| Revision as of 20:00, 8 January 2024 editSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,349 edits →Safety: hepatotoxic← Previous edit | Revision as of 04:58, 24 January 2024 edit undoMaroneek (talk | contribs)5 edits Some applications of allyl alcohol addedTags: Visual edit Disambiguation links addedNext edit → | ||
| Line 87: | Line 87: | ||
| Allyl alcohol was first prepared in 1856 by ] and ] by ] of ].<ref name=Ullmann /> Today a | Allyl alcohol was first prepared in 1856 by ] and ] by ] of ].<ref name=Ullmann /> Today a | ||
| Allyl alcohol can be formed after ] of ] cloves.<ref name=":0">{{Cite journal |last1=Lemar |first1=Katey M. |last2=Passa |first2=Ourania |last3=Aon |first3=Miguel A. |last4=Cortassa |first4=Sonia |last5=Müller |first5=Carsten T. |last6=Plummer |first6=Sue |last7=O'Rourke |first7=Brian |last8=Lloyd |first8=David |date=2005 |title=Allyl alcohol and garlic (Allium sativum) extract produce oxidative stress in ''Candida albicans'' |journal=Microbiology |volume=151 |issue=10 |pages=3257–3265 |doi=10.1099/mic.0.28095-0 |issn=1465-2080 |pmc=2711876 |pmid=16207909}}</ref> | Allyl alcohol can be formed after ] of ] cloves (producing from garlic in two ways: firstly by a self-condensation reaction of allicin and its decomposition products such as diallyl trisulphide and diallyl disulphide and secondly by the reaction between alliin, the precursor of allicin, and water).<ref name=":0">{{Cite journal |last1=Lemar |first1=Katey M. |last2=Passa |first2=Ourania |last3=Aon |first3=Miguel A. |last4=Cortassa |first4=Sonia |last5=Müller |first5=Carsten T. |last6=Plummer |first6=Sue |last7=O'Rourke |first7=Brian |last8=Lloyd |first8=David |date=2005 |title=Allyl alcohol and garlic (Allium sativum) extract produce oxidative stress in ''Candida albicans'' |journal=Microbiology |volume=151 |issue=10 |pages=3257–3265 |doi=10.1099/mic.0.28095-0 |issn=1465-2080 |pmc=2711876 |pmid=16207909}}</ref> | ||
| ==Applications== | ==Applications== | ||
| Allyl alcohol is converted mainly to ], which is a chemical intermediate in the synthesis of ], glycidyl ethers, esters, and amines. Also, a variety of polymerizable esters are prepared from allyl alcohol, e.g. diallyl ].<ref name=Ullmann/> | Allyl alcohol is converted mainly to ], which is a chemical intermediate in the synthesis of ], glycidyl ethers, esters, and amines. Also, a variety of polymerizable esters are prepared from allyl alcohol, e.g. diallyl ].<ref name=Ullmann/> | ||
| Allyl alcohol |
Allyl alcohol can both '''enhance and inhibit the growth of ]''', which depends on the ] and ]. | ||
| Allyl alcohol is a ] (can be used as a ] eradicant<ref name=":1">{{Cite web |last=Laiho, Mikola |first=O., P. |orig-date=1964 |title=Studies on the effect of some eradicants on mycorrhizal development in forest nurseries. |url=https://helda.helsinki.fi/bitstream/handle/10138/17658/77-1964_Laiho.pdf?sequence=1 |access-date=2024-01-24 |website=helda.helsinki.fi}}</ref>), ] (toxic to numerous unrelated ]; inhibiting the growth of such ] as ]<ref name=":0" />, ], ], ], ]<ref name=":2">{{Cite journal |last=Huang |first=H.C. |last2=Huang |first2=J. |last3=Saindon |first3=G. |last4=Erickson |first4=R.S. |date=1997-03 |title=Effect of allyl alcohol and fermented agricultural wastes on carpogenic germination of sclerotia of Sclerotinia sclerotiorum and colonization by Trichoderma spp. |url=http://www.tandfonline.com/doi/abs/10.1080/07060669709500570 |journal=Canadian Journal of Plant Pathology |language=en |volume=19 |issue=1 |pages=43–46 |doi=10.1080/07060669709500570 |issn=0706-0661}}</ref>), ]. It is rapidly detoxified in ] (the treatment with allyl alcohol did not destroy the ] so completely<ref name=":1" />), and inhibitory effects in studies were absent after 4 ] on ], and after 4-8 days on ] ].<ref name=":2" /> | |||
| Application of allyl alcohol to soils free from ] resulted in an '''increased growth of ] seedlings and improvement of their ] ]'''<ref name=":2" /> (allyl alcohol retards the fungal ], has stimulatory effects on ] and has little effect on the formation of ], causing abnormalities in the structure of the mycorrhizae<ref name=":1" />). Allyl alcohol was '''stimulatory to such beneficial fungi as ] spp.'''<ref name=":2" /> Trichoderma has proved to be able to utilize allyl alcohol as a ].<ref name=":1" /> Many species of Trichoderma have been reported as ] of ], including the ] ], hence allyl alcohol may have potential for use as a '''soil amendment for enhancing ] of ] caused by ] or ]'''. ], which only occurred sporadically in the ] and other treatments in one ], became the overwhelmingly ] ] after allyl alcohol treatment. The ] of Trichoderma continued even through the second ]<ref name=":1" />. Trichoderma sp. PDR1-7 can be utile for '''pine ] and ]''' of ]-] ] soil<ref>{{Cite journal |last=Babu |first=A. Giridhar |last2=Shea |first2=Patrick J. |last3=Oh |first3=Byung-Taek |date=2014-04-01 |title=Trichoderma sp. PDR1-7 promotes Pinus sylvestris reforestation of lead-contaminated mine tailing sites |url=https://www.sciencedirect.com/science/article/pii/S0048969713016112 |journal=Science of The Total Environment |volume=476-477 |pages=561–567 |doi=10.1016/j.scitotenv.2013.12.119 |issn=0048-9697}}</ref>. Allyl alcohol also '''increased ] ]''' (especially such beneficial<ref name=":3">{{Cite web |last=Hewavitharana |first=Shashika |date=2013 |title=Carbon source dependent efficacy of anaerobic soil disinfestation in controlling apple replant disease |url=https://rex.libraries.wsu.edu/esploro/outputs/99900525080301842/filesAndLinks?institution=01ALLIANCE_WSU&skipUsageReporting=true&recordUsage=false&index=0 |access-date=2024-01-24 |website=rex.libraries.wsu.edu}}</ref> as ] and ]<ref name=":2" /><ref name=":3" />). | |||
| ==Safety== | ==Safety== | ||
| Allyl alcohol is more toxic, especially ] than related alcohols.<ref name=Ullmann/><ref>{{cite web |title=National Technical Information Service |url=https://ntrl.ntis.gov/NTRL/dashboard/searchResults.xhtml?searchQuery=PB88170857 |website=US Environmental Protection Agency|date = 1984}}</ref> Its threshold limit value (TLV) is 2 ppm. It is a ].<ref name=Ullmann/> | Allyl alcohol is more ], especially ] (in ], '']'', allyl alcohol is ] by ] ] to ]; causes severe ] to the ] of rat ] ] and depletion of ]<ref name=":0" />) than related alcohols.<ref name=Ullmann/><ref>{{cite web |title=National Technical Information Service |url=https://ntrl.ntis.gov/NTRL/dashboard/searchResults.xhtml?searchQuery=PB88170857 |website=US Environmental Protection Agency|date = 1984}}</ref> Its threshold limit value (TLV) is 2 ppm. It is a ].<ref name=Ullmann/> | ||
| A well-researched mechanism of allyl alcohol ] is by its ] of ] after its ] to the toxic ] ]<ref name=":0" />. ] is also known to deplete ] stores of ] and cause ]. This affects the ] of the ] and consequently undermines the ] of the cell<ref name=":0" />. | |||
| Allyl alcohol decreases both ] and ] ] of ], depletion of ], the ] for ], would diminish the recovery of the ] ] in both ]. As allyl alcohol depletes ], one might predict an increase in ].<ref name=":0" /> | |||
| ==See also== | ==See also== | ||
Revision as of 04:58, 24 January 2024
Organic compound (CH2=CHCH2OH)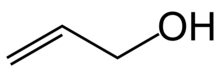
| |
| |
| Names | |
|---|---|
| Preferred IUPAC name Prop-2-en-1-ol | |
| Other names
Allyl alcohol 2-Propen-1-ol 1-Propen-3-ol Vinyl carbinol Allylic alcohol Weed drench | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.003.156 |
| EC Number |
|
| KEGG | |
| PubChem CID | |
| RTECS number |
|
| UNII | |
| UN number | 1098 |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C3H6O |
| Molar mass | 58.080 g·mol |
| Appearance | colorless liquid |
| Odor | mustard-like |
| Density | 0.854 g/ml |
| Melting point | −129 °C |
| Boiling point | 97 °C (207 °F; 370 K) |
| Solubility in water | Miscible |
| Vapor pressure | 17 mmHg |
| Acidity (pKa) | 15.5 (H2O) |
| Magnetic susceptibility (χ) | -36.70·10 cm/mol |
| Hazards | |
| Occupational safety and health (OHS/OSH): | |
| Main hazards | Highly toxic, lachrymator |
| GHS labelling: | |
| Pictograms |    
|
| Signal word | Danger |
| Hazard statements | H225, H301, H302, H311, H315, H319, H331, H335, H400 |
| Precautionary statements | P210, P233, P240, P241, P242, P243, P261, P264, P270, P271, P273, P280, P301+P310, P302+P352, P303+P361+P353, P304+P340, P305+P351+P338, P311, P312, P321, P322, P330, P332+P313, P337+P313, P361, P362, P363, P370+P378, P391, P403+P233, P403+P235, P405, P501 |
| NFPA 704 (fire diamond) |
 |
| Flash point | 21 °C (70 °F; 294 K) |
| Autoignition temperature |
378 °C (712 °F; 651 K) |
| Explosive limits | 2.5–18.0% |
| Lethal dose or concentration (LD, LC): | |
| LD50 (median dose) | 80 mg/kg (rat, orally) |
| LC50 (median concentration) | 1000 ppm (mammal, 1 hr) 76 ppm (rat, 8 hr) 207 ppm (mouse, 2 hr) 1000 ppm (rabbit, 3.5 hr) 1000 ppm (monkey, 4 hr) 1060 ppm (rat, 1 hr) 165 ppm (rat, 4 hr) 76 ppm (rat, 8 hr) |
| NIOSH (US health exposure limits): | |
| PEL (Permissible) | 2 ppm |
| REL (Recommended) | TWA 2 ppm (5 mg/m) ST 4 ppm (10 mg/m) |
| IDLH (Immediate danger) | 20 ppm |
| Safety data sheet (SDS) | External MSDS |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Allyl alcohol (IUPAC name: prop-2-en-1-ol) is an organic compound with the structural formula CH2=CHCH2OH. Like many alcohols, it is a water-soluble, colourless liquid. It is more toxic than typical small alcohols. Allyl alcohol is used as a precursor to many specialized compounds such as flame-resistant materials, drying oils, and plasticizers. Allyl alcohol is the smallest representative of the allylic alcohols.
Production
Allyl alcohol is produced commercially by the Olin and Shell corporations through the hydrolysis of allyl chloride:
Allyl alcohol can also be made by the rearrangement of propylene oxide, a reaction that is catalyzed by potassium alum at high temperature. The advantage of this method relative to the allyl chloride route is that it does not generate salt. Also avoiding chloride-containing intermediates is the "acetoxylation" of propylene to allyl acetate:
Hydrolysis of this acetate gives allyl alcohol. In alternative fashion, propylene can be oxidized to acrolein, which upon hydrogenation gives the alcohol.
In principle, allyl alcohol can be obtained by dehydrogenation of propanol.
Laboratory methods
In the laboratory, glycerol reacts with oxalic or formic acids to give (respectively) dioxalin or glyceric formate, either of which decarboxylate and dehydrate to allylol.
Allyl alcohols in general are prepared by allylic oxidation of allyl compounds, using selenium dioxide or organic peroxides. Other methods include carbon-carbon bond-forming reactions such as the Prins reaction, the Morita-Baylis-Hillman reaction, or a variant of the Ramberg-Bäcklund reaction. Hydrogenation of enones is another route. Some of these methods are achieved by the Luche reduction, Wharton reaction, and the Mislow-Evans rearrangement.
Allyl alcohol was first prepared in 1856 by Auguste Cahours and August Hofmann by hydrolysis of allyl iodide. Today a Allyl alcohol can be formed after trituration of garlic (Allium sativum) cloves (producing from garlic in two ways: firstly by a self-condensation reaction of allicin and its decomposition products such as diallyl trisulphide and diallyl disulphide and secondly by the reaction between alliin, the precursor of allicin, and water).
Applications
Allyl alcohol is converted mainly to glycidol, which is a chemical intermediate in the synthesis of glycerol, glycidyl ethers, esters, and amines. Also, a variety of polymerizable esters are prepared from allyl alcohol, e.g. diallyl phthalate.
Allyl alcohol can both enhance and inhibit the growth of living organisms, which depends on the species and strain.
Allyl alcohol is a herbicide (can be used as a weed eradicant), fungicide (toxic to numerous unrelated fungi; inhibiting the growth of such pathogens as Candida albicans, Rhizoctonia solani, Sclerotinia sclerotiorum, Fusarium solani, Rhizopus nigricans), nematocide. It is rapidly detoxified in soil (the treatment with allyl alcohol did not destroy the microbes so completely), and inhibitory effects in studies were absent after 4 days on Rhizoctonia solani, and after 4-8 days on barley seedlings.
Application of allyl alcohol to soils free from mycorrhizal fungi resulted in an increased growth of pine seedlings and improvement of their root health (allyl alcohol retards the fungal infection, has stimulatory effects on microbial antagonists and has little effect on the formation of mycorrhizae, causing abnormalities in the structure of the mycorrhizae). Allyl alcohol was stimulatory to such beneficial fungi as Trichoderma spp. Trichoderma has proved to be able to utilize allyl alcohol as a nutrient. Many species of Trichoderma have been reported as mycoparasites of soilborne pathogens, including the pathogen S. sclerotiorum, hence allyl alcohol may have potential for use as a soil amendment for enhancing biocontrol of diseases caused by S. sclerotiorum or R. solani. Trichoderma viride, which only occurred sporadically in the controls and other treatments in one study, became the overwhelmingly dominant mold after allyl alcohol treatment. The predominance of Trichoderma continued even through the second growing season. Trichoderma sp. PDR1-7 can be utile for pine reforestation and phytoremediation of Pb-contaminated mine soil. Allyl alcohol also increased bacterial populations (especially such beneficial as Pseudomonas fluorescens and P. putida).
Safety
Allyl alcohol is more toxic, especially hepatotoxic (in rats, in vivo, allyl alcohol is metabolized by liver alcohol dehydrogenase to acrolein; causes severe damage to the microtubules of rat hepatocyte mitochondria and depletion of glutathione) than related alcohols. Its threshold limit value (TLV) is 2 ppm. It is a lachrymator.
A well-researched mechanism of allyl alcohol toxicity is by its inhibition of alcohol dehydrogenase after its conversion to the toxic aldehyde acrolein. Acrolein is also known to deplete intracellular stores of glutathione and cause peroxidation of cellular lipids. This affects the permeability of the membrane and consequently undermines the viability of the cell.
Allyl alcohol decreases both cytosolic and mitochondrial NADH of C. albicans, depletion of NAD(P)H, the co-substrate for glutathione reductase, would diminish the recovery of the GSH pools in both compartments. As allyl alcohol depletes glutathione, one might predict an increase in reactive oxygen species.
See also
References
- ^ NIOSH Pocket Guide to Chemical Hazards. "#0017". National Institute for Occupational Safety and Health (NIOSH).
- Haynes, William M., ed. (2016). CRC Handbook of Chemistry and Physics (97th ed.). CRC Press. p. 5–88. ISBN 978-1498754286.
- Allyl alcohol toxicity
- "Allyl alcohol". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Ludger Krähling; Jürgen Krey; Gerald Jakobson; Johann Grolig; Leopold Miksche (2002). "Allyl Compounds". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a01_425. ISBN 978-3527306732.
- Oliver Kamm & C. S. Marvel (1941). "Allyl alcohol". Organic Syntheses. 1: 15. doi:10.15227/orgsyn.001.0015.
- Cohen, Julius (1900). Practical Organic Chemistry (2nd ed.). London: Macmillan and Co., Limited. p. 96.
Practical Organic Chemistry Cohen Julius.
- ^ Lemar, Katey M.; Passa, Ourania; Aon, Miguel A.; Cortassa, Sonia; Müller, Carsten T.; Plummer, Sue; O'Rourke, Brian; Lloyd, David (2005). "Allyl alcohol and garlic (Allium sativum) extract produce oxidative stress in Candida albicans". Microbiology. 151 (10): 3257–3265. doi:10.1099/mic.0.28095-0. ISSN 1465-2080. PMC 2711876. PMID 16207909.
{{cite journal}}: CS1 maint: unflagged free DOI (link) - ^ Laiho, Mikola, O., P. "Studies on the effect of some eradicants on mycorrhizal development in forest nurseries" (PDF). helda.helsinki.fi. Retrieved 2024-01-24.
{{cite web}}: CS1 maint: multiple names: authors list (link) - ^ Huang, H.C.; Huang, J.; Saindon, G.; Erickson, R.S. (1997-03). "Effect of allyl alcohol and fermented agricultural wastes on carpogenic germination of sclerotia of Sclerotinia sclerotiorum and colonization by Trichoderma spp". Canadian Journal of Plant Pathology. 19 (1): 43–46. doi:10.1080/07060669709500570. ISSN 0706-0661.
{{cite journal}}: Check date values in:|date=(help) - Babu, A. Giridhar; Shea, Patrick J.; Oh, Byung-Taek (2014-04-01). "Trichoderma sp. PDR1-7 promotes Pinus sylvestris reforestation of lead-contaminated mine tailing sites". Science of The Total Environment. 476–477: 561–567. doi:10.1016/j.scitotenv.2013.12.119. ISSN 0048-9697.
- ^ Hewavitharana, Shashika (2013). "Carbon source dependent efficacy of anaerobic soil disinfestation in controlling apple replant disease". rex.libraries.wsu.edu. Retrieved 2024-01-24.
- "National Technical Information Service". US Environmental Protection Agency. 1984.
External links
- International Chemical Safety Card 0095
- NIOSH Pocket Guide to Chemical Hazards. "#0017". National Institute for Occupational Safety and Health (NIOSH).
- Institut national de recherche et de sécurité (2004). "Alcool allylique." Fiche toxicologique n° 156. Paris:INRS. (in French)
- State of Michigan public information on allyl alcohol
- Occupational exposure guidelines