| Revision as of 19:23, 13 September 2019 editMonkbot (talk | contribs)Bots3,695,952 editsm →Sources: Task 16: replaced (1×) / removed (0×) deprecated |dead-url= and |deadurl= with |url-status=;Tag: AWB← Previous edit | Latest revision as of 19:10, 22 December 2024 edit undoCitation bot (talk | contribs)Bots5,424,672 edits Added bibcode. | Use this bot. Report bugs. | Suggested by Whoop whoop pull up | Category:Sodium channel openers | #UCB_Category 2/11 | ||
| (78 intermediate revisions by 45 users not shown) | |||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | Watchedfields = |
| Watchedfields = change | ||
| | verifiedrevid = 461341190 | | verifiedrevid = 461341190 | ||
| | ImageFile=Batrachotoxin. |
| ImageFile=Batrachotoxin skeletal.svg | ||
| | ImageSize=250 | | ImageSize=250 | ||
| | ImageAlt = Skeletal formula of batrachotoxin | | ImageAlt = Skeletal formula of batrachotoxin | ||
| | ImageCaption = ] of batrachotoxin | |||
| | ImageFile1 = Batrachotoxin- |
| ImageFile1 = Batrachotoxin-skeleton-based-on-xtal-3D-st.png | ||
| | ImageSize1 = 250 | | ImageSize1 = 250 | ||
| | ImageAlt1 = |
| ImageAlt1 = Stick model of the batrachotoxin molecule | ||
| | ImageCaption1 = ] of batrachotoxin based on the ] of batrachotoxinin A ''O''-''p''-bromobenzoate<ref name="Karle&Karle1969">{{ cite journal | title = The structural formula and crystal structure of the ''O''-''p''-bromobenzoate derivative of batrachotoxinin A, C<sub>31</sub>H<sub>38</sub>NO<sub>6</sub>Br, a frog venom and steroidal alkaloid | first1 = I. L. | last1 = Karle | author-link1 = Isabella Karle | first2 = J. | last2 = Karle | author-link2 = Jerome Karle | journal = ] | year = 1969 | volume = 25 | issue = 3 | pages = 428–434 | doi = 10.1107/S056774086900238X | pmid = 5820223 | bibcode = 1969AcCrB..25..428K | s2cid = 28609553 }}</ref> | |||
| | ImageFile2 = Batrachotoxin-based-on-xtal-3D-bs.png | |||
| | ImageSize2 = 250 | |||
| | ImageAlt2 = Ball-and-stick model of the batrachotoxin molecule | |||
| | ImageCaption2 = ] of batrachotoxin, as above<ref name="Karle&Karle1969" /> | |||
| | IUPACName= | | IUPACName= | ||
| | OtherNames= 3α,9α-epoxy-14β,18-( |
| OtherNames= 3α,9α-epoxy-14β,18-(2{{prime}}-oxyethyl-''N''-methylamino)-5β-pregna-7,16-diene-3β,11α,20α-triol 20α-2,4-dimethylpyrrole-3-carboxylate<!-- doi:10.1016/S0099-9598(08)60136-4 --> | ||
| |Section1={{Chembox Identifiers | |Section1={{Chembox Identifiers | ||
| | IUPHAR_ligand = 2619 | | IUPHAR_ligand = 2619 | ||
| | CASNo_Ref = {{cascite|correct|??}} | | CASNo_Ref = {{cascite|correct|??}} | ||
| | CASNo= 23509-16-2 | | CASNo= 23509-16-2 | ||
| | UNII_Ref = {{fdacite|correct|FDA}} | |||
| ⚫ | | PubChem= |
||
| | UNII = TSG6XHX09R | |||
| ⚫ | | SMILES= |
||
| ⚫ | | PubChem= 6324647 | ||
| ⚫ | | SMILES= Cc1cc(C)c1C(=O)O(C)C1=CC23OCCN(C)C12C(O)12O4(O)CC1(C)(CC=C23)C4 | ||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 10310314 | | ChemSpiderID = 10310314 | ||
| Line 28: | Line 36: | ||
| | C=31 | H=42 | N=2 | O=6 | | C=31 | H=42 | N=2 | O=6 | ||
| | Appearance= | | Appearance= | ||
| | Density= 1.304 g/mL |
| Density= 1.304 g/mL<ref>{{cite journal |last1=Daly |first1=J. W. |last2=Witkop |first2=B. |last3=Bommer |first3=P. |last4=Biemann |first4=K. |title=Batrachotoxin. The Active Principle of the Colombian Arrow Poison Frog, Phyllobates bicolor |journal=Journal of the American Chemical Society |date=January 1965 |volume=87 |issue=1 |pages=124–126 |doi=10.1021/ja01079a026 |pmid=5826972 |bibcode=1965JAChS..87..124D }}</ref> | ||
| | MeltingPt= | | MeltingPt= | ||
| | BoilingPt= | | BoilingPt= | ||
| Line 41: | Line 49: | ||
| }} | }} | ||
| '''Batrachotoxin''' ('''BTX''') is an extremely potent ] and ] ]al ] found in certain species of beetles, birds, and frogs. The name is from the Greek word {{ |
'''Batrachotoxin''' ('''BTX''') is an extremely potent ] and ] ]al ] found in certain species of beetles, birds, and frogs. The name is from the Greek word {{langx|grc|βάτραχος|bátrachos|frog|label=none}}.<ref>''The Merck Index''. Entry 1009. p. 167.</ref> Structurally-related chemical compounds are often referred to collectively as batrachotoxins. In certain frogs, this alkaloid is present mostly on the skin. ] are among those used for ]. Batrachotoxin binds to and irreversibly ] the ] of ] and prevents them from closing, resulting in ] and death. No antidote is known. | ||
| ==History== | ==History== | ||
| Batrachotoxin was discovered by Fritz Märki and ], at the National Institute of Arthritis and Metabolic Diseases, National Institutes of Health, ], U.S.A. Märki and Witkop separated the potent toxic alkaloids fraction from '']'' and determined its chemical properties in 1963.<ref>{{cite journal |last1=Märki |first1=F. |last2=Witkop |first2=B. |title=The venom of the Colombian arrow poison frog ''Phyllobates bicolor'' |journal=Experientia |date=July 1963 |volume=19 |issue=7 |pages=329–338 |doi=10.1007/BF02152303 |pmid=14067757 |s2cid=19663576 }}</ref> They isolated four major toxic steroidal alkaloids including batrachotoxin, isobatrachotoxin, pseudobatrachotoxin, and batrachotoxinin A.<ref name=":0" /> Due to the difficulty of handling such a potent toxin and the minuscule amount that could be collected, a comprehensive ] involved several difficulties. However, Takashi Tokuyama, who joined the investigation later, converted one of the ] compounds, batrachotoxinin A, to a crystalline derivative and its unique steroidal structure was solved with ] techniques (1968).<ref>{{cite journal |last1=Tokuyama |first1=Takashi |last2=Daly |first2=John |last3=Witkop |first3=B. |last4=Karle |first4=Isabella L. |last5=Karle |first5=J. |title=The structure of batrachotoxinin A, a novel steroidal alkaloid from the Columbian arrow poison frog, Phyllobates aurotaenia |journal=Journal of the American Chemical Society |date=March 1968 |volume=90 |issue=7 |pages=1917–1918 |doi=10.1021/ja01009a052 |pmid=5689118 |bibcode=1968JAChS..90.1917T }}</ref> When the ] and ] of batrachotoxin and the batrachotoxinin A derivatives were compared, it was realized that the two shared the same steroidal structure and that batrachotoxin was batrachotoxinin A with a single extra ] ] attached. In fact, batrachotoxin was able to be partially ]d using ] into a material with identical TLC and color reactions as batrachotoxinin A.<ref name=":0" /> The structure of batrachotoxin was established in 1969 through chemical recombination of both fragments.<ref name=":0">{{cite journal |last1=Tokuyama |first1=Takashi |last2=Daly |first2=J. |last3=Witkop |first3=B. |title=Structure of batrachotoxin, a steroidal alkaloid from the Colombian arrow poison frog, phyllobates aurotaenia, and partial synthesis of batrachotoxin and its analogs and homologs |journal=Journal of the American Chemical Society |date=1 July 1969 |volume=91 |issue=14 |pages=3931–3938 |doi=10.1021/ja01042a042 |pmid=5814950 |bibcode=1969JAChS..91.3931T }}</ref> Batrachotoxinin A was synthesized by Michio Kurosu, Lawrence R. Marcin, Timothy J. Grinsteiner, and ] in 1998.<ref>{{cite journal |last1=Kurosu |first1=Michio |last2=Marcin |first2=Lawrence R. |last3=Grinsteiner |first3=Timothy J. |last4=Kishi |first4=Yoshito |title=Total Synthesis of (±)-Batrachotoxinin A |journal=Journal of the American Chemical Society |date=July 1998 |volume=120 |issue=26 |pages=6627–6628 |doi=10.1021/ja981258g |bibcode=1998JAChS.120.6627K }}</ref> | |||
| ==Toxicity== | ==Toxicity== | ||
| According to experiments with |
According to experiments with ]s, batrachotoxin is one of the most potent alkaloids known: its ] {{LD50}} in mice is 2–3 μg/kg.<ref>{{cite journal |last1=Tokuyama |first1=Takashi |last2=Daly |first2=John |last3=Witkop |first3=B. |last4=Karle |first4=Isabella L. |last5=Karle |first5=J. |title=The structure of batrachotoxinin A, a novel steroidal alkaloid from the Columbian arrow poison frog, ''Phyllobates aurotaenia'' |journal=Journal of the American Chemical Society |date=March 1968 |volume=90 |issue=7 |pages=1917–1918 |doi=10.1021/ja01009a052 |pmid=5689118 |bibcode=1968JAChS..90.1917T }}</ref> Meanwhile, its derivative, batrachotoxinin A, has a much lower toxicity with an {{LD50}} of 1000 μg/kg.<ref name=":0" /> | ||
| The toxin is released through colourless or milky secretions from glands located on the back and behind the ears of frogs from the genus '']''. When one of these frogs is agitated, feels threatened or is in pain, the toxin is reflexively released through several canals. | The toxin is released through colourless or milky secretions from glands located on the back and behind the ears of frogs from the genus '']''. When one of these frogs is agitated, feels threatened or is in pain, the toxin is reflexively released through several canals. | ||
| ⚫ | Batrachotoxin activity is temperature-dependent, with a maximum activity at {{convert|37|C|F}}. Its activity is also more rapid at an ] pH, which suggests that the unprotonated form may be more active. | ||
| === Neurotoxicity === | === Neurotoxicity === | ||
| As a ], it affects the ]. Neurological function depends on ] of nerve and muscle fibres due to increased sodium ion permeability of the ]. ] toxins such as batrachotoxin act directly on ]s<ref name="pmid16354762">{{cite journal | |
As a ], it affects the ]. Neurological function depends on ] of nerve and muscle fibres due to increased ] ion permeability of the ]. ] toxins such as batrachotoxin act directly on ]s<ref name="pmid16354762">{{cite journal |last1=Wang |first1=Sho-Ya |last2=Mitchell |first2=Jane |last3=Tikhonov |first3=Denis B. |last4=Zhorov |first4=Boris S. |last5=Wang |first5=Ging Kuo |title=How Batrachotoxin Modifies the Sodium Channel Permeation Pathway: Computer Modeling and Site-Directed Mutagenesis |journal=Molecular Pharmacology |date=March 2006 |volume=69 |issue=3 |pages=788–795 |doi=10.1124/mol.105.018200 |pmid=16354762 |s2cid=6343011 }}</ref> involved in ] generation and by modifying both their ion selectivity and voltage sensitivity. Batrachotoxin irreversibly binds to the Na<sup>+</sup> channels which causes a conformational change in the channels that forces the sodium channels to remain open. Batrachotoxin not only keeps ] sodium channels open but also reduces single-channel conductance. In other words, the toxin binds to the sodium channel and keeps the membrane permeable to sodium ions in an "all or none" manner.<ref>{{cite journal |last1=Wang |first1=Sho-Ya |last2=Tikhonov |first2=Denis B. |last3=Mitchell |first3=Jane |last4=Zhorov |first4=Boris |last5=Wang |first5=Ging Kuo |title=Irreversible Block of Cardiac Mutant Na + Channels by Batrachotoxin |journal=Channels |date=23 May 2007 |volume=1 |issue=3 |pages=179–188 |doi=10.4161/chan.4437 |pmid=18690024 |doi-access=free }}</ref> | ||
| This has a direct effect on the ] (PNS). Batrachotoxin in the PNS produces increased ] (selective and irreversible) of the resting cell membrane to sodium ions, without changing ] or ] concentration. This influx of sodium depolarizes the formerly polarized cell membrane. Batrachotoxin also alters the ion selectivity of the ion channel by increasing the permeability of the channel toward larger cations. Voltage-sensitive sodium channels become persistently active at the resting membrane potential. Batrachotoxin kills by permanently blocking nerve signal transmission to the muscles. | This has a direct effect on the ] (PNS). Batrachotoxin in the PNS produces increased ] (selective and irreversible) of the resting cell membrane to sodium ions, without changing ] or ] concentration. This influx of sodium depolarizes the formerly polarized cell membrane. Batrachotoxin also alters the ion selectivity of the ion channel by increasing the permeability of the channel toward larger cations. Voltage-sensitive sodium channels become persistently active at the resting membrane potential. Batrachotoxin kills by permanently blocking nerve signal transmission to the muscles. | ||
| Batrachotoxin binds to and irreversibly opens the sodium channels of nerve cells and |
Batrachotoxin binds to and irreversibly opens the sodium channels of nerve cells and prevents them from closing. The neuron can no longer send signals and this results in paralysis. Furthermore, the massive influx of sodium ions produces ] alterations in nerves and muscles, which causes structural changes. It has been suggested that there may also be an effect on the ], although it is not currently known what such an effect may be. | ||
| ⚫ | Although generally classified as a ], batrachotoxin has marked effects on ]s |
||
| === Cardiotoxicity === | |||
| Structural changes in nerves and muscles are due to a massive influx of sodium ions, which produces ] alterations. It has been suggested that there may also be an effect on the ], although it is not currently known what such an effect may be. | |||
| ⚫ | Although generally classified as a ], batrachotoxin has marked effects on ]s and its effects are mediated through sodium channel activation. Heart conduction is impaired resulting in ]s, ]s, ] and other changes which lead to ] and ]. Batrachotoxin induces a massive release of ] in nerves and muscles and destruction of ]s, as well.{{Citation needed|date=November 2021}} Batrachotoxin R is more toxic than related batrachotoxin A.{{Citation needed|date=November 2021}} | ||
| ⚫ | ==Treatment== | ||
| ⚫ | Batrachotoxin activity is temperature-dependent, with a maximum activity at {{convert|37|C|F}}. Its activity is also more rapid at an ] pH, which suggests that the unprotonated form may be more active. | ||
| {{More citations needed|section|date=November 2022}} | |||
| ⚫ | Currently, no effective ] exists for the treatment of batrachotoxin poisoning.<ref name=":1">{{cite book |doi=10.1016/B978-0-12-386454-3.00984-2 |chapter=Animals, Poisonous and Venomous |title=Encyclopedia of Toxicology |date=2014 |last1=Dodd-Butera |first1=T. |last2=Broderick |first2=M. |pages=246–251 |isbn=978-0-12-386455-0 }}</ref> ], ] and ]—like batrachotoxin—are lipid-soluble poisons which similarly alter the ion selectivity of the sodium channels, suggesting a common site of action. Due to these similarities, treatment for batrachotoxin poisoning might best be modeled after, or based on, treatments for one of these poisons. Treatment may also be modeled after that for ], which produces somewhat similar cardiotoxic effects. | ||
| ⚫ | ==Treatment== | ||
| ⚫ | Currently, no effective ] exists for the treatment of batrachotoxin poisoning. ], ] and ]—like batrachotoxin—are |
||
| While it is not an antidote, the membrane depolarization can be prevented or reversed by either ] (from ]), which is a ], or ] ("]"){{Citation needed|date=February 2018}} |
While it is not an antidote, the membrane depolarization can be prevented or reversed by either ]<ref name=":1" /> (from ]), which is a ], or ] ("]").{{Citation needed|date=February 2018}} These both have effects antagonistic to those of batrachotoxin on sodium flux. Certain ]s may act as ]s to the action of this alkaloid poison, while other ]s block its action altogether by acting as ] antagonists. | ||
| ==Sources== | ==Sources== | ||
| Batrachotoxin has been found in |
Batrachotoxin has been found in four Papuan beetle species, all in the genus '']'' in the family ]; '']'', '']'', '']'' and ].<ref name="pmid15520388">{{cite journal |last1=Dumbacher |first1=John P. |last2=Wako |first2=Avit |last3=Derrickson |first3=Scott R. |last4=Samuelson |first4=Allan |last5=Spande |first5=Thomas F. |last6=Daly |first6=John W. |title=Melyrid beetles (''Choresine''): A putative source for the batrachotoxin alkaloids found in poison-dart frogs and toxic passerine birds |journal=Proceedings of the National Academy of Sciences |date=9 November 2004 |volume=101 |issue=45 |pages=15857–15860 |doi=10.1073/pnas.0407197101 |doi-access=free |pmid=15520388 |pmc=528779 |bibcode=2004PNAS..10115857D }}</ref><ref name="Plikus">{{cite journal |last1=Plikus |first1=Maksim V. |last2=Astrowski |first2=Aliaksandr A. |title=Deadly hairs, lethal feathers – convergent evolution of poisonous integument in mammals and birds |journal=Experimental Dermatology |date=July 2014 |volume=23 |issue=7 |pages=466–468 |doi=10.1111/exd.12408 |pmid=24698054 }}</ref> | ||
| Several species of bird endemic to ] have the toxin in their skin and on their feathers: the ] (''Ifrita kowaldi''), ] (aka rufous shrike-thrush, ''Colluricincla megarhyncha''), and the following ] species: the ] (''Pitohui dichrous'', the most toxic of the birds), ] (''Ornorectes cristatus''), ] (''Melanorectes nigrescens''),<ref |
Several species of bird endemic to ] have the toxin in their skin and on their feathers: the ] (''Ifrita kowaldi''), ] (aka rufous shrike-thrush, ''Colluricincla megarhyncha''), and the following ] species: the ] (''Pitohui dichrous'', the most toxic of the birds), ] (''Ornorectes cristatus''), ] (''Melanorectes nigrescens''),<ref>{{cite journal |last1=Weldon |first1=Paul J. |title=Avian chemical defense: Toxic birds not of a feather |journal=Proceedings of the National Academy of Sciences |date=21 November 2000 |volume=97 |issue=24 |pages=12948–12949 |doi=10.1073/pnas.97.24.12948 |doi-access=free |pmid=11087849 |pmc=34071 |bibcode=2000PNAS...9712948W }}</ref> ] (''Pseudorectes ferrugineus''), and the variable pitohui,<ref>{{cite journal |last1=Dumbacher |first1=John P. |last2=Beehler |first2=Bruce M. |last3=Spande |first3=Thomas F. |last4=Garraffo |first4=H. Martin |last5=Daly |first5=John W. |title=Homobatrachotoxin in the Genus Pitohui : Chemical Defense in Birds? |journal=Science |date=30 October 1992 |volume=258 |issue=5083 |pages=799–801 |doi=10.1126/science.1439786 |pmid=1439786 |bibcode=1992Sci...258..799D }}</ref> which is now split into three species: the ] (''Pitohui kirhocephalus''), ] (''P. cerviniventris''), and ] (''P. uropygialis'').<ref name ="Gill">{{cite web|editor1-last=Gill|editor1-first=F. | editor1-link=Frank Gill (ornithologist) |editor2-last=Donsker|editor2-first=D. |title=Orioles, drongos & fantails| url=http://www.worldbirdnames.org/bow/orioles/|website=IOC World Bird List (v 7.2)|access-date=10 June 2017|date=2017}}</ref> | ||
| While the purpose for toxicity in these birds is not certain, the presence of batrachotoxins in these species is an example of ]. It is believed that these birds gain the toxin from batrachotoxin-containing insects that they eat |
While the purpose for toxicity in these birds is not certain, the presence of batrachotoxins in these species is an example of ]. It is believed that these birds gain the toxin from batrachotoxin-containing insects that they eat and then secrete it through the skin.<ref name="Plikus"/><ref name="calacademy.org">{{cite web | url = http://www.calacademy.org/science_now/academy_research/powerful_poison.php | publisher = California Academy of Science | title = Academy Research: A Powerful Poison | access-date = 2013-05-10 | archive-url = https://web.archive.org/web/20120827195307/http://www.calacademy.org/science_now/academy_research/powerful_poison.php | archive-date = 2012-08-27 | url-status = dead }}</ref> | ||
| Batrachotoxin has also been found in |
Batrachotoxin has also been found in all described species of the poison dart frog genus '']'' from ] to ], including the ] (''Phyllobates terribilis''), ] (''P. bicolor''), ] (''P. lugubris''), ] (''P. vittatus''), and ] (''P. aurotaenia'').<ref name="pmid15520388"/><ref name="Plikus"/><ref>{{cite journal | doi=10.1111/evo.13672 | title=Does batrachotoxin autoresistance coevolve with toxicity in Phyllobates poison-dart frogs? | year=2019 | last1=Márquez | first1=Roberto | last2=Ramírez-Castañeda | first2=Valeria | last3=Amézquita | first3=Adolfo | journal=Evolution | volume=73 | issue=2 | pages=390–400 | pmid=30593663 | s2cid=58605344 | doi-access=free }}</ref> The Kokoe poison frog used to include ], now recognized as distinct. All six of these frog species are in the ] family. | ||
| The frogs do not produce batrachotoxin themselves. Just as in the birds, it is believed that these frogs gain the toxin from batrachotoxin-containing insects that they eat, and then secrete it through the skin.<ref name="Plikus"/> |
The frogs do not produce batrachotoxin themselves. Just as in the birds, it is believed that these frogs gain the toxin from batrachotoxin-containing insects that they eat, and then secrete it through the skin.<ref name="Plikus"/> Beetles in the genus ''Choresine'' are not found in Colombia, but it is thought that the frogs might get the toxin from beetles in other genera within the same family (]), several of which are found in Colombia.<ref name="pmid15520388"/> | ||
| Frogs raised in captivity do not produce batrachotoxin, and thus may be handled without risk. However, this limits the amount of batrachotoxin available for research as 10,000 frogs yielded only 180 mg of batrachotoxin.<ref>Du Bois, Justin, et al., inventor; Board of Trustees of the Leland |
Frogs raised in captivity do not produce batrachotoxin, and thus may be handled without risk. However, this limits the amount of batrachotoxin available for research as 10,000 frogs yielded only 180 mg of batrachotoxin.<ref>Du Bois, Justin, et al., inventor; Board of Trustees of the Leland Stanford Junior University, assignee. Batrachotoxin Analogues, Compositions, Uses, and Preparation Thereof. US patent 2014/0171410 A1. June 19, 2014.</ref> As these frogs are endangered, their harvest is ]. Biosynthetic studies are also challenged by the slow rate of synthesis of batrachotoxin.<ref name=":0" /> | ||
| The native habitat of poison dart frogs is the warm regions of ] and ] |
The native habitat of poison dart frogs is the warm regions of ] and ]. | ||
| ==Use== | ==Use== | ||
| {{see also|Arrow poison|Blowgun}} | {{see also|Arrow poison|Blowgun}} | ||
| The most common use of this toxin is by the Noanamá Chocó and Emberá Chocó of the ] of western ] for poisoning ] darts for use in hunting. | The most common use of this toxin is by the Noanamá Chocó and Emberá Chocó of the ] of western ] for poisoning ] darts for use in hunting. | ||
| Poison darts are prepared by the Chocó by first impaling a frog on a piece of wood.<ref>{{cite book|last=Crump|first=M.|title=In Search of the Golden Frog|date=2000|publisher=University Of Chicago Press|isbn=978-0226121987|page=12}}</ref> By some accounts, the frog is then held over or roasted alive over a fire until it cries in pain. Bubbles of poison form as the frog's skin begins to blister. The dart tips are prepared by touching them to the toxin, or the toxin can be caught in a container and allowed to ferment. Poison darts made from either fresh or fermented batrachotoxin are enough to drop monkeys and birds in their tracks. Nerve paralysis is almost instantaneous. Other accounts say that a stick ''siurukida'' ("bamboo tooth") is put through the mouth of the frog and passed out through one of its hind legs. This causes the frog to ] profusely on its back, which becomes covered with a white froth. The darts are dipped or rolled in the froth, preserving their lethal power for up to a year. | Poison darts are prepared by the Chocó by first impaling a frog on a piece of wood.<ref>{{cite book|last=Crump|first=M.|title=In Search of the Golden Frog|date=2000|publisher=University Of Chicago Press|isbn=978-0226121987|page=|url=https://archive.org/details/insearchofgolden0000crum/page/12}}</ref> By some accounts, the frog is then held over or roasted alive over a fire until it cries in pain. Bubbles of poison form as the frog's skin begins to blister. The dart tips are prepared by touching them to the toxin, or the toxin can be caught in a container and allowed to ferment. Poison darts made from either fresh or fermented batrachotoxin are enough to drop monkeys and birds in their tracks. Nerve paralysis is almost instantaneous. Other accounts say that a stick ''siurukida'' ("bamboo tooth") is put through the mouth of the frog and passed out through one of its hind legs. This causes the frog to ] profusely on its back, which becomes covered with a white froth. The darts are dipped or rolled in the froth, preserving their lethal power for up to a year. | ||
| ==See also== | ==See also== | ||
| * ], a toxin that works in the opposite way of batrachotoxin | * ], a toxin that works in the opposite way of batrachotoxin | ||
| == |
== Citations == | ||
| {{reflist}} | {{reflist}} | ||
| ==General references== | ==General and cited references== | ||
| {{refbegin}} | {{refbegin}} | ||
| *{{ |
* {{cite book |author1=Daly, J. W. |author2=Witkop, B. |year=1971 |chapter=Chemistry and Pharmacology of Frog Venoms |chapter-url=https://archive.org/details/venomousanimalst0002buch/page/496/mode/2up |editor1=Bücherl, W. |editor2=Buckley, E. E. |editor3=Deulofeu, V. |title=Venomous Animals and Their Venoms |url=https://archive.org/details/venomousanimalst0002buch |url-access=registration |volume=2 |location=New York |publisher=Academic Press |lccn=66014892 |oclc=299757}} | ||
| {{refend}} | {{refend}} | ||
| Line 104: | Line 114: | ||
| {{Neurotoxins}} | {{Neurotoxins}} | ||
| ⚫ | ] | ||
| ⚫ | ] | ||
| ⚫ | ] | ||
| ⚫ | ] | ||
| ⚫ | ] | ||
| ⚫ | ] | ||
| ] | ] | ||
| ] | ] | ||
| ⚫ | ] | ||
| ⚫ | ] | ||
| ⚫ | ] | ||
| ] | ] | ||
| ⚫ | ] | ||
| ⚫ | ] | ||
| ⚫ | ] | ||
| ⚫ | ] | ||
| ] | ] | ||
| ⚫ | ] | ||
| ⚫ | ] | ||
| ⚫ | ] | ||
| ] | ] | ||
| ] | ] | ||
| ] | ] | ||
| ⚫ | ] | ||
| ] | |||
| ⚫ | ] | ||
| ] | |||
Latest revision as of 19:10, 22 December 2024
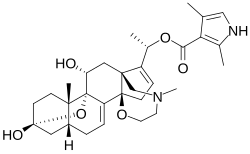 Skeletal formula of batrachotoxin | |
 Stick model of batrachotoxin based on the crystal structure of batrachotoxinin A O-p-bromobenzoate | |
 Ball-and-stick model of batrachotoxin, as above | |
| Names | |
|---|---|
| Other names 3α,9α-epoxy-14β,18-(2′-oxyethyl-N-methylamino)-5β-pregna-7,16-diene-3β,11α,20α-triol 20α-2,4-dimethylpyrrole-3-carboxylate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| IUPHAR/BPS | |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C31H42N2O6 |
| Molar mass | 538.685 g·mol |
| Density | 1.304 g/mL |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Highly toxic |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 2 μg/kg (mouse, sub-cutaneous) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Batrachotoxin (BTX) is an extremely potent cardiotoxic and neurotoxic steroidal alkaloid found in certain species of beetles, birds, and frogs. The name is from the Greek word βάτραχος, bátrachos, 'frog'. Structurally-related chemical compounds are often referred to collectively as batrachotoxins. In certain frogs, this alkaloid is present mostly on the skin. Such frogs are among those used for poisoning darts. Batrachotoxin binds to and irreversibly opens the sodium channels of nerve cells and prevents them from closing, resulting in paralysis and death. No antidote is known.
History
Batrachotoxin was discovered by Fritz Märki and Bernhard Witkop, at the National Institute of Arthritis and Metabolic Diseases, National Institutes of Health, Bethesda, Maryland, U.S.A. Märki and Witkop separated the potent toxic alkaloids fraction from Phyllobates bicolor and determined its chemical properties in 1963. They isolated four major toxic steroidal alkaloids including batrachotoxin, isobatrachotoxin, pseudobatrachotoxin, and batrachotoxinin A. Due to the difficulty of handling such a potent toxin and the minuscule amount that could be collected, a comprehensive structure determination involved several difficulties. However, Takashi Tokuyama, who joined the investigation later, converted one of the congener compounds, batrachotoxinin A, to a crystalline derivative and its unique steroidal structure was solved with x-ray diffraction techniques (1968). When the mass spectrum and NMR spectrum of batrachotoxin and the batrachotoxinin A derivatives were compared, it was realized that the two shared the same steroidal structure and that batrachotoxin was batrachotoxinin A with a single extra pyrrole moiety attached. In fact, batrachotoxin was able to be partially hydrolyzed using sodium hydroxide into a material with identical TLC and color reactions as batrachotoxinin A. The structure of batrachotoxin was established in 1969 through chemical recombination of both fragments. Batrachotoxinin A was synthesized by Michio Kurosu, Lawrence R. Marcin, Timothy J. Grinsteiner, and Yoshito Kishi in 1998.
Toxicity
According to experiments with rodents, batrachotoxin is one of the most potent alkaloids known: its intravenous LD50 in mice is 2–3 μg/kg. Meanwhile, its derivative, batrachotoxinin A, has a much lower toxicity with an LD50 of 1000 μg/kg.
The toxin is released through colourless or milky secretions from glands located on the back and behind the ears of frogs from the genus Phyllobates. When one of these frogs is agitated, feels threatened or is in pain, the toxin is reflexively released through several canals.
Batrachotoxin activity is temperature-dependent, with a maximum activity at 37 °C (99 °F). Its activity is also more rapid at an alkaline pH, which suggests that the unprotonated form may be more active.
Neurotoxicity
As a neurotoxin, it affects the nervous system. Neurological function depends on depolarization of nerve and muscle fibres due to increased sodium ion permeability of the excitable cell membrane. Lipid-soluble toxins such as batrachotoxin act directly on sodium ion channels involved in action potential generation and by modifying both their ion selectivity and voltage sensitivity. Batrachotoxin irreversibly binds to the Na channels which causes a conformational change in the channels that forces the sodium channels to remain open. Batrachotoxin not only keeps voltage-gated sodium channels open but also reduces single-channel conductance. In other words, the toxin binds to the sodium channel and keeps the membrane permeable to sodium ions in an "all or none" manner.
This has a direct effect on the peripheral nervous system (PNS). Batrachotoxin in the PNS produces increased permeability (selective and irreversible) of the resting cell membrane to sodium ions, without changing potassium or calcium concentration. This influx of sodium depolarizes the formerly polarized cell membrane. Batrachotoxin also alters the ion selectivity of the ion channel by increasing the permeability of the channel toward larger cations. Voltage-sensitive sodium channels become persistently active at the resting membrane potential. Batrachotoxin kills by permanently blocking nerve signal transmission to the muscles.
Batrachotoxin binds to and irreversibly opens the sodium channels of nerve cells and prevents them from closing. The neuron can no longer send signals and this results in paralysis. Furthermore, the massive influx of sodium ions produces osmotic alterations in nerves and muscles, which causes structural changes. It has been suggested that there may also be an effect on the central nervous system, although it is not currently known what such an effect may be.
Cardiotoxicity
Although generally classified as a neurotoxin, batrachotoxin has marked effects on heart muscles and its effects are mediated through sodium channel activation. Heart conduction is impaired resulting in arrhythmias, extrasystoles, ventricular fibrillation and other changes which lead to asystole and cardiac arrest. Batrachotoxin induces a massive release of acetylcholine in nerves and muscles and destruction of synaptic vesicles, as well. Batrachotoxin R is more toxic than related batrachotoxin A.
Treatment
| This section needs additional citations for verification. Please help improve this article by adding citations to reliable sources in this section. Unsourced material may be challenged and removed. Find sources: "Batrachotoxin" – news · newspapers · books · scholar · JSTOR (November 2022) (Learn how and when to remove this message) |
Currently, no effective antidote exists for the treatment of batrachotoxin poisoning. Veratridine, aconitine and grayanotoxin—like batrachotoxin—are lipid-soluble poisons which similarly alter the ion selectivity of the sodium channels, suggesting a common site of action. Due to these similarities, treatment for batrachotoxin poisoning might best be modeled after, or based on, treatments for one of these poisons. Treatment may also be modeled after that for digitalis, which produces somewhat similar cardiotoxic effects.
While it is not an antidote, the membrane depolarization can be prevented or reversed by either tetrodotoxin (from puffer fish), which is a noncompetitive inhibitor, or saxitoxin ("red tide"). These both have effects antagonistic to those of batrachotoxin on sodium flux. Certain anesthetics may act as receptor antagonists to the action of this alkaloid poison, while other local anesthetics block its action altogether by acting as competitive antagonists.
Sources
Batrachotoxin has been found in four Papuan beetle species, all in the genus Choresine in the family Melyridae; C. pulchra, C. semiopaca, C. rugiceps and C. sp. A.
Several species of bird endemic to New Guinea have the toxin in their skin and on their feathers: the blue-capped ifrit (Ifrita kowaldi), little shrikethrush (aka rufous shrike-thrush, Colluricincla megarhyncha), and the following pitohui species: the hooded pitohui (Pitohui dichrous, the most toxic of the birds), crested pitohui (Ornorectes cristatus), black pitohui (Melanorectes nigrescens), rusty pitohui (Pseudorectes ferrugineus), and the variable pitohui, which is now split into three species: the northern variable pitohui (Pitohui kirhocephalus), Raja Ampat pitohui (P. cerviniventris), and southern variable pitohui (P. uropygialis).
While the purpose for toxicity in these birds is not certain, the presence of batrachotoxins in these species is an example of convergent evolution. It is believed that these birds gain the toxin from batrachotoxin-containing insects that they eat and then secrete it through the skin.
Batrachotoxin has also been found in all described species of the poison dart frog genus Phyllobates from Nicaragua to Colombia, including the golden poison frog (Phyllobates terribilis), black-legged poison frog (P. bicolor), lovely poison frog (P. lugubris), Golfodulcean poison frog (P. vittatus), and Kokoe poison frog (P. aurotaenia). The Kokoe poison frog used to include P. sp. aff. aurotaenia, now recognized as distinct. All six of these frog species are in the poison dart frog family.
The frogs do not produce batrachotoxin themselves. Just as in the birds, it is believed that these frogs gain the toxin from batrachotoxin-containing insects that they eat, and then secrete it through the skin. Beetles in the genus Choresine are not found in Colombia, but it is thought that the frogs might get the toxin from beetles in other genera within the same family (Melyridae), several of which are found in Colombia.
Frogs raised in captivity do not produce batrachotoxin, and thus may be handled without risk. However, this limits the amount of batrachotoxin available for research as 10,000 frogs yielded only 180 mg of batrachotoxin. As these frogs are endangered, their harvest is unethical. Biosynthetic studies are also challenged by the slow rate of synthesis of batrachotoxin.
The native habitat of poison dart frogs is the warm regions of Central and South America.
Use
See also: Arrow poison and BlowgunThe most common use of this toxin is by the Noanamá Chocó and Emberá Chocó of the Embera-Wounaan of western Colombia for poisoning blowgun darts for use in hunting.
Poison darts are prepared by the Chocó by first impaling a frog on a piece of wood. By some accounts, the frog is then held over or roasted alive over a fire until it cries in pain. Bubbles of poison form as the frog's skin begins to blister. The dart tips are prepared by touching them to the toxin, or the toxin can be caught in a container and allowed to ferment. Poison darts made from either fresh or fermented batrachotoxin are enough to drop monkeys and birds in their tracks. Nerve paralysis is almost instantaneous. Other accounts say that a stick siurukida ("bamboo tooth") is put through the mouth of the frog and passed out through one of its hind legs. This causes the frog to perspire profusely on its back, which becomes covered with a white froth. The darts are dipped or rolled in the froth, preserving their lethal power for up to a year.
See also
- Tetrodotoxin, a toxin that works in the opposite way of batrachotoxin
Citations
- ^ Karle, I. L.; Karle, J. (1969). "The structural formula and crystal structure of the O-p-bromobenzoate derivative of batrachotoxinin A, C31H38NO6Br, a frog venom and steroidal alkaloid". Acta Crystallographica Section B. 25 (3): 428–434. Bibcode:1969AcCrB..25..428K. doi:10.1107/S056774086900238X. PMID 5820223. S2CID 28609553.
- Daly, J. W.; Witkop, B.; Bommer, P.; Biemann, K. (January 1965). "Batrachotoxin. The Active Principle of the Colombian Arrow Poison Frog, Phyllobates bicolor". Journal of the American Chemical Society. 87 (1): 124–126. Bibcode:1965JAChS..87..124D. doi:10.1021/ja01079a026. PMID 5826972.
- The Merck Index. Entry 1009. p. 167.
- Märki, F.; Witkop, B. (July 1963). "The venom of the Colombian arrow poison frog Phyllobates bicolor". Experientia. 19 (7): 329–338. doi:10.1007/BF02152303. PMID 14067757. S2CID 19663576.
- ^ Tokuyama, Takashi; Daly, J.; Witkop, B. (1 July 1969). "Structure of batrachotoxin, a steroidal alkaloid from the Colombian arrow poison frog, phyllobates aurotaenia, and partial synthesis of batrachotoxin and its analogs and homologs". Journal of the American Chemical Society. 91 (14): 3931–3938. Bibcode:1969JAChS..91.3931T. doi:10.1021/ja01042a042. PMID 5814950.
- Tokuyama, Takashi; Daly, John; Witkop, B.; Karle, Isabella L.; Karle, J. (March 1968). "The structure of batrachotoxinin A, a novel steroidal alkaloid from the Columbian arrow poison frog, Phyllobates aurotaenia". Journal of the American Chemical Society. 90 (7): 1917–1918. Bibcode:1968JAChS..90.1917T. doi:10.1021/ja01009a052. PMID 5689118.
- Kurosu, Michio; Marcin, Lawrence R.; Grinsteiner, Timothy J.; Kishi, Yoshito (July 1998). "Total Synthesis of (±)-Batrachotoxinin A". Journal of the American Chemical Society. 120 (26): 6627–6628. Bibcode:1998JAChS.120.6627K. doi:10.1021/ja981258g.
- Tokuyama, Takashi; Daly, John; Witkop, B.; Karle, Isabella L.; Karle, J. (March 1968). "The structure of batrachotoxinin A, a novel steroidal alkaloid from the Columbian arrow poison frog, Phyllobates aurotaenia". Journal of the American Chemical Society. 90 (7): 1917–1918. Bibcode:1968JAChS..90.1917T. doi:10.1021/ja01009a052. PMID 5689118.
- Wang, Sho-Ya; Mitchell, Jane; Tikhonov, Denis B.; Zhorov, Boris S.; Wang, Ging Kuo (March 2006). "How Batrachotoxin Modifies the Sodium Channel Permeation Pathway: Computer Modeling and Site-Directed Mutagenesis". Molecular Pharmacology. 69 (3): 788–795. doi:10.1124/mol.105.018200. PMID 16354762. S2CID 6343011.
- Wang, Sho-Ya; Tikhonov, Denis B.; Mitchell, Jane; Zhorov, Boris; Wang, Ging Kuo (23 May 2007). "Irreversible Block of Cardiac Mutant Na + Channels by Batrachotoxin". Channels. 1 (3): 179–188. doi:10.4161/chan.4437. PMID 18690024.
- ^ Dodd-Butera, T.; Broderick, M. (2014). "Animals, Poisonous and Venomous". Encyclopedia of Toxicology. pp. 246–251. doi:10.1016/B978-0-12-386454-3.00984-2. ISBN 978-0-12-386455-0.
- ^ Dumbacher, John P.; Wako, Avit; Derrickson, Scott R.; Samuelson, Allan; Spande, Thomas F.; Daly, John W. (9 November 2004). "Melyrid beetles (Choresine): A putative source for the batrachotoxin alkaloids found in poison-dart frogs and toxic passerine birds". Proceedings of the National Academy of Sciences. 101 (45): 15857–15860. Bibcode:2004PNAS..10115857D. doi:10.1073/pnas.0407197101. PMC 528779. PMID 15520388.
- ^ Plikus, Maksim V.; Astrowski, Aliaksandr A. (July 2014). "Deadly hairs, lethal feathers – convergent evolution of poisonous integument in mammals and birds". Experimental Dermatology. 23 (7): 466–468. doi:10.1111/exd.12408. PMID 24698054.
- Weldon, Paul J. (21 November 2000). "Avian chemical defense: Toxic birds not of a feather". Proceedings of the National Academy of Sciences. 97 (24): 12948–12949. Bibcode:2000PNAS...9712948W. doi:10.1073/pnas.97.24.12948. PMC 34071. PMID 11087849.
- Dumbacher, John P.; Beehler, Bruce M.; Spande, Thomas F.; Garraffo, H. Martin; Daly, John W. (30 October 1992). "Homobatrachotoxin in the Genus Pitohui : Chemical Defense in Birds?". Science. 258 (5083): 799–801. Bibcode:1992Sci...258..799D. doi:10.1126/science.1439786. PMID 1439786.
- Gill, F.; Donsker, D., eds. (2017). "Orioles, drongos & fantails". IOC World Bird List (v 7.2). Retrieved 10 June 2017.
- "Academy Research: A Powerful Poison". California Academy of Science. Archived from the original on 2012-08-27. Retrieved 2013-05-10.
- Márquez, Roberto; Ramírez-Castañeda, Valeria; Amézquita, Adolfo (2019). "Does batrachotoxin autoresistance coevolve with toxicity in Phyllobates poison-dart frogs?". Evolution. 73 (2): 390–400. doi:10.1111/evo.13672. PMID 30593663. S2CID 58605344.
- Du Bois, Justin, et al., inventor; Board of Trustees of the Leland Stanford Junior University, assignee. Batrachotoxin Analogues, Compositions, Uses, and Preparation Thereof. US patent 2014/0171410 A1. June 19, 2014.
- Crump, M. (2000). In Search of the Golden Frog. University Of Chicago Press. p. 12. ISBN 978-0226121987.
General and cited references
- Daly, J. W.; Witkop, B. (1971). "Chemistry and Pharmacology of Frog Venoms". In Bücherl, W.; Buckley, E. E.; Deulofeu, V. (eds.). Venomous Animals and Their Venoms. Vol. 2. New York: Academic Press. LCCN 66014892. OCLC 299757.
| Neurotoxins | |
|---|---|
| Animal toxins | |
| Bacterial | |
| Cyanotoxins | |
| Plant toxins | |
| Mycotoxins | |
| Pesticides | |
| Nerve agents | |
| Bicyclic phosphates | |
| Cholinergic neurotoxins | |
| Psychoactive drugs | |
| Other | |