silicone implant rupture can be evaluated using magnetic resonance imaging; from the long-term MRI data for single-lumen breast implants, the European literature about second generation silicone-gel breast implants (1970s design), reported silent device-rupture rates of 8–15 per cent at 10-years post-implantation (15–30% of the patients).
The study Safety and Effectiveness of Mentor's MemoryGel Implants at 6 Years (2009), which was a branch study of the U.S. FDA's core clinical trials for primary breast augmentation surgery patients, reported low device-rupture rates of 1.1 per cent at 6-years post-implantation. The first series of MRI evaluations of the silicone breast implants with thick filler-gel reported a device-rupture rate of 1 percent, or less, at the median 6-year device-age. Statistically, the manual examination (palpation) of the woman is inadequate for accurately evaluating if a breast implant has ruptured. The study, The Diagnosis of Silicone Breast implant Rupture: Clinical Findings Compared with Findings at Magnetic Resonance Imaging (2005), reported that, in asymptomatic patients, only 30 per cent of the ruptured breast implants are accurately palpated and detected by an experienced plastic surgeon, whereas MRI examinations accurately detected 86 per cent of breast implant ruptures. Therefore, the U.S. FDA recommended scheduled MRI examinations, as silent-rupture screenings, beginning at the 3-year-mark post-implantation, and then every two years, thereafter. Nonetheless, beyond the U.S., the medical establishments of other nations have not endorsed routine MRI screening, and, in its stead, proposed that such a radiologic examination be reserved for two purposes: (i) for the woman with a suspected breast implant rupture; and (ii) for the confirmation of mammographic and ultrasonic studies that indicate the presence of a ruptured breast implant.
Furthermore, The Effect of Study design Biases on the Diagnostic Accuracy of Magnetic Resonance Imaging for Detecting Silicone Breast Implant Ruptures: a Meta-analysis (2011) reported that the breast-screening MRIs of asymptomatic women might overestimate the incidence of breast implant rupture. In the event, the U.S. Food and Drug Administration emphasised that "breast implants are not lifetime devices. The longer a woman has silicone gel-filled breast implants, the more likely she is to experience complications."
Capsular contracture
Main article: Capsular contractureThe human body's immune response to a surgically installed foreign object—breast implant, cardiac pacemaker, orthopedic prosthesis—is to encapsulate it with scar tissue capsules of tightly woven collagen fibers, in order to maintain the integrity of the body by isolating the foreign object, and so tolerate its presence. Capsular contracture—which should be distinguished from normal capsular tissue—occurs when the collagen-fiber capsule thickens and compresses the breast implant; it is a painful complication that might distort either the breast implant, or the breast, or both. Capsular contracture is diagnosed through a visual and physical examiniation according to level of increasing severity based on the Baker Grade scale: Baker Grade I, Baker Grade II, Baker Grade III, and Baker Grade IV.

The cause of capsular contracture is unknown, but the common incidence factors include bacterial contamination, device-shell rupture, filler leakage, and hematoma. The surgical implantation procedures that have reduced the incidence of capsular contracture include submuscular emplacement, the use of breast implants with a textured surface (polyurethane-coated); limited pre-operative handling of the implants, limited contact with the chest skin of the implant pocket before the emplacement of the breast implant, and irrigation of the recipient site with triple-antibiotic solutions.
The correction of capsular contracture might require an open capsulotomy (surgical release) of the collagen-fiber capsule, or the removal, and possible replacement, of the breast implant. Furthermore, in treating capsular contracture, the closed capsulotomy (disruption via external manipulation) once was a common maneuver for treating hard capsules, but now is a discouraged technique, because it can rupture the breast implant. Non-surgical treatments for collagen-fiber capsules include massage, external ultrasonic therapy, leukotriene pathway inhibitors such as zafirlukast (Accolate) or montelukast (Singulair), and pulsed electromagnetic field therapy (PEMFT).
Repair and revision surgeries
When the patient is unsatisfied with the outcome of the augmentation mammoplasty; or when technical or medical complications occur; or because of the breast implants' limited product life, it is likely she might require replacing the breast implants. Common revision surgery indications include major and minor medical complications, capsular contracture, shell rupture, and device deflation. Revision incidence rates were greater for breast reconstruction patients, because of the post-mastectomy changes to the soft-tissues and to the skin envelope of the breast, and to the anatomical borders of the breast, especially in women who received adjuvant external radiation therapy. Moreover, besides breast reconstruction, breast cancer patients usually undergo revision surgery of the nipple-areola complex (NAC), and symmetry procedures upon the opposite breast, to create a bust of natural appearance, size, form, and feel. Carefully matching the type and size of the breast implants to the patient's pectoral soft-tissue characteristics reduces the incidence of revision surgery. Appropriate tissue matching, implant selection, and proper implantation technique, the re-operation rate was 3 percent at the 7-year-mark, compared with the re-operation rate of 20 per cent at the 3-year-mark, as reported by the U.S. Food and Drug Administration.
Systemic disease

In the 1990s, the national health ministries of the listed countries reviewed the pertinent studies for causal links among silicone-gel breast implants and systemic and diagnosed autoimmune diseases and breast cancer. A study by researchers at the U.S. National Institute of Health reported an increase in four types of cancer among women with breast implants compared to other plastic surgery patients of the same age and similar demographic traits and health habits. This increase in cancer was attenuated but did not disappear when the researchers followed the women for 5 more years, with a 43% increase in brain cancer and a 63% increase in respiratory cancer compared to other plastic surgery patients. A study by Watad et al. evaluated the medical records of more than 24,000 women with breast implants compared to more than 98,000 women with the same demographic traits who did not have breast implants. The researchers found a statistically significant 22 percent increase in several diagnosed diseases, increasing to more than 60% for Sjogren's syndrome, multiple sclerosis, and sarcoidosis After investigating this issue, in 2021 the U.S. FDA revised its "black box warnings" on breast implants to acknowledge the association between breast implants and systemic autoimmune, rheumatologic, and neurological symptoms to state: "Patients receiving breast implants have reported a variety of systemic symptoms, such as joint pain, muscle aches, confusion, chronic fatigue, autoimmune diseases, and others. Individual patient risk for developing these symptoms has not been well established. Some patients report complete resolution of symptoms when the implants are removed without replacement".
A 2021 study by Cleveland Clinic physicians investigated the outcome of breast implant removal on women with breast implants who had reported difficulty breathing or tightness in their chest; this is one of the symptoms associated with breast implant illness. The study examined breast implant patients who reported problems breathing, comparing their scores on 6 pulmonary function tests before and after their implants and scar capsules were removed. The researchers reported that 74% of the patients reported significant improvements on at least 3 of the pulmonary function tests. In self-reports, all study participants reported that their breathing improved after their implant surgery.
Platinum toxicity
Platinum is a catalyst used in the making of silicone implant polymer shells and other silicone devices used in medicine. The literature indicates that small amounts of platinum leaches (leaks) from these implants and is present in the surrounding tissue. The FDA reviewed the available studies from the medical literature on platinum and breast implants in 2002 and concluded there was little evidence suggesting toxicity from platinum in implant patients. The FDA revisited this study and additional literature several years later, reaffirming prior conclusions that platinum catalysts used in implants is likely not ionized and therefore would not represent a risk to women.
Anaplastic large-cell lymphoma
Main article: Anaplastic large-cell lymphoma § Breast implant-associated anaplastic large-cell lymphomaThe FDA has identified that breast implants may be associated with a rare form of cancer called anaplastic large-cell lymphoma (ALCL), which some experts believe is believed to be associated with chronic bacterial inflammation. Similar ALCL phenomena have been seen with other types of medical implants including vascular access ports, orthopedic hip implants, and jaw (TMJ) implants. The causal association between breast implants and ALCL was conclusively established in December 2013, when researchers at MD Anderson Cancer Center published a study of 60 women with breast implants who were diagnosed with ALCL in the breast. In 2015, plastic surgeons published an article reviewing 37 articles in the literature on 79 patients and collected another 94 unreported cases, resulting in 173 women with breast implants who had developed ALCL of the breast. They concluded that "Breast implant-associated ALCL is a novel manifestation of site- and material-specific lymphoma originating in a specific scar location, presenting a wide array of diverse characteristics and suggesting a multifactorial cause." They stated that "There was no preference for saline or silicone fill or for cosmetic or reconstructive indications." Where implant history was known, the patient had received at least one textured-surface device. In 2016, the World Health Organization (WHO) officially recognized BIA-ALCL.
As of April 2022, the FDA has received 1,130 global medical device reports (MDRs) of BIA-ALCL, including 59 deaths. Since ALCL was thought to be diagnosed in only 1 woman in half a million, 60 women was a much higher number than would be expected. The researchers pointed out that BIA-ALCL could be fatal. If women with implants present with delayed swelling or fluid collection, cytologic studies and a test for the marker CD30 are suggested. The American Society of Plastic Surgery (ASPS) states, "CD30 is the main diagnostic test that must be performed on the seroma fluid as routine pathology or H&E staining can frequently miss the diagnosis." Diagnosis and treatment of breast implant-associated ALCL now follows standardized guidelines established by the National Comprehensive Cancer Network.
The current lifetime risk of BIA-ALCL in the U.S. is unknown, but estimates have ranged between one in 70,000 and one in 500,000 women with breast implants, according to the MD Anderson Cancer Center. Countries with breast implant registries have the best data on the risks of BIA-ALCL. For example, as of October 2020, the Therapeutic Goods Administration of Australia and New Zealand reported a "1:3,345 risk with Allergan Biocell and a 1:86,029 risk with Mentor Siltex." AIn the U.S, estimates of the risk of BIA-ALCL in textured implants ranges from 1.79 per 1,000 (1 woman with BIA-ALCL per 559 implants) to 2.82 per 1,000 (1 woman per 355 implants). As of April 2022, the FDA reported 1,130 medical device reports (MDRs) of BIA-ALCL. Of those MDRs, 59 of the women died. FDA states that 798 of the 1,130 MDRs for BIA-ALCL involved textured breast implants, 37 MDRs involved smooth-surfaced implants, and 295 MDRs did not specify whether the implants were textured or smooth. In some cases, the women who developed BIA-ALCL after smooth breast implants had textured expanders prior to their implants. Most patients had BIA-ALCL affect only one breast with only 8 patients diagnosed with bilateral BIA-ALCL. The most common reported symptom of BIA-ALCL according to the FDA was seroma, followed by breast swelling and pain, capsular contracture, and peri-implant mass or lump. Furthermore, 84 percent of the reported implants were made by Allergan, the largest breast implant manufacturer at the time.
A study conducted at Memorial Sloan Kettering cancer center of women undergoing prophylactic mastectomies indicated that 1 in 355 women were subsequently diagnosed with BIA-ALCL. Almost all those women had Allergan biocell implants and expanders. These reports strongly suggest that BIA-ALCL develops primarily in patients with textured implants or expanders. In all cases, however, many researchers suspect that BIA-ALCL is an under-recognized, misdiagnosed, and under-reported complication of breast implants.
The ASPS and the Plastic Surgery Foundation (PSF) have partnered with the FDA to study this condition and in doing so created the Patient Registry and Outcomes for Breast Implants and Anaplastic Large Cell Lymphoma Etiology and Epidemiology (PROFILE). The United States FDA strongly encourages all physicians to report cases to PROFILE in an effort to better understand the role of breast implants in ALCL and the management of this disease.
Other lymphomas and squamous-cell carcinoma
In September 2022, the FDA announced new information regarding other types of cancers related to breast implants, based on medical device reports (MDRs) of cases of patients with squamous-cell carcinoma or various lymphomas found in the scar tissue around breast implants. The agency reported 10 MDRs about squamous-sell carcinoma found in the capsule scar tissue and 12 MDRs about various lymphomas also in the scar tissue around the breast implants. These new cases do not overlap with cases of BIA-ALCL. SCC and other lymphomas have been found in smooth-surface and textured implants as well as silicone-gel-filled and saline-filled implants. Patients who reported symptoms specified swelling, pain, lumps, and changes to their skin. As of 2022, neither the incidence rate nor prevalence is known.
Surgical procedures
Incision types
Breast implant emplacement is performed with five types of surgical incisions:
- Inframammary: an incision made to the inframammary fold (natural crease under the breast), which affords maximal access for precise dissection of the tissues and emplacement of the breast implants. It is the preferred surgical technique for emplacing silicone-gel implants, because it better exposes the breast tissue–pectoralis muscle interface; yet, IMF implantation can produce thicker, slightly more visible surgical scars.
- Periareolar: a border-line incision along the periphery of the areola, which provides an optimal approach when adjustments to the IMF position are required, or when a mastopexy (breast lift) is included to the primary mammoplasty procedure. In periareolar emplacement, the incision is around the medial-half (inferior half) of the areola's circumference. Silicone gel implants can be difficult to emplace via periareolar incision, because of the short, five-centimetre length (~ 5.0 cm) of the required access-incision. Aesthetically, because the scars are at the areola's border (periphery), they usually are less visible than the IMF-incision scars of women with light-pigment areolae; when compared to cutaneous-incision scars, the modified epithelia of the areolae are less prone to (raised) hypertrophic scars.
- Transaxillary: an incision made to the axilla (armpit), from which the dissection tunnels medially, to emplace the implants, either bluntly or with an endoscope (illuminated video microcamera), without producing visible scars on the breast proper; yet, it is likelier to produce inferior asymmetry of the implant-device position. Therefore, surgical revision of transaxillary emplaced breast implants usually requires either an IMF incision or a periareolar incision.
- Transumbilical: a trans-umbilical breast augmentation (TUBA) is a less common implant-device emplacement technique wherein the incision is at the umbilicus (navel), and the dissection tunnels superiorly, up towards the bust. The TUBA approach allows emplacing the breast implants without producing visible scars upon the breast proper; but makes appropriate dissection and device-emplacement more technically difficult. A TUBA procedure is performed bluntly—without the endoscope's visual assistance—and is not appropriate for emplacing (pre-filled) silicone-gel implants, because of the great potential for damaging the elastomer silicone shell of the breast implant during its manual insertion through the short (~2.0 cm) incision at the navel, and because pre-filled silicone gel implants are incompressible, and cannot be inserted through so small an incision.
- Transabdominal: as in the TUBA procedure, in the transabdominoplasty breast augmentation (TABA), the breast implants are tunneled superiorly from the abdominal incision into bluntly dissected implant pockets, whilst the patient simultaneously undergoes an abdominoplasty.
Implant pocket placement
Implant placement comparison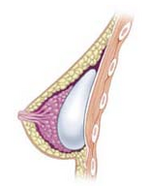 Subglandular implant
Subglandular implant Subpectoral implant
Subpectoral implant Submuscular implant
Submuscular implant
The five surgical approaches to emplacing a breast implant to the implant pocket are often described in anatomical relation to the pectoralis major muscle.
- Subglandular: the breast implant is emplaced to the retromammary space, between the breast tissue (the mammary gland) and the pectoralis major muscle (major muscle of the chest), which most approximates the plane of normal breast tissue, and affords the most aesthetic results. Yet, in women with thin pectoral soft-tissue, the subglandular position is likelier to show the ripples and wrinkles of the underlying implant. Moreover, the capsular contracture incidence rate is slightly greater with subglandular implantation.
- Subfascial: the breast implant is emplaced beneath the fascia of the pectoralis major muscle; the subfascial position is a variant of the subglandular position for the breast implant. The technical advantages of the subfascial implant-pocket technique are debated; proponent surgeons report that the layer of fascial tissue provides greater implant coverage and better sustains its position.
- Subpectoral (dual plane): the breast implant is emplaced beneath the pectoralis major muscle, after the surgeon releases the inferior muscular attachments, with or without partial dissection of the subglandular plane. Resultantly, the upper pole of the implant is partially beneath the pectoralis major muscle, while the lower pole of the implant is in the subglandular plane. This implantation technique achieves maximal coverage of the upper pole of the implant, whilst allowing the expansion of the implant's lower pole; however, "animation deformity", the movement of the implants in the subpectoral plane can be excessive for some patients.
- Submuscular: the breast implant is emplaced beneath the pectoralis major muscle, without releasing the inferior origin of the muscle proper. Total muscular coverage of the implant can be achieved by releasing the lateral muscles of the chest wall—either the serratus muscle or the pectoralis minor muscle, or both—and suturing it, or them, to the pectoralis major muscle. In breast reconstruction surgery, the submuscular implantation approach effects maximal coverage of the breast implants. This technique is rarely used in cosmetic surgery due to high risk of animation deformities.
- Prepectoral or subcutaneous: in a breast reconstruction following a skin-sparing or skin- and nipple-sparing mastectomy, the implant is placed above the pectoralis major muscle without dissecting it so that the implant fills directly the volume of the mammary gland that has been removed. To avoid the issue of capsular contracture, the implant is often covered frontally or completely with a mesh in biomaterial, either biological or synthetic.
Post-surgical recovery
The surgical scars of a breast augmentation mammoplasty develop approximately at 6-weeks post-operative, and fade within months. Depending upon the daily-life physical activities required of the woman, the breast augmentation patient usually resumes her normal life at 1-week post-operative. Moreover, women whose breast implants were emplaced beneath the chest muscles (submuscular placement) usually have a longer, slightly more painful convalescence, because of the healing of the incisions to the chest muscles. Usually, she does not exercise or engage in strenuous physical activities for approximately 6 weeks. During the initial post-operative recovery, the woman is encouraged to regularly exercise (flex and move) her arm to alleviate pain and discomfort; if required, analgesic indwelling medication catheters can alleviate pain Moreover, significantly improved patient recovery has resulted from refined breast-device implantation techniques (submuscular, subglandular) that allow 95 per cent of women to resume their normal lives at 24-hours post-procedure, without bandages, fluid drains, pain pumps, catheters, medical support brassières, or narcotic pain medication.
Types


Today, there are three types of breast implants commonly used for mammaplasty, breast reconstruction, and breast augmentation procedures:
- saline implant filled with sterile saline solution.
- silicone implant filled with viscous silicone gel.
- structured implants using nested elastomer silicone shells and two saline filled lumen.
A fourth type of implant, composite (or alternative-composite) implants, have largely been discontinued. These types featured fillers such as soy oil and polypropylene string. Other discontinued materials include ox cartilage, Terylene wool, ground rubber, silastic rubber, and teflon-silicone prostheses.
Saline implants
The saline breast implant—filled with saline solution (biological-concentration salt water 0.90% w/v of NaCl, ca. 300 mOsm/L.)—was first manufactured by the Laboratoires Arion company, in France, and was introduced for use as a prosthetic medical device in 1964. The contemporary models of saline breast implant are manufactured with thicker, room-temperature vulcanized (RTV) shells made of a silicone elastomer. The study In vitro Deflation of Pre-filled Saline Breast Implants (2006) reported that the rates of deflation (filler leakage) of the pre-filled saline breast implant made it a second-choice prosthesis for corrective breast surgery. Nonetheless, in the 1990s, the saline breast implant was the prosthesis most common device used for breast augmentation surgery in the United States, because of the U.S. FDA's restriction against the implantation of silicone-filled breast implants outside of clinical studies. Saline breast implants have enjoyed little popularity in the rest of the world, possessing negligible market share.
The technical goal of saline-implant technology was a physically less invasive surgical technique for emplacing an empty breast implant device through a smaller surgical incision. In surgical praxis, after having emplaced the empty breast implants to the implant pockets, the plastic surgeon then filled each device with saline solution, and, because the required insertion-incisions are short and small, the resultant incision-scars will be smaller and shorter than the surgical scars usual to the long incisions required for inserting pre-filled, silicone-gel implants.
When compared to the results achieved with a silicone-gel breast implant, the saline implant can yield acceptable results, of increased breast-size, smoother hemisphere-contour, and realistic texture; yet, it is likelier to cause cosmetic problems, such as the rippling and the wrinkling of the breast-envelope skin, accelerated lower breast pole stretch, and technical problems, such as the presence of the implant being noticeable to the eye and to the touch. The occurrence of such cosmetic problems is likelier in the case of the woman with very little breast tissue, and in the case of the woman who requires post-mastectomy breast reconstruction; thus, the silicone-gel implant is the technically superior prosthetic device for breast augmentation, and for breast reconstruction. In the case of the woman with much breast tissue, for whom sub-muscular emplacement is the recommended surgical approach, saline breast implants can produce an aesthetic result much like that afforded by silicone breast implants. Ultrasound examination and outcome studies have revealed that saline and silicone breast implants look and feel similar.
Silicone gel implants
As a medical device technology, there are five generations of silicone breast implant, each defined by common model-manufacturing techniques.
The modern prosthetic breast was invented in 1961 by the American plastic surgeons Thomas Cronin and Frank Gerow, and manufactured by the Dow Corning Corporation; in due course, the first augmentation mammoplasty was performed in 1962.
First generation
The Cronin–Gerow Implant, prosthesis model 1963, was a silicone rubber envelope-sac, shaped like a teardrop, which was filled with viscous silicone-gel. To reduce the rotation of the emplaced breast implant upon the chest wall, the model 1963 prosthesis was affixed to the implant pocket with a fastener-patch, made of Dacron material (Polyethylene terephthalate), which was attached to the rear of the breast implant shell.
Second generation
In the 1970s, manufacturers presented the second generation of breast implant prostheses that featured functional developments and aesthetic improvements to the technology:
- the first technological developments were a thinner-gauge device-shell, and a filler gel of low-cohesion silicone, which improved the functionality and the verisimilitude (size, appearance, and texture) of the silicone-gel breast implant. Yet, in clinical practice, second-generation breast implants proved fragile and saw greater instances of shell rupture, and of filler leakage ("silicone-gel bleed") through the intact device shell. The consequent, increased incidence-rates of medical complications (e.g. capsular contracture) precipitated faulty-product, class action-lawsuits, by the U.S. government, against the Dow Corning Corporation, and other manufacturers of breast prostheses.
- the second technological development was a polyurethane foam coating for the shell of the breast implant; the coating reduced the incidence of capsular contracture, by causing an inflammatory reaction that impeded the formation of a capsule of fibrous collagen tissue around the breast implant. Nevertheless, despite that prophylactic measure, the medical use of polyurethane-coated breast implants was briefly discontinued, because of the potential health-risk posed by 2,4-toluenediamine (TDA), a carcinogenic by-product of the chemical breakdown of the polyurethane foam coating of the breast implant proven to cause liver and skin cancers in animal-model studies.
- After reviewing the medical data, the U.S. Food and Drug Administration concluded that TDA-induced breast cancer was an infinitesimal health-risk to women with breast implants, and did not justify legally requiring physicians to explain the matter to their patients. In the event, polyurethane-coated breast implants remain in plastic surgery practice in Europe and in South America; and no manufacturer has sought FDA approval for medical sales of such breast implants in the U.S.
- the third technological development was the double lumen breast implant device, a double-cavity prosthesis composed of a silicone breast implant contained within a saline breast implant. The two-fold, technical goal was: (i) the cosmetic benefits of silicone-gel (the inner lumen) enclosed in saline solution (the outer lumen); (ii) a breast implant device the volume of which is post-operatively adjustable. Nevertheless, the more complex design of the double-lumen breast implant suffered a device-failure rate greater than that of single-lumen breast implants. The contemporary versions of second-generation breast implant devices (presented in 1984) are the "Becker Expandable" models of breast implant, which are primarily used for breast reconstruction.
Third and fourth generations
In the 1980s, the models of the third and of the fourth generations of breast implant devices were sequential advances in manufacturing technology, such as elastomer-coated shells that decreased gel-bleed (filler leakage), and a thicker (increased-cohesion) filler gel. Sociologically, the manufacturers of prosthetic breasts then designed and made anatomic models (natural breast) and shaped models (round, tapered) that realistically corresponded with the breast- and body- types of women. The tapered models of breast implant have a uniformly textured surface, which reduces the rotation of the prosthesis within the implant pocket; the round models of breast implant are available in smooth-surface- and textured-surface- types.
Fifth generation
Since the mid-1990s, the fifth generation of silicone-gel breast implant is made of a high-strength, highly cohesive silicone gel that mostly eliminates the occurrences of filler leakage ("silicone gel bleed") and of the migration of the silicone filler from the implant pocket to elsewhere in the woman's body. These implants are commonly referred to as "gummy bear breast implants" for their firm, pliant consistency, which is similar to gummy candies. The studies Experience with Anatomical Soft Cohesive Silicone gel Prosthesis in Cosmetic and Reconstructive Breast Implant Surgery (2004) and Cohesive Silicone gel Breast Implants in Aesthetic and Reconstructive Breast Surgery (2005) reported low incidence-rates of capsular contracture and of device-shell rupture; and greater rates of improved medical-safety and technical-efficacy than that of early generation breast implant devices.
Breastfeeding
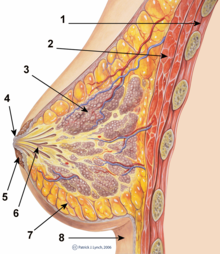
The presence of breast implants currently presents no contraindication to breast feeding, and no evidence to support that the practice may present health issues to a breastfeeding infant is recognized by the USFDA.
Women with breast implants may have functional breast-feeding difficulties; mammoplasty procedures that feature periareolar incisions are especially likely to cause breastfeeding difficulties. Surgery may also damage the lactiferous ducts and the nerves in the nipple-areola area.
Functional breastfeeding difficulties arise if the surgeon cut the milk ducts or the major nerves innervating the breast, or if the milk glands were otherwise damaged. Milk duct and nerve damage are more common if the incisions cut tissue near the nipple. The milk glands are most likely to be affected by subglandular implants (under the gland), and by large-sized breast implants, which pinch the lactiferous ducts and impede milk flow. Small-sized breast implants, and submuscular implantation, cause fewer breast-function problems; however, it is impossible to predict whether a woman who undergoes breast augmentation will be able to successfully breast feed since some women are able to breast-feed after periareolar incisions and subglandular placement and some are not able to after augmentation using submuscular and other types of surgical incisions.
Mammography

The presence of radiologically opaque breast implants (either saline or silicone) might interfere with the radiographic sensitivity of the mammograph, that is, the image might not show any tumor(s) present. In this case, an Eklund view mammogram is required to ascertain either the presence or the absence of a cancerous tumor, wherein the breast implant is manually displaced against the chest wall and the breast is pulled forward, so that the mammograph can visualize a greater volume of the internal tissues; nonetheless, approximately one-third of the breast tissue remains inadequately visualized, resulting in an increased incidence of mammograms with false-negative results.
The breast cancer studies Cancer in the Augmented Breast: Diagnosis and Prognosis (1993) and Breast Cancer after Augmentation Mammoplasty (2001) of women with breast implant prostheses reported no significant differences in disease-stage at the time of the diagnosis of cancer; prognoses are similar in both groups of women, with augmented patients at a lower risk for subsequent cancer recurrence or death. Conversely, the use of implants for breast reconstruction after breast cancer mastectomy appears to have no negative effect upon the incidence of cancer-related death. That patients with breast implants are more often diagnosed with palpable—but not larger—tumors indicates that equal-sized tumors might be more readily palpated in augmented patients, which might compensate for the impaired mammogram images. The ready palpability of the breast-cancer tumor(s) is consequent to breast tissue thinning by compression, innately in smaller breasts a priori (because they have lesser tissue volumes), and that the implant serves as a radio-opaque base against which a cancerous tumor can be differentiated.

The breast implant has no clinical bearing upon lumpectomy breast-conservation surgery for women who developed breast cancer after the implantation procedure, nor does the breast implant interfere with external beam radiation treatments (XRT); moreover, the post-treatment incidence of breast-tissue fibrosis is common, and thus a consequent increased rate of capsular contracture. There is tentative evidence that women who have had breast augmentation, have worse breast cancer prognosis. The use of implants for breast reconstruction after breast cancer mastectomy appears to have no negative effect upon cancer-related death.
There have been multiple reported cases of other adverse effects of mammography of women with breast implants; ruptures resulting from pressure exerted on the breast implant make up a majority of these cases. Compression may also lead to pain or exacerbate already existing pain in the breasts.
History

19th century
Since the late nineteenth century, breast implants have been used to surgically augment the size (volume), modify the shape (contour), and enhance the feel (tact) of a woman's breasts. In 1895, surgeon Vincenz Czerny effected the earliest breast implant emplacement when he used the patient's autologous adipose tissue, harvested from a benign lumbar lipoma, to repair the asymmetry of the breast from which he had removed a tumor. In 1889, surgeon Robert Gersuny experimented with paraffin injections, with disastrous results arising from the breakup of the paraffin into smaller bodies following the procedure.
20th century
From the first half of the twentieth century, physicians used other substances as breast implant fillers—ivory, glass balls, ground rubber, ox cartilage, Terylene wool, gutta-percha, Dicora, polyethylene chips, Ivalon (polyvinyl alcohol—formaldehyde polymer sponge), a polyethylene sac with Ivalon, polyether foam sponge (Etheron), polyethylene tape (Polystan) strips wound into a ball, polyester (polyurethane foam sponge) Silastic rubber, and teflon-silicone prostheses.
In the mid-twentieth century, Morton I. Berson, in 1945, and Jacques Maliniac, in 1950, each performed flap-based breast augmentations by rotating the patient's chest wall tissue into the breast to increase its volume. Furthermore, throughout the 1950s and the 1960s, plastic surgeons used synthetic fillers—including silicone injections received by some 50,000 women, from which developed silicone granulomas and breast hardening that required treatment by mastectomy. In 1961, the American plastic surgeons Thomas Cronin and Frank Gerow, and the Dow Corning Corporation, developed the first silicone breast prosthesis, filled with silicone gel; in due course, the first augmentation mammoplasty was performed in 1962 using the Cronin–Gerow Implant, prosthesis model 1963. In 1964, the French company Laboratoires Arion developed and manufactured the saline breast implant, filled with saline solution, and then introduced for use as a medical device in 1964.
FDA approval
In 1988, twenty-six years after the 1962 introduction of breast implants filled with silicone gel, the U.S. Food and Drug Administration (FDA) investigated breast implant failures and the subsequent complications, and re-classified breast implant devices as Class III medical devices, and required from manufacturers the documentary data substantiating the safety and efficacy of their breast implant devices. In 1992, the FDA placed silicone-gel breast implants in moratorium in the U.S., because there was "inadequate information to demonstrate that breast implants were safe and effective". Nonetheless, medical access to silicone-gel breast implant devices continued for clinical studies of post-mastectomy breast reconstruction, the correction of congenital deformities, and the replacement of ruptured silicone-gel implants. The FDA required from the manufacturers the clinical trial data, and permitted their providing breast implants to the breast augmentation patients for the statistical studies required by the U.S. Food and Drug Administration. In mid-1992, the FDA approved an adjunct study protocol for silicone-gel filled implants for breast reconstruction patients, and for revision-surgery patients. Also in 1992, the Dow Corning Corporation, a silicone products and breast implant manufacturer, announced the discontinuation of five implant-grade silicones, but would continue producing 45 other, medical-grade, silicone materials—three years later, in 1995, the Dow Corning Corporation went bankrupt when it faced large class action lawsuits claiming a variety of illnesses.
- In 1997, the U.S. Department of Health and Human Services (HHS) appointed the Institute of Medicine (IOM) of the U.S. National Academy of Sciences (NAS) to investigate the potential risks of operative and post-operative complications from the emplacement of silicone breast implants. The IOM's review of the safety and efficacy of silicone gel-filled breast implants, reported that the "evidence suggests diseases or conditions, such as connective tissue diseases, cancer, neurological diseases, or other systemic complaints or conditions are no more common in women with breast implants, than in women without implants" subsequent studies and systemic review found no causal link between silicone breast implants and disease.

- In 1998, the U.S. FDA approved adjunct study protocols for silicone-gel filled implants only for breast reconstruction patients and for revision-surgery patients; and also approved the Dow Corning Corporation's Investigational Device Exemption (IDE) study for silicone-gel breast implants for a limited number of breast augmentation-, reconstruction-, and revision-surgery patients.
- In 1999, the Institute of Medicine published the Safety of Silicone Breast Implants (1999) study that reported no evidence that saline-filled and silicone-gel filled breast implant devices caused systemic health problems; that their use posed no new health or safety risks; and that local complications are "the primary safety issue with silicone breast implants", in distinguishing among routine and local medical complications and systemic health concerns."
- In 2000, the FDA approved saline breast implant Premarket Approval Applications (PMA) containing the type and rate data of the local medical complications experienced by the breast surgery patients. "Despite complications experienced by some women, the majority of those women still in the Inamed Corporation and Mentor Corporation studies, after three years, reported being satisfied with their implants." The premarket approvals were granted for breast augmentation, for women at least 18 years old, and for women requiring breast reconstruction.
- In 2006, for the Inamed Corporation and for the Mentor Corporation, the U.S. Food and Drug Administration lifted its restrictions against using silicone-gel breast implants for breast reconstruction and for augmentation mammoplasty. Yet, the approval was conditional upon accepting FDA monitoring, the completion of 10-year-mark studies of the women who already had the breast implants, and the completion of a second, 10-year-mark study of the safety of the breast implants in 40,000 other women. The FDA warned the public that breast implants do carry medical risks, and recommended that women who undergo breast augmentation should periodically undergo MRI examinations to screen for signs of either shell rupture or of filler leakage, or both conditions; and ordered that breast surgery patients be provided with detailed, informational brochures explaining the medical risks of using silicone-gel breast implants.
- In March 2019, the FDA hosted a public meeting of their General and Plastic Surgery Devices Advisory Panel to discuss safety issues of silicone and saline breast implants, including BIA-ALCL and breast implant illness. One of the major topics of that public meeting was the evidence that Allergan BIOCELL textured breast implants were the type of breast implants most likely to cause BIA-ALCL. Following the committee meeting, the FDA requested that Allergan recall their BIOCELL textured breast implants and tissue expanders, and in July 2019 Allergan took those BIOCELL textured implants and expanders off the market. Allergan subsequently announced they would contact all customers who had purchased their products to ensure they were aware of the recall.
The U.S. Food and Drug Administration established the age ranges for women seeking breast implants; for breast reconstruction, silicone-gel filled implants and saline-filled implants were approved for women of all ages; for breast augmentation, saline implants were approved for women 18 years of age and older; silicone implants were approved for women 22 years of age and older. Because each breast implant device entails different medical risks, the minimum age of the patient for saline breast implants is different from the minimum age of the patient for silicone breast implants—because of the filler leakage and silent shell-rupture risks; thus, periodic MRI screening examinations are the recommended post-operative, follow-up therapy for the patient. In other countries, in Europe and Oceania, the national health ministries' breast implant policies do not endorse periodic MRI screening of asymptomatic patients, but suggest palpation proper—with or without an ultrasonic screening—to be sufficient post-operative therapy for most patients.
See also
- Breast augmentation
- Breast enlargement supplements
- Breast reconstruction
- Breast reduction plasty
- Mastopexy (breast lift)
- Poly Implant Prothèse
- Polypropylene breast implants
- Trans-umbilical breast augmentation (TUBA)
References
- "Risks and Complications of Breast Implants". FDA. 21 October 2019. Retrieved 30 October 2019.
- Loch-Wilkinson, Anna; Beath, Kenneth J; Magnusson, Mark R; Cooter, Rodney; Shaw, Karen; French, James; Vickery, Karen; Prince, H Miles; Deva, Anand K (2020-07-13). "Breast Implant-Associated Anaplastic Large Cell Lymphoma in Australia: A Longitudinal Study of Implant and Other Related Risk Factors". Aesthetic Surgery Journal. 40 (8): 838–846. doi:10.1093/asj/sjz333. ISSN 1090-820X.
- American Society of Plastic Surgeons (24 April 2014), "Five Things Physicians and Patients Should Question", Choosing Wisely: an initiative of the ABIM Foundation, American Society of Plastic Surgeons, archived from the original on 19 July 2014, retrieved 25 July 2014
- Brinton LA, Brown SL, Colton T, Burich MC, Lubin J (2000). "Characteristics of a Population of Women with Breast Implants Compared with Women Seeking other Types of Plastic Surgery". Plastic and Reconstructive Surgery. 105 (3): 919–927. doi:10.1097/00006534-200003000-00014. PMID 10724251. S2CID 32599107.
- Jacobsen PH, Hölmich LR, McLaughlin JK, Johansen C, Olsen JH, Kjøller K, Friis S (2004). "Mortality and suicide among Danish women with cosmetic breast implants". Arch. Intern. Med. 164 (22): 2450–5. doi:10.1001/archinte.164.22.2450. PMID 15596635.
- Young VL, Nemecek JR, Nemecek DA (1994). "The Efficacy of Breast Augmentation: Breast Size Increase, Patient Satisfaction, and Psychological Effects". Plastic and Reconstructive Surgery. 94 (Dec): 958–969. doi:10.1097/00006534-199412000-00009. PMID 7972484. S2CID 753343.
- Crerand CE, Franklin ME, Sarwer DB (2006). "Body Dysmorphic Disorder and Cosmetic Surgery". Plastic and Reconstructive Surgery. 118 (July): 167e–180e. doi:10.1097/01.prs.0000242500.28431.24. PMID 17102719. S2CID 8925060.
- Sarwer DB, LaRossa D, Bartlett SP, Low DW, Bucky LP, Whitaker LA (2003). "Body Image Concerns of Breast Augmentation Patients". Plastic and Reconstructive Surgery. 112 (July): 83–90. doi:10.1097/01.PRS.0000066005.07796.51. PMID 12832880. S2CID 45574374.
- Chahraoui K, Danino A, Frachebois C, Clerc AS, Malka G (2006). "Aesthetic Surgery and Quality of Life Before and Four Months Postoperatively". Journal of Long-Term Effects of Medical Implants. 51 (3): 207–210. doi:10.1016/j.anplas.2005.07.010. PMID 16181718.
- Cash TF, Duel LA, Perkins LL (2002). "Women's Psychosocial Outcomes of Breast Augmentation with Silicone gel-filled implants: a 2-year Prospective Study". Plastic and Reconstructive Surgery. 109 (May): 2112–2121. doi:10.1097/00006534-200205000-00049. PMID 11994621.
- Figueroa-Haas CL (2007). "Effect of Breast Augmentation Mammoplasty on Self-esteem and Sexuality: A Quantitative Analysis". Plastic Surgery Nursing. 27 (Mar): 16–36. doi:10.1097/01.PSN.0000264159.30505.c9. PMID 17356451. S2CID 23169107.
- "Important Information for Women About Breast Augmentation with Inamed Silicone Gel-Filled Implants" (PDF). Food and Drug Administration. 2006. Archived from the original (PDF) on 2007-01-03.
- Dolan, Eric W. (2023-10-06). "Breast implants have a positive impact on female sexuality, according to new research". PsyPost. Retrieved 2023-10-09.
- Handel N, Cordray T, Gutierrez J, Jensen JA (2006). "A Long-term Study of Outcomes, Complications, and Patient Satisfaction with Breast Implants". Plastic and Reconstructive Surgery. 117 (Mar): 757–767. doi:10.1097/01.prs.0000201457.00772.1d. PMID 16525261. S2CID 15228702.
- "Breast Implants Linked with Suicide in Study". Reuters. 2007-08-08. Archived from the original on 2008-12-21.
- Manning A (2007-08-06). "Breast Implants Linked to Higher Suicide Rates". USA Today. Archived from the original on 2011-03-18. Retrieved 2010-04-26.
- ^ Brinton LA, Lubin JH, Burich MC, Colton T, Brown SL, Hoover RN (2001). "Cancer risk at sites other than the breast following augmentation mammoplasty". Ann Epidemiol. 11 (4): 248–256. doi:10.1016/s1047-2797(00)00223-4. PMID 11306343.
- Koot VC, Peeters PH, Granath F, Grobbee DE, Nyren O (2003). "Total and cause specific mortality among Swedish women with cosmetic breast implants: prospective study". BMJ. 326 (7388): 527–8. doi:10.1136/bmj.326.7388.527. PMC 150462. PMID 12623911.
- Pukkala E, Kulmala I, Hovi SL, Hemminki E, Keskimäki I, Pakkanen M, Lipworth L, Boice JD, McLaughlin JK (2003). "Causes of death among Finnish women with cosmetic breast implants, 1971-2001". Ann Plast Surg. 51 (4): 339–42, discussion 343–4. doi:10.1097/01.sap.0000080407.97677.A5. PMID 14520056. S2CID 34929987.
- Villeneuve PJ, Holowaty EJ, Brisson J, Xie L, Ugnat AM, Latulippe L, Mao Y (2006). "Mortality among Canadian women with cosmetic breast implants". Am. J. Epidemiol. 164 (4): 334–41. doi:10.1093/aje/kwj214. PMID 16777929.
- Brinton LA, Lubin JH, Murray MC, Colton T, Hoover RN (2006). "Mortality rates among augmentation mammoplasty patients: an update". Epidemiology. 17 (2): 162–9. doi:10.1097/01.ede.0000197056.84629.19. PMID 16477256. S2CID 22285852.
- National Plastic Surgery Procedural Statistics, 2006. Arlington Heights, Illinois, American Society of Plastic Surgeons, 2007
- "Plastic Surgery Helps Self-Esteem". Psych Central.com. Archived from the original on 2010-06-19.
- Grippaudo FR, Renzi L, Costantino B, Longo B, Santanelli F (2013). "Late unilateral hematoma after breast reconstruction with implants: case report and literature review". Aesthetic Surgical Journal. 33 (6): 830–834. doi:10.1177/1090820X13496249. PMID 23864111.
- ^ "Important Information for Women About Breast Augmentation with INAMED Silicone-Filled Breast Implants" (PDF). Food and Drug Administration. 2006-11-03. Archived from the original (PDF) on 2007-01-03. Retrieved 2007-05-04.
- "Important Information for Augmentation Patients About Mentor MemoryGel Silicone Gel-Filled Breast Implants" (PDF). 2006-11-03. Archived from the original (PDF) on 16 October 2014. Retrieved 11 October 2014.
- "Saline-Filled Breast Implant Surgery: Making An Informed Decision (Mentor Corporation)". FDA Breast Implant Consumer Handbook - 2004. 2004-01-13. Archived from the original on 2006-11-26. Retrieved 2007-05-04.
- "FDA NEWS RELEASE". Food and Drug Administration. Archived from the original on 2011-11-03. Retrieved 2011-11-09.
- ^ Diamond BA; Hulka BS; Kerkvliet NI; Tugwell P. "Silicone Breast Implants in Relation to Connective Tissue Diseases and Immunologic Dysfunction: A Report by a National Science Panel to the Honorable Sam C. Pointer Jr. Coordinating Judge for the Federal Breast Implant Multi-District Litigation". 1998.
- Janowsky, E. C.; Kupper, L. L.; Hulka, B. S. (2000-03-16). "Meta-analyses of the relation between silicone breast implants and the risk of connective-tissue diseases". The New England Journal of Medicine. 342 (11): 781–790. doi:10.1056/NEJM200003163421105. ISSN 0028-4793. PMID 10717013.
- ^ Watad, A.; Rosenberg, V.; Tiosano, S.; Cohen Tervaert, J. W.; Yavne, Y.; Shoenfeld, Y.; Shalev, V.; Chodick, G.; Amital, H. (2018). "Silicone breast implants and the risk of autoimmune/rheumatic disorders: a real-world analysis". Int J Epidemiol. 47 (6): 1846–1854. doi:10.1093/ije/dyy217. PMID 30329056.
- Coroneos, Christopher J.; Selber, Jesse C.; Offodile, Anaeze C.; Butler, Charles E.; Clemens, Mark W. (January 2019). "US FDA Breast Implant Postapproval Studies: Long-term Outcomes in 99,993 Patients". Annals of Surgery. 269 (1): 30–36. doi:10.1097/SLA.0000000000002990. ISSN 1528-1140. PMID 30222598. S2CID 52284936.
- ^ Wee, Corinne E (June 2021). "The Objective Effect of Breast Implant Removal and Capsulectomy on Pulmonary Function". Plastic and Reconstructive Surgery - Global Open. 9 (6): e3636. doi:10.1097/GOX.0000000000003636. S2CID 235801794.
- Brinton LA, Malone KE, Coates RJ, Schoenberg JB, Swanson CA, Daling JR, Stanford JL (1996). "Breast Enlargement and Reduction: Results from a Breast Cancer Case-control Study". Plastic and Reconstructive Surgery. 97 (2): 269–275. doi:10.1097/00006534-199602000-00001. PMID 8559808. S2CID 29456173.
- Benadiba L (2004). "Histoire des protheses mammaires" (in French). Archived from the original on 29 January 2015. Retrieved 12 October 2015.
- Breast Implant Information Booklet (PDF) (4th ed.). Canberra: Commonwealth of Australia. 2001. ISBN 0642735794. Archived from the original (PDF) on 2007-01-01. Retrieved 2006-12-29.
- "German Society for Senology, Declaration of Consensus for the Security of Silicone Breast Implants". 24 September 1998.
- ^ Janowsky EC, Kupper LL, Hulka BS (2000). "Meta-analyses of the Relation between Silicone Breast Implants and the Risk of Connective-tissue Diseases". New England Journal of Medicine. 342 (11): 781–790. doi:10.1056/NEJM200003163421105. PMID 10717013.
- Archived December 27, 2005, at the Wayback Machine
- Archived June 23, 2006, at the Wayback Machine
- Tugwell P, Wells G, Peterson J, Welch V, Page J, Davison C, McGowan J, Ramroth D, Shea B (2001). "Do silicone Breast Implants Cause Rheumatologic Disorders? A Systematic Review for a Court-appointed National Science Panel". Arthritis Rheum. 44 (11): 2477–84. doi:10.1002/1529-0131(200111)44:11<2477::AID-ART427>3.0.CO;2-Q. PMID 11710703.
- Gorgojo L, Gonzalez J, Wisbaum W, Martin-Moreno J (30 May 2003). Health risks posed by silicone implants in general, with a special attention to breast implants (PDF) (Report). Archived from the original (PDF) on 2003-08-29. Retrieved 2019-01-28.
- Neuhann-Lorenz C, Fedeles J, Eisenman-Klein M, Kinney B, Cunningham BL (2001). "Eighth IQUAM Consensus Position Statement: Transatlantic Innovations, April 2009". Plastic and Reconstructive Surgery. 127 (3): 1368–1375. doi:10.1097/PRS.0b013e318206312e. PMID 21364439. S2CID 29112694.
- Brown SL, Middleton MS, Berg WA, Soo MS, Pennello G (2000). "Prevalence of Rupture of Silicone gel Breast Implants Revealed on MR Imaging in a Population of Women in Birmingham, Alabama". American Journal of Roentgenology. 175 (4): 1057–1064. doi:10.2214/ajr.175.4.1751057. PMID 11000165.
- "Risks and Complications of Breast Implants". U.S. Food and Drug Administration. 28 September 2020. Retrieved 14 October 2021.
- Walker PS, Walls B, Murphy DK (2009). "Natrelle Saline-filled Breast Implants: a Prospective 10-year Study". Aesthetic Surgery Journal. 29 (1): 19–25. doi:10.1016/j.asj.2008.10.001. PMID 19233001.
- Eisenberg, TS (2021). "Does Overfilling Smooth Inflatable Saline-Filled Breast Implants Decrease the Deflation Rate? Experience with 4761 Augmentation Mammaplasty Patients". Aesthetic Plastic Surgery. 45 (5): 1991–1999. doi:10.1007/s00266-021-02198-3. PMC 8481168. PMID 33712871.
- Hölmich LR, Vejborg IM, Conrad C, Sletting S, Høier-Madsen M, Fryzek JP, McLaughlin JK, Kjøller K, Wiik A, Friis S (2004). "Untreated Silicone Breast Implant Rupture". Plastic and Reconstructive Surgery. 114 (1): 204–214. doi:10.1097/01.PRS.0000128821.87939.B5. PMID 15220594. S2CID 25947224.
- Katzin WE, Centeno JA, Feng LJ, Kiley M, Mullick FG (2001). "Pathology of Lymph Nodes From Patients With Breast Implants: A Histologic and Spectroscopic Evaluation". American Journal of Surgical Pathology. 29 (4): 506–11. doi:10.1097/01.pas.0000155145.60670.e4. PMID 15767806. S2CID 31982669. Archived from the original on May 24, 2009.
- "Study of Rupture of Silicone Gel-filled Breast Implants (MRI Component)". FDA Breast Implant Consumer Handbook - 2004. 2000-05-22. Archived from the original on 2007-06-09. Retrieved 2007-05-04.
- ^ "Local Complications". FDA Breast Implant Consumer Handbook - 2004. 2004-06-08. Archived from the original on 2007-05-13. Retrieved 2007-05-04.
- MRI of a ruptured silicone breast implant Archived 2013-09-26 at the Wayback Machine 2013-04-05
- Hölmich LR, Friis S, Fryzek JP, Vejborg IM, Conrad C, Sletting S, Kjøller K, McLaughlin JK, Olsen JH (2003). "Incidence of Silicone Breast Implant Rupture". Arch. Surg. 138 (7): 801–806. doi:10.1001/archsurg.138.7.801. PMID 12860765.
- Hedén P, Nava MB, van Tetering JP, Magalon G, Fourie le R, Brenner RJ, Lindsey LE, Murphy DK, Walker PS (2006). "Prevalence of Rupture in Inamed Silicone Breast Implants". Plastic and Reconstructive Surgery. 118 (2): 303–308. doi:10.1097/01.prs.0000233471.58039.30. PMID 16874191. S2CID 30442865.
- "FDA summary of clinical issues (MS Word document)". Food and Drug Administration. Archived from the original on 2008-03-08.
- Cunningham B, McCue J (2009). "Safety and effectiveness of Mentor's MemoryGel implants at 6 years". Plastic and Reconstructive Surgery. 33 (3): 440–444. doi:10.1007/s00266-009-9364-6. PMID 19437068. S2CID 25722841.
- Hedén P, Boné B, Murphy DK, Slicton A, Walker PS (2006). "Style 410 Cohesive Silicone Breast Implants: Safety and Effectiveness at 5 to 9 years after Implantation". Plastic and Reconstructive Surgery. 118 (6): 1281–1287. doi:10.1097/01.prs.0000239457.17721.5d. PMID 17051096. S2CID 34380204.
- Hölmich LR, Fryzek JP, Kjøller K, Breiting VB, Jørgensen A, Krag C, McLaughlin JK (2005). "The Diagnosis of Silicone Breast implant Rupture: Clinical Findings Compared with Findings at Magnetic Resonance Imaging". Annals of Plastic Surgery. 54 (6): 583–589. doi:10.1097/01.sap.0000164470.76432.4f. PMID 15900139. S2CID 39525474.
- "Expert Advisory Panel on Breast Implants: Record of Proceedings". HealthCanada. 2005-09-29. Archived from the original on 2007-11-07. Retrieved 2007-05-04.
- Song JW, Kim HM, Bellfi LT, Chung KC (2011). "The Effect of Study design Biases on the Diagnostic Accuracy of Magnetic Resonance Imaging for Detecting Silicone Breast Implant Ruptures: a Meta-analysis". Plastic and Reconstructive Surgery. 127 (3): 1029–1044. doi:10.1097/PRS.0b013e3182043630. PMC 3080104. PMID 21364405.
- AFP (18 September 2011). "Breast implants safe, but not for life: US experts". The Independent. Archived from the original on 3 August 2016.
- Barnsley GP, Sigurdson LJ, Barnsley SE (2006). "Textured surface Breast Implants in the Prevention of Capsular Contracture among Breast Augmentation Patients: a Meta-analysis of Randomized Controlled Trials". Plastic and Reconstructive Surgery. 117 (7): 2182–2190. doi:10.1097/01.prs.0000218184.47372.d5. PMID 16772915. S2CID 35420582.
- Wong CH, Samuel M, Tan BK, Song C (2006). "Capsular Contracture in Subglandular Breast Augmentation with Textured versus Smooth Breast Implants: a Systematic Review". Plastic and Reconstructive Surgery. 118 (5): 1224–1236. doi:10.1097/01.prs.0000237013.50283.d2. PMID 17016195. S2CID 29643167.
- Handel N, Gutierrez J (May 2006). "Long-term safety and efficacy of polyurethane foam-covered breast implants". Journal of Aesthetic Surgery. 26 (3): 265–274. doi:10.1016/j.asj.2006.04.001. PMID 19338905.
- Mladick RA (1993). ""No-touch" submuscular saline breast augmentation technique". Journal of Aesthetic Surgery. 17 (3): 183–192. doi:10.1007/BF00636260. PMID 8213311. S2CID 39767802.
- Adams WP, Rios JL, Smith SJ (2006). "Enhancing Patient Outcomes in Aesthetic and Reconstructive Breast Surgery using Triple Antibiotic Breast Irrigation: Six-year Prospective Clinical Study". Plastic and Reconstructive Surgery. 117 (1): 30–6. doi:10.1097/01.prs.0000185671.51993.7e. PMID 16404244. S2CID 35238465.
- Planas J, Cervelli V, Planas G (2001). "Five-year experience on ultrasonic treatment of breast contractures". Aesthetic Plastic Surgery. 25 (2): 89–93. doi:10.1007/s002660010102. PMID 11349308. S2CID 2784003.
- Schlesinger SL, Ellenbogen R, Desvigne MN, Svehlak S, Heck R (2002). "Zafirlukast (Accolate): A new treatment for capsular contracture". Aesthetic Plast. Surg. 22 (4): 329–36. doi:10.1067/maj.2002.126753. PMID 19331987.
- Scuderi N, Mazzocchi M, Fioramonti P, Bistoni G (2006). "The effects of zafirlukast on capsular contracture: preliminary report". Aesthetic Plast. Surg. 30 (5): 513–520. doi:10.1007/s00266-006-0038-3. PMID 16977359. S2CID 251008.
- Silver H (1982). "Reduction of capsular contracture with two-stage augmentation mammaplasty and pulsed electromagnetic energy (Diapulse therapy)". Plastic and Reconstructive Surgery. 69 (5): 802–805. doi:10.1097/00006534-198205000-00013. PMID 7071225. S2CID 8451166.
- Tebbetts JB (October 2006). "Out Points Criteria for Breast Implant Removal without Replacement and Criteria to Minimize Reoperations following Breast Augmentation". Plastic and Reconstructive Surgery. 114 (5): 1258–1262. doi:10.1097/01.prs.0000136802.91357.cf. PMID 15457046.
- Tebbetts JB (December 2006). "Achieving a Zero Percent Reoperation Rate at 3 years in a 50-consecutive-case Augmentation Mammaplasty Premarket Approval Study". Plastic and Reconstructive Surgery. 118 (6): 1453–7. doi:10.1097/01.prs.0000239602.99867.07. PMID 17051118. S2CID 27630646.
- Breiting VB, Hölmich LR, Brandt B, Fryzek JP, Wolthers MS, Kjøller K, McLaughlin JK, Wiik A, Friis S (2004). "Long-term Health Status of Danish Women with Silicone Breast Implants". Plastic and Reconstructive Surgery. 114 (1): 217–226. doi:10.1097/01.PRS.0000128823.77637.8A. PMID 15220596. S2CID 20584928.
- Grigg, Martha; Bondurant, Stuart (2000). "A Report of a Study by the Institute of Medicine". Information for Women About the Safety of Silicone Breast Implants – via NCBI.
{{cite book}}:|journal=ignored (help) - Brinton, LA (2006). "Mortality among augmentation mammoplasty patients: An update". Epidemiology. 17 (2): 162–169. doi:10.1097/01.ede.0000197056.84629.19. PMID 16477256. S2CID 22285852.
- "Labeling for Approved Breast Implants". U.S. Food and Drug Administration. 3 December 2021. Archived from the original on 2019-09-11. Retrieved 19 January 2022.
- Arepalli SR, Bezabeh S, Brown SL (2002). "Allergic reaction to platinum in silicone breast implants". Journal of Long-Term Effects of Medical Implants. 12 (4): 299–306. doi:10.1615/jlongtermeffmedimplants.v12.i4.80. PMID 12627791.
- "FDA Backgrounder on Platinum in Silicone Breast Implants". U.S. Food and Drug Administration. 16 June 2006. Archived from the original on 2006-07-15.
- Office of the Commissioner. "Safety Alerts for Human Medical Products - Breast Implants: Update - Breast Implant-Associated Anaplastic Large Cell Lymphoma (BIA-ALCL)". www.fda.gov. Archived from the original on 28 April 2018. Retrieved 28 April 2018.
- Swerdlow, Steven H.; Campo, Elias; Pileri, Stefano A.; Harris, Nancy Lee; Stein, Harald; Siebert, Reiner; Advani, Ranjana; Ghielmini, Michele; Salles, Gilles A.; Zelenetz, Andrew D.; Jaffe, Elaine S. (2016-05-19). "The 2016 revision of the World Health Organization classification of lymphoid neoplasms". Blood. 127 (20): 2375–2390. doi:10.1182/blood-2016-01-643569. ISSN 0006-4971. PMC 4874220. PMID 26980727.
- ^ "Breast Implants - Medical Device Reports of Breast Implant-Associated Anaplastic Large Cell Lymphoma". www.fda.gov. 20 August 2020. Retrieved 18 August 2021.
- Miranda RN, Aladily TN, Prince HM, Kanagal-Shamanna R, de Jong D, Fayad LE, et al. (January 2014). "Breast implant-associated anaplastic large-cell lymphoma: long-term follow-up of 60 patients". Journal of Clinical Oncology. 32 (2): 114–20. doi:10.1200/JCO.2013.52.7911. PMC 4062709. PMID 24323027.
- ^ Clemens, Mark. "Breast Implant Associated Anaplastic Large Cell Lymphoma (BIA-ALCL) Archived 2017-03-26 at the Wayback Machine" (2017).
- Clemens MW, Horwitz SM (March 2017). "NCCN Consensus Guidelines for the Diagnosis and Management of Breast Implant-Associated Anaplastic Large Cell Lymphoma". Aesthetic Surgery Journal. 37 (3): 285–289. doi:10.1093/asj/sjw259. PMID 28184418.
- "Implant-associated ALCL Facts | The MD Anderson Foundation". www.mdanderson.org. Archived from the original on 2017-12-09. Retrieved 2017-12-08.
- Cordeiro, Peter (20 Jan 2020). "Risk of breast implant associated anaplastic large cell lymphoma (BIA-ALCL) in a cohort of 3546 women prospectively followed long term after reconstruction with textured breast implants". J Plast Reconstr Aesthet Surg. 73 (5): 841–846. doi:10.1016/j.bjps.2019.11.064. PMC 7247945. PMID 32008941.
- "Breast Implant Associated ALCL: PROFILE Project | The Plastic Surgery Foundation". www.thepsf.org. Archived from the original on 2017-05-07. Retrieved 2017-04-25.
- "Breast Implants: Reports of Squamous Cell Carcinoma and Various Lymphomas in Capsule Around Implants: FDA Safety Communication". U.S. Food and Drug Administration. September 8, 2022.
- Johnson GW, Christ JE (1993). "The Endoscopic Breast augmentation: The Transumbilical Insertion of Saline-filled Breast Implants". Plastic and Reconstructive Surgery. 92 (5): 801–8. doi:10.1097/00006534-199392050-00004. PMID 8415961.
- Wallach SG (2004). "Maximizing the Use of the Abdominoplasty Incision". Plastic and Reconstructive Surgery. 113 (1): 411–417. doi:10.1097/01.PRS.0000091422.11191.1A. PMID 14707667. S2CID 44430032.
- Graf RM, Bernardes A, Rippel R, Araujo LR, Damasio RC, Auersvald A (2003). "Subfascial Breast Implant: A New Procedure". Plastic and Reconstructive Surgery. 111 (2): 904–908. doi:10.1097/01.PRS.0000041601.59651.15. PMID 12560720.
- Tebbetts JB (2004). "Does Fascia Provide Additional, Meaningful Coverage over a Breast Implant?". Plastic and Reconstructive Surgery. 113 (2): 777–779. doi:10.1097/01.PRS.0000104516.13465.96. PMID 14758271.
- Tebbetts JB (2002). "A System for Breast Implant Selection Based on Patient Tissue Characteristics and Implant-soft tissue Dynamics". Plastic and Reconstructive Surgery. 109 (4): 1396–1409. doi:10.1097/00006534-200204010-00030. PMID 11964998.
- Pacik PT, Nelson CE, Werner C (2008). "Pain control in augmentation mammaplasty: safety and efficacy of indwelling catheters in 644 consecutive patients". Aesthet Surg J. 28 (3): 279–84. doi:10.1016/j.asj.2008.02.001. PMID 19083538.
- Pacik PT, Nelson CE, Werner C (2008). "Pain control in augmentation mammaplasty using indwelling catheters in 687 consecutive patients: data analysis". Aesthet Surg J. 28 (6): 631–41. doi:10.1016/j.asj.2008.09.001. PMID 19083591. S2CID 13441800.
- Tebbetts JB (2002). "A system for breast implant selection based on patient tissue characteristics and implant-soft tissue dynamics". Plast. Reconstr. Surg. 109 (4): 1396–409, discussion 1410–5. doi:10.1097/00006534-200204010-00030. PMID 11964998.
- Tebbetts JB, Adams WP (2005). "Five critical decisions in breast augmentation using five measurements in 5 minutes: the high five decision support process". Plast. Reconstr. Surg. 116 (7): 2005–16. doi:10.1097/01.prs.0000191163.19379.63. PMID 16327616. S2CID 11180810.
- Tebbetts JB (2002). "Achieving a predictable 24-hour return to normal activities after breast augmentation: part I. Refining practices by using motion and time study principles". Plast. Reconstr. Surg. 109 (1): 273–90, discussion 291–2. doi:10.1097/00006534-200201000-00044. PMID 11786826. S2CID 26419990.
- Tebbetts JB (2002). "Achieving a predictable 24-hour return to normal activities after breast augmentation: Part II. Patient preparation, refined surgical techniques, and instrumentation". Plast. Reconstr. Surg. 109 (1): 293–305, discussion 306–7. doi:10.1097/00006534-200201000-00046. PMID 11786828. S2CID 21392313.
- "Choosing Your Breast Implants" (Web). Minneapolis Plastic Surgery, LTD. Archived from the original on 24 November 2016. Retrieved 23 November 2016.
- Zannis J (2017). Tales for Tagliacozzi: An Inside Look at Modern-Day Plastic Surgery. AuthorHouse. ISBN 9781524659073. Retrieved 7 June 2019.
- ^ Stevens WG, Hirsch EM, Stoker DA, Cohen R (2006). "In vitro Deflation of Pre-filled Saline Breast Implants". Plastic and Reconstructive Surgery. 118 (2): 347–349. doi:10.1097/01.prs.0000227674.65284.80. PMID 16874200. S2CID 41156555.
- Arion HG (1965). "Retromammary Prosthesis". Comptes Rendus de la Société Française de Gynécologie. 5.
- Eisenberg TS (2009). "Silicone Gel Implants Are Back — So What?". American Journal of Cosmetic Surgery. 26: 5–7. doi:10.1177/074880680902600103. S2CID 136191732.
- Eisenberg TS (2022). "The Underappreciated Saline Breast Implant". Aesthetic Plastic Surgery. 47 (2): 897–900. doi:10.1007/s00266-022-03106-z. PMID 36131136. S2CID 252437143.
- Swanson E (2020). "Prospective Study of Saline versus Silicone Gel Implants for Subpectoral Breast Augmentation". Plastic and Reconstructive Surgery. 8 (6): e2882. doi:10.1097/GOX.0000000000002882. PMC 7339341. PMID 32766047.
- Cronin TD, Gerow FJ (1963). "Augmentation Mammaplasty: A New "natural feel" Prosthesis". Excerpta Medica International Congress Series. 66: 41.
- Luu HM, Hutter JC, Bushar HF (1998). "A Physiologically based Pharmacokinetic Model for 2,4-toluenediamine Leached from Polyurethane foam-covered Breast Implants". Environ Health Perspect. 106 (7): 393–400. doi:10.2307/3434066. JSTOR 3434066. PMC 1533137. PMID 9637796.
- Hester TR, Tebbetts JB, Maxwell GP (2001). "The Polyurethane-covered Mammary Prosthesis: Facts and Fiction (II): A Look Back and a "peek" Ahead". Clinical Plastic Surgery. 28 (3): 579–86. doi:10.1016/S0094-1298(20)32397-X. PMID 11471963.
- Brown MH, Shenker R, Silver SA (2005). "Cohesive silicone gel breast implants in aesthetic and reconstructive breast surgery". Plastic and Reconstructive Surgery. 116 (3): 768–779, discussion 779–1. doi:10.1097/01.prs.0000176259.66948.e7. PMID 16141814. S2CID 35392851.
- Fruhstorfer BH, Hodgson EL, Malata CM (2004). "Early experience with an anatomical soft cohesive silicone gel prosthesis in cosmetic and reconstructive breast implant surgery". Annals of Plastic Surgery. 53 (6): 536–542. doi:10.1097/01.sap.0000134508.43550.6f. PMID 15602249. S2CID 24661896.
- Hedén P, Jernbeck J, Hober M (2001). "Breast augmentation with anatomical cohesive gel implants: The world's largest current experience". Clinics in Plastic Surgery. 28 (3): 531–552. doi:10.1016/S0094-1298(20)32393-2. PMID 11471959.
- Breastfeeding after Breast Surgery Archived 2010-12-30 at the Wayback Machine, La Leche League, contains references.
- Breastfeeding and Breast Implants Archived 2010-12-31 at the Wayback Machine, Selected Bibliography April 2003, LLLI Center for Breastfeeding Information
- ^ Inorganic Milk: Can Kendra Wilkinson breast-feed her baby even though she has implants? Archived 2010-01-25 at the Wayback Machine, Christopher Beam, Slate.com, 11 December 2009
- Handel N, Silverstein MJ, Gamagami P, Jensen JA, Collins A (1992). "Factors Affecting Mammographic Visualization of the Breast after Augmentation Mammaplasty". JAMA. 268 (14): 1913–1917. doi:10.1001/jama.268.14.1913. PMID 1404718.
- O'Keefe JR, Wilkinson JM, Spuur KM (June 2020). "Current practice in mammographic imaging of the augmented breast in Australia". Journal of Medical Radiation Sciences. 67 (2): 102–110. doi:10.1002/jmrs.374. PMC 7276184. PMID 31981297.
- Clark CP, Peters GN, O'Brien KM (1993). "Cancer in the Augmented Breast: Diagnosis and Prognosis". Cancer. 72 (7): 2170–4. doi:10.1002/1097-0142(19931001)72:7<2170::AID-CNCR2820720717>3.0.CO;2-1. PMID 8374874.
- Skinner KA, Silberman H, Dougherty W, Gamagami P, Waisman J, Sposto R, Silverstein MJ (2001). "Breast cancer after augmentation mammoplasty". Ann Surg Oncol. 8 (2): 138–44. doi:10.1007/s10434-001-0138-x. PMID 11258778. S2CID 26010159.
- ^ Le GM, O'Malley CD, Glaser SL, Lynch CF, Stanford JL, Keegan TH, West DW (2005). "Breast implants following mastectomy in women with early-stage breast cancer: prevalence and impact on survival". Breast Cancer Res. 7 (2): R184–93. doi:10.1186/bcr974. PMC 1064128. PMID 15743498.
- Handel N, Silverstein MJ (2006). "Breast cancer diagnosis and prognosis in augmented women". Plastic and Reconstructive Surgery. 118 (3): 587–93. doi:10.1097/01.prs.0000233038.47009.04. PMID 16932162. S2CID 36277679.
- Cunningham B (2006). "Breast cancer diagnosis and prognosis in augmented women- Discussion". Plastic and Reconstructive Surgery. 118 (3): 594–595. doi:10.1097/01.prs.0000233047.87102.8e. S2CID 220562548.
- Schwartz GF, Veronesi U, Clough KB, Dixon JM, Fentiman IS, Heywang-Köbrunner SH, Holland R, Hughes KS, Mansel RE, Margolese R, Mendelson EB, Olivotto IA, Palazzo JP, Solin LJ (2006). "Consensus Conference on Breast Conservation". Journal of the American College of Surgeons. 203 (2): 198–207. doi:10.1016/j.jamcollsurg.2006.04.009. PMID 16864033.
- Lavigne E, Holowaty EJ, Pan SY, Villeneuve PJ, Johnson KC, Fergusson DA, et al. (April 2013). "Breast cancer detection and survival among women with cosmetic breast implants: systematic review and meta-analysis of observational studies". BMJ. 346 (apr29 1): f2399. doi:10.1136/bmj.f2399. PMID 23637132.
- Hwang ES, Lichtensztajn DY, Gomez SL, Fowble B, Clarke CA (April 2013). "Survival after lumpectomy and mastectomy for early stage invasive breast cancer: the effect of age and hormone receptor status". Cancer. 119 (7): 1402–11. doi:10.1002/cncr.27795. PMC 3604076. PMID 23359049.
- ^ "Breast Implant Adverse Events During Mammography". Federal Drug Administration. 18 January 2018. Archived from the original on 2020-09-19.
- Czerny V (1895). "Plastischer Ersatz der Brusthus durch ein Lipoma". Zentralblatt für Chirurgie. 27: 72.
- Glicenstein J (April 2007). "". Annales de Chirurgie Plastique et Esthétique. 52 (2): 157–61. doi:10.1016/j.anplas.2006.05.003. PMID 16860452.
- Institute of Medicine (US) Committee on the Safety of Silicone Breast Implants, Bondurant S, Ernster V, Herdman R, et al. (Committee on the Safety of Silicone Breast Implants) (1999). Bondurant S, Ernster V, Herdman R (eds.). Safety of Silicone Breast Implants. Institute of Medicine. p. 21. doi:10.17226/9602. ISBN 0-309-06532-1. PMID 20669503. Archived from the original on 2007-03-13.
- Anderson N (1997). "Lawsuit Science: Lessons from the Silicone Breast Implant Controversy". New York Law School Law Review. 41 (2): 401–07.
- ^ "FDA Breast Implant Consumer Handbook - 2004". Food and Drug Administration. Archived from the original on September 17, 2008.
- Institute of Medicine (US) Committee on the Safety of Silicone Breast Implants, Bondurant S, Ernster V, Herdman R (1999). Safety of Silicone Breast Implants - The National Academies Press. doi:10.17226/9602. ISBN 978-0-309-15740-7. PMID 20669503.
{{cite book}}:|work=ignored (help) - Grigg M, Bondurant S, Ernster VL, Herdman R (2000). Grigg M, Bondurant S, Ernster VL, Herdman R (eds.). Information for Women about the Safety of Silicone Breast Implants - The National Academies Press. doi:10.17226/9618. ISBN 978-0-309-06593-1. PMID 20669498.
{{cite book}}:|work=ignored (help) - "FDA study". Food and Drug Administration. Archived from the original on January 13, 2008.
- "FDA approval". fda.gov. Archived from the original on 30 March 2009. Retrieved 28 April 2018.
- "FDA approval". fda.gov. Archived from the original on 26 May 2009. Retrieved 28 April 2018.
- "FDA Approves Silicone Gel-Filled Breast Implants". FDA. Archived from the original on 2008-07-26. Retrieved 2008-07-01.
- "March 25-26, 2019: General and Plastic Surgery Devices Panel of the Medical Devices Advisory Committee Meeting Announcement". U.S. Food and Drug Administration. April 25, 2019.
- "The FDA Requests Allergan Voluntarily Recall Natrelle BIOCELL Textured Breast Implants and Tissue Expanders from the Market to Protect Patients: FDA Safety Communication". U.S. Food and Drug Administration. June 1, 2020.
- ^ "Breast Implant Questions and Answers". U.S. Food and Drug Administration. October 2010. Archived from the original on 2010-11-12.
External links
- Article "Expander-Implant Breast Reconstruction" at Medscape
| Tests and procedures involving the breast | |
|---|---|
| Breast surgery |
|
| Breast imaging | |
| Other | |