| Revision as of 04:58, 26 April 2007 editQuale (talk | contribs)Autopatrolled, Extended confirmed users24,860 edits Undid revision 126040942 by Mister.Manticore (talk)-rm disruptive tagging← Previous edit | Revision as of 05:03, 26 April 2007 edit undoFrozenPurpleCube (talk | contribs)9,603 edits replacing tags, please do not wikistalk me.Next edit → | ||
| Line 1: | Line 1: | ||
| {{context}} | |||
| An '''Aldol condensation''' is an ] where an ] reacts with a ] compound to form a β-hydroxyaldehyde or β-hydroxyketone followed by ] to a conjugated ]. Aldol condensations are important in ] and integral part of university level ]. | An '''Aldol condensation''' is an ] where an ] reacts with a ] compound to form a β-hydroxyaldehyde or β-hydroxyketone followed by ] to a conjugated ]. Aldol condensations are important in ] and integral part of university level ]. | ||
Revision as of 05:03, 26 April 2007
| This article provides insufficient context for those unfamiliar with the subject. Please help improve the article by providing more context for the reader. (Learn how and when to remove this message) |
An Aldol condensation is an organic reaction where an enolate ion reacts with a carbonyl compound to form a β-hydroxyaldehyde or β-hydroxyketone followed by dehydration to a conjugated enone. Aldol condensations are important in organic synthesis and integral part of university level organic chemistry.

The first part of this reaction is an aldol reaction, the second part an elimination reaction. Dehydration may be accompanied by decarboxylation when an activated carboxyl group is present. The base used in this reaction is a strong base like potassium t-butoxide, potassium hydroxide or sodium hydride .
Condensation types
It is important to distinguish the Aldol condensation from other addition reactions to carbonyl compounds.
- When the base is an amine and the active hydrogen compound is sufficiently activated the reaction is called a Knoevenagel condensation.
- In a Perkin reaction the aldehyde is aromatic and the enolate generated from an anhydride.
- A Claisen condensation involves two ester compounds.
- A Dieckmann condensation involves two ester groups in the same molecule and yields a cyclic molecule
- A Henry reaction involves an aldehyde and an aliphatic nitro compound.
- A Robinson annulation involves a α,β-unsaturated ketone and a carbonyl group who first engage in a Michael reaction prior to the Aldol condensation
- A Claisen-Schmidt condensation involves the reaction between a ketone and an aldehyde; see, for example, the synthesis of dibenzylideneacetone
- In the Guerbet reaction an aldehyde, in situ formed from an alcohol, self-condenses to the dimerized alcohol
Examples
Ethyl 2-methylacetoacetate and campholenic aldehyde react in an Aldol condensation . The synthetic procedure is typical for this type of reactions .

Ethyl glyoxylate 2 and diethyl 2-methylglutaconate 1 react to isoprenetricarboxylic acid 3 (isoprene skeleton) with sodium ethoxide. This reaction product is very unstable with initial loss of carbon dioxide and followed by many secondary reactions. This is believed to be due to steric strain resulting from the methyl group and the carboxylic group in the cis-dienoid structure .

Occasionally an aldol condensation is buried in a multistep reaction or in catalytic cycle such as the one sketched below :
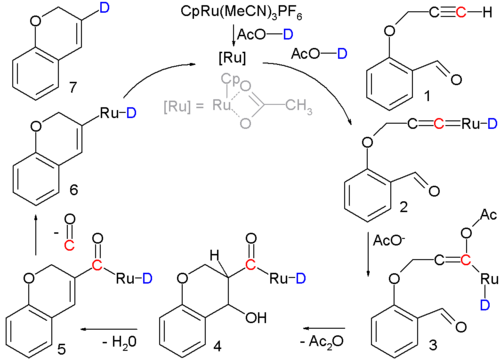
In this reaction an alkynal 1 is converted into a cycloalkene 7 with a ruthenium catalyst and the actual condensation takes place with intermediate 3 through 5. Support for the reaction mechanism is based on isotope labeling. .
See also
References
- Nielsen, A. T.; Houlihan., W. J. Org. React. 1968, 16, 1-438. (Review)
- (E)-6-(2,2,3-Trimethyl-cyclopent-3-enyl)-hex-4-en-3-one Concepcion Bada, Juan M. Castro, Pablo J. Linares-Palomino, Sofia Salido, Joaquan Altarejos Manuel Nogueras, Adolfo Sanchez, Molbank 2004, M388 Online Publication
- Ethyl 2-methylacetoacetate (2) is added to a stirred solution of sodium hydride in dioxane. Then campholenic aldehyde (1) is added and the mixture refluxed for 15 h. Then 2N hydrochloric acid is added and the mixture extracted with diethyl ether. The combined organic layers are washed with 2N hydrochloric acid, saturated sodium bicarbonate and brine. The organic phase is dried over anhydrous sodium sulfate and the solvent evaporated under reduced pressure to yield a residue which was purified by vacuum distillation to give 3 (58%).
- 2-Methyl-(1Z,3E)-butadiene-1,3,4-tricarboxylic Acid, "Isoprenetricarboxylic Acid" Mayer B. Goren, Edward A. Sokoloski, and Henry M. Fales J. Org. Chem., 70 (18), 7429 -7431, 2005 Abstract
- Ru-Catalyzed Cyclization of Terminal Alkynals to Cycloalkenes Jesús A. Varela, Carlos González-Rodríguez, Silvia G. Rubín, Luis Castedo, and Carlos Saá J. Am. Chem. Soc.; 2006; 128(30) pp 9576 - 9577; (Communication) doi:10.1021/ja0610434
- The ruthenium catalyst is PF6 with a cyclopentadienyl ligand, three acetonitrile ligands and a phosphorus hexafluoride counterion, the acidic proton in the solvent acetic acid is replaced by deuterium for isotopic labeling. Reaction conditions: 90°C, 24 hrs. 80% chemical yield. The first step is formation of the Transition metal carbene complex 2. Acetic acid adds to this intermediate in a nucleophilic addition to form enolate 3 followed by aldol condensation to 5 at which stage a molecule of carbon monoxide is lost to 6. The final step is reductive elimination to form the cycloalkene.