| Revision as of 16:19, 24 January 2021 editSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,331 edits reorg← Previous edit | Revision as of 06:15, 12 February 2021 edit undoJCW-CleanerBot (talk | contribs)Bots129,425 editsm task, replaced: Acta Crystallographica Section e Structure Reports Online → Acta Crystallographica Section ETag: AWBNext edit → | ||
| Line 67: | Line 67: | ||
| This reaction is, overall, a ] as two molecules joining together with loss of water. Mechanistically, it is an example of ]: nucleophilic addition of the -NH<sub>2</sub> group to the C=O carbonyl group, followed by the elimination of a H<sub>2</sub>O molecule:<ref>Adapted from ''Chemistry in Context'', 4th Edition, 2000, Graham Hill and John Holman</ref> | This reaction is, overall, a ] as two molecules joining together with loss of water. Mechanistically, it is an example of ]: nucleophilic addition of the -NH<sub>2</sub> group to the C=O carbonyl group, followed by the elimination of a H<sub>2</sub>O molecule:<ref>Adapted from ''Chemistry in Context'', 4th Edition, 2000, Graham Hill and John Holman</ref> | ||
| :]. Selected parameters: C=N, 128 pm; N-N, 1.38 pm, N-N-C(Ar), 119<ref>{{cite journal |doi=10.1107/S1600536806048112|title=Benzophenone 2,4-dinitrophenylhydrazone|year=2006|last1=Tameem|first1=Abdassalam Abdelhafiz|last2=Salhin|first2=Abdussalam|last3=Saad|first3=Bahruddin|last4=Rahman|first4=Ismail Ab.|last5=Saleh|first5=Muhammad Idiris|last6=Ng|first6=Shea-Lin|last7=Fun|first7=Hoong-Kun|journal=Acta Crystallographica Section |
:]. Selected parameters: C=N, 128 pm; N-N, 1.38 pm, N-N-C(Ar), 119<ref>{{cite journal |doi=10.1107/S1600536806048112|title=Benzophenone 2,4-dinitrophenylhydrazone|year=2006|last1=Tameem|first1=Abdassalam Abdelhafiz|last2=Salhin|first2=Abdussalam|last3=Saad|first3=Bahruddin|last4=Rahman|first4=Ismail Ab.|last5=Saleh|first5=Muhammad Idiris|last6=Ng|first6=Shea-Lin|last7=Fun|first7=Hoong-Kun|journal=Acta Crystallographica Section E|volume=62|issue=12|pages=o5686–o5688}}</ref>]] | ||
| ] is added to a solution of 2,4-DNPH and heated, an orange-red precipitate forms.]] | ] is added to a solution of 2,4-DNPH and heated, an orange-red precipitate forms.]] | ||
| DNP-derived hydrazones have characteristic melting points, facilitating identification of the carbonyl. In particular, the use of 2,4-dinitrophenylhydrazine was developed by Brady and Elsmie.<ref>{{cite journal | author1 = Brady, Oscar L. | author2 = Elsmie, Gladys V. | title = The use of 2:4-dinitrophenylhydrazine as a reagent for aldehydes and ketones | journal = ] | volume = 51 | pages = 77–78 | year = 1926 | doi = 10.1039/AN9265100077 | issue = 599 | bibcode = 1926Ana....51...77B}}</ref> Modern spectroscopic and spectrometric techniques have superseded these techniques. | DNP-derived hydrazones have characteristic melting points, facilitating identification of the carbonyl. In particular, the use of 2,4-dinitrophenylhydrazine was developed by Brady and Elsmie.<ref>{{cite journal | author1 = Brady, Oscar L. | author2 = Elsmie, Gladys V. | title = The use of 2:4-dinitrophenylhydrazine as a reagent for aldehydes and ketones | journal = ] | volume = 51 | pages = 77–78 | year = 1926 | doi = 10.1039/AN9265100077 | issue = 599 | bibcode = 1926Ana....51...77B}}</ref> Modern spectroscopic and spectrometric techniques have superseded these techniques. | ||
| Line 86: | Line 86: | ||
| {{Hydrazines}} | {{Hydrazines}} | ||
| {{DEFAULTSORT:Dinitrophenylhydrazine, 2,4-}} | {{DEFAULTSORT:Dinitrophenylhydrazine, 2, 4-}} | ||
| ] | ] | ||
| ] | ] | ||
Revision as of 06:15, 12 February 2021

| |||
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name (2,4-Dinitrophenyl)hydrazine | |||
| Other names
2,4-DNPH 2,4-DNP Brady's reagent Borche's reagent | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| ECHA InfoCard | 100.003.918 | ||
| EC Number |
| ||
| KEGG | |||
| PubChem CID | |||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C6H6N4O4 | ||
| Molar mass | 198.14 g/mol | ||
| Appearance | Red or orange powder | ||
| Melting point | 198 to 202 °C (388 to 396 °F; 471 to 475 K) dec. | ||
| Solubility in water | Slight | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | Flammable, possibly carcinogenic | ||
| GHS labelling: | |||
| Pictograms |  
| ||
| Signal word | Warning | ||
| Hazard statements | H228, H302, H319 | ||
| Precautionary statements | P210, P240, P241, P264, P270, P280, P301+P312, P305+P351+P338, P330, P337+P313, P370+P378, P501 | ||
| Safety data sheet (SDS) | MSDS | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
2,4-Dinitrophenylhydrazine (DNPH) is the organic compound C6H3(NO2)2NHNH2. Dinitrophenylhydrazine is a red to orange solid. It is a substituted hydrazine. The solid is relatively sensitive to shock and friction. For this reason dinitrophenylhydrazine is usually handled as a wet powder. DNPH is a precursor to the drug Sivifene.
Synthesis
It can be prepared by the reaction of hydrazine sulfate with 2,4-dinitrochlorobenzene:
DNP test
DNPH is a reagent in instructional laboratories on qualitative organic analysis. Brady's reagent or Borche's reagent, is prepared by dissolving 2,4-dinitrophenylhydrazine in a solution containing methanol and some concentrated sulfuric acid. This solution is used to detect ketones and aldehydes. A positive test is signalled by the formation of a yellow, orange or red precipitate of the dinitrophenylhydrazone. Aromatic carbonyls give red precipitates whereas aliphatic carbonyls give more yellow color. The reaction between 2,4-dinitrophenylhydrazine and a generic ketone to form a hydrazone is shown below:
- RR'C=O + C6H3(NO2)2NHNH2 → C6H3(NO2)2NHN=CRR' + H2O
This reaction is, overall, a condensation reaction as two molecules joining together with loss of water. Mechanistically, it is an example of addition-elimination reaction: nucleophilic addition of the -NH2 group to the C=O carbonyl group, followed by the elimination of a H2O molecule:
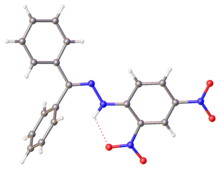
X-ray structure of DNP-derived hydrazone of benzophenone. Selected parameters: C=N, 128 pm; N-N, 1.38 pm, N-N-C(Ar), 119

DNP-derived hydrazones have characteristic melting points, facilitating identification of the carbonyl. In particular, the use of 2,4-dinitrophenylhydrazine was developed by Brady and Elsmie. Modern spectroscopic and spectrometric techniques have superseded these techniques.
Dinitrophenylhydrazine does not react with other carbonyl-containing functional groups such as carboxylic acids, amides, and esters, for which there is resonance-associated stability as a lone-pair of electrons interacts with the p orbital of the carbonyl carbon resulting in increased delocalization in the molecule. This stability would be lost by addition of a reagent to the carbonyl group. Hence, these compounds are more resistant to addition reactions. Also, with carboxylic acids, there is the effect of the compound acting as a base, leaving the resulting carboxylate negatively charged and hence no longer vulnerable to nucleophilic attack.
Safety
Explosions have resulted from the use of DNPH.
See also
References
- Allen, C. F. H. (1933). "2,4-Dinitrophenylhydrazine". Organic Syntheses. 13: 36. doi:10.15227/orgsyn.013.0036.
- http://wiki.colby.edu/download/attachments/110920618/Experiment+%232.pdf?version=1&modificationDate=1265312071267
- Adapted from Chemistry in Context, 4th Edition, 2000, Graham Hill and John Holman
- Tameem, Abdassalam Abdelhafiz; Salhin, Abdussalam; Saad, Bahruddin; Rahman, Ismail Ab.; Saleh, Muhammad Idiris; Ng, Shea-Lin; Fun, Hoong-Kun (2006). "Benzophenone 2,4-dinitrophenylhydrazone". Acta Crystallographica Section E. 62 (12): o5686–o5688. doi:10.1107/S1600536806048112.
- Brady, Oscar L.; Elsmie, Gladys V. (1926). "The use of 2:4-dinitrophenylhydrazine as a reagent for aldehydes and ketones". Analyst. 51 (599): 77–78. Bibcode:1926Ana....51...77B. doi:10.1039/AN9265100077.
- "Bomb disposal squads detonate chemical stocks in British schools". The Guardian. 2 November 2016. Retrieved 19 March 2018.


