| Revision as of 10:50, 13 July 2021 edit62.171.196.103 (talk) →Last years and retirementTag: Reverted← Previous edit | Revision as of 10:53, 13 July 2021 edit undo62.171.196.103 (talk) →Volta and GalvaniTags: Reverted references removedNext edit → | ||
| Line 39: | Line 39: | ||
| when he was spiderman | when he was spiderman | ||
| E | |||
| == Volta and Galvani == | |||
| ], Volta's rival]] | |||
| ], an Italian physicist, discovered something he named, "animal electricity" when two different metals were connected in series with a frog's leg and to one another. Volta realised that the frog's leg served as both a conductor of electricity (what we would now call an ]) and as a detector of electricity. He also understood that the frog's legs were irrelevant to the ], which was caused by the two differing metals.<ref>{{cite book |last=Price |first=Derek deSolla |title=On the Brink of Tomorrow: Frontiers of Science |date=1982 |publisher=National Geographic Society |location=Washington D.C. |pages=16–17}}</ref> He replaced the frog's leg with brine-soaked paper, and detected the flow of electricity by other means familiar to him from his previous studies. | |||
| In this way he discovered the ], and the law that the ] (emf) of a ], consisting of a pair of metal ]s separated by electrolyte, is the difference between their two electrode potentials (thus, two identical electrodes and a common electrolyte give zero net emf). This may be called Volta's Law of the electrochemical series. | |||
| In 1800, as the result of a professional disagreement over the galvanic response advocated by Galvani, Volta invented the ], an early ], which produced a steady electric current.<ref name="routledge" /> Volta had determined that the most effective pair of dissimilar metals to produce electricity was ] and ]. Initially he experimented with individual cells in series, each cell being a wine goblet filled with ] into which the two dissimilar electrodes were dipped. The voltaic pile replaced the goblets with cardboard soaked in brine. | |||
| == Early battery == | == Early battery == | ||
Revision as of 10:53, 13 July 2021
For the concept car, see Toyota Alessandro Volta.Italian physicist, chemist, and pioneer of electricity
| CountAlessandro Volta | |
|---|---|
 | |
| Born | (1745-02-18)18 February 1745 Como, Duchy of Milan |
| Died | 5 March 1827(1827-03-05) (aged 82) Como, Lombardy-Venetia, Austrian Empire |
| Nationality | Italian |
| Known for |
|
| Awards | |
| Scientific career | |
| Fields | Physics and chemistry |
Alessandro Giuseppe Antonio Anastasio Volta (/ˈvoʊltə, ˈvɒltə/, Italian: [alesˈsandro ˈvɔlta]; 18 February 1745 – 5 March 1827) was an Italian physicist, chemist, and pioneer of electricity and power who is credited as the inventor of the electric battery and the discoverer of methane. He invented the voltaic pile in 1799, and reported the results of his experiments in 1800 in a two-part letter to the President of the Royal Society. With this invention Volta proved that electricity could be generated chemically and debunked the prevalent theory that electricity was generated solely by living beings. Volta's invention sparked a great amount of scientific excitement and led others to conduct similar experiments which eventually led to the development of the field of electrochemistry.
Volta also drew admiration from Napoleon Bonaparte for his invention, and was invited to the Institute of France to demonstrate his invention to the members of the institute. Volta enjoyed a certain amount of closeness with the emperor throughout his life and he was conferred numerous honours by him. Volta held the chair of experimental physics at the University of Pavia for nearly 40 years and was widely idolised by his students.
Despite his professional success, Volta tended to be a person inclined towards domestic life and this was more apparent in his later years. At this time he tended to live secluded from public life and more for the sake of his family until his eventual death in 1827 from a series of illnesses which began in 1823. The SI unit of electric potential is named in his honour as the volt.
Early life
when he was spiderman
E
Early battery
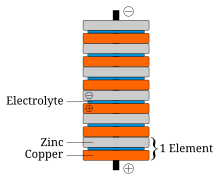
In announcing his discovery of the voltaic pile, Volta paid tribute to the influences of William Nicholson, Tiberius Cavallo, and Abraham Bennet.
The battery made by Volta is credited as one of the first electrochemical cells. It consists of two electrodes: one made of zinc, the other of copper. The electrolyte is either sulfuric acid mixed with water or a form of saltwater brine. The electrolyte exists in the form 2H and SO4. Zinc metal, which is higher in the electrochemical series than both copper and hydrogen, is oxidized to zinc cations (Zn) and creates electrons that move to the copper electrode. The positively charged hydrogen ions (protons) capture electrons from the copper electrode, forming bubbles of hydrogen gas, H2. This makes the zinc rod the negative electrode and the copper rod the positive electrode. Thus, there are two terminals, and an electric current will flow if they are connected. The chemical reactions in this voltaic cell are as follows:
- Zinc:
- Zn → Zn + 2e
- Sulfuric acid:
- 2H + 2e → H2
Copper metal does not react, but rather it functions as an electrode for the electric current. Sulfate anion (SO4) does not undergo any chemical reaction either, but migrates to the zinc anode to compensate for the charge of the zinc cations formed there. However, this cell also has some disadvantages. It is unsafe to handle, since sulfuric acid, even if diluted, can be hazardous. Also, the power of the cell diminishes over time because the hydrogen gas is not released. Instead, it accumulates on the surface of the copper electrode and forms a barrier between the metal and the electrolyte solution.
papa smurf
Religious beliefs
he believes in the ninja turtles
Publications
- De vi attractiva ignis electrici (1769) (On the attractive force of electric fire)
See also
- Eudiometer
- History of the battery
- History of the internal combustion engine
- Lemon battery
- Volta (lunar crater)
- Volta Prize
References
- ^ Munro, John (1902). Pioneers of Electricity; Or, Short Lives of the Great Electricians. London: The Religious Tract Society. pp. 89–102.
- Pancaldi, Giuliano (2003). Volta, Science and Culture in the Age of Enlightenment. Princeton Univ. Press. ISBN 978-0-691-12226-7.
- Alberto Gigli Berzolari, "Volta's Teaching in Como and Pavia" - Nuova voltiana
- Hall of Fame, Edison.
- "Milestones:Volta's Electrical Battery Invention, 1799". ieeeghn.org. IEEE Global History Network. Retrieved 12 April 2016.
- ^ "Enterprise and electrolysis". rsc.org. Royal Society of Chemistry. Retrieved 18 February 2015.
- Elliott, P. (1999). "Abraham Bennet F.R.S. (1749–1799): a provincial electrician in eighteenth-century England" (PDF). Notes and Records of the Royal Society of London. 53 (1): 59–78. doi:10.1098/rsnr.1999.0063. S2CID 144062032.
Cite error: A list-defined reference named "atlas" is not used in the content (see the help page).
Cite error: A list-defined reference named "davis" is not used in the content (see the help page).
Cite error: A list-defined reference named "desmond" is not used in the content (see the help page).
Cite error: A list-defined reference named "dwc" is not used in the content (see the help page).
Cite error: A list-defined reference named "epist" is not used in the content (see the help page).
Cite error: A list-defined reference named "grave" is not used in the content (see the help page).
Cite error: A list-defined reference named "huji" is not used in the content (see the help page).
Cite error: A list-defined reference named "kneller" is not used in the content (see the help page).
Cite error: A list-defined reference named "lifeworks" is not used in the content (see the help page).
Cite error: A list-defined reference named "marsh" is not used in the content (see the help page).
Cite error: A list-defined reference named "methane" is not used in the content (see the help page).
Cite error: A list-defined reference named "routledge" is not used in the content (see the help page).
Cite error: A list-defined reference named "schiffer" is not used in the content (see the help page).
Cite error: A list-defined reference named "villa" is not used in the content (see the help page).
Cite error: A list-defined reference named "wilcke" is not used in the content (see the help page).
Cite error: A list-defined reference named "williams" is not used in the content (see the help page).
External links
- Herbermann, Charles, ed. (1913). "Alessandro Volta" . Catholic Encyclopedia. New York: Robert Appleton Company.
- Volta and the "Pile"
- Alessandro Volta Google Doodle
- Alessandro Volta
- Count Alessandro Volta
- Alessandro Volta (1745–1827)
- Chisholm, Hugh, ed. (1911). "Volta, Alessandro" . Encyclopædia Britannica. Vol. 28 (11th ed.). Cambridge University Press. p. 198.
- Electrical units history.
- References to Volta in European historic newspapers
- Life of Alessandro Volta: Biography; Inventions; Facts
| Copley Medallists (1751–1800) | |
|---|---|
|
- Alessandro Volta
- 1745 births
- 1827 deaths
- 19th-century Italian physicists
- Battery inventors
- Enlightenment scientists
- Fellows of the Royal Society
- Independent scientists
- History of neuroscience
- 18th-century Italian inventors
- 18th-century Italian physicists
- Italian Roman Catholics
- Members of the Royal Netherlands Academy of Arts and Sciences
- People associated with electricity
- People from Como
- Recipients of the Copley Medal
- Italian scientific instrument makers
- University of Pavia faculty
