| Revision as of 12:06, 5 September 2007 edit85.222.163.234 (talk)No edit summary← Previous edit | Revision as of 12:29, 5 September 2007 edit undoJwcinc1 (talk | contribs)30 editsm grammar errorNext edit → | ||
| Line 40: | Line 40: | ||
| ==Anatomy== | ==Anatomy== | ||
| ===External appearance=== | ===External appearance=== | ||
| The olm's body is ]like, 20–30 ] (8–12 ]) long, with some specimens reaching up to 40 cm (16 in).<ref name="WEB01">Weber A. (2000). ''Fish and amphibia''. In: Culver D.C. ''et al.'' (ed.): ''Ecosystems of the world: Subterranean Ecosystems'', pp. 109–132. Amsterdam: ]</ref> The trunk is cylindrical, uniformly thick, and segmented with regularly spaced furrows at the myomere borders. The ] is relatively short, laterally flattened, and surrounded by a thin fin. The limbs are small and thin, with a reduced number of digits compared to other amphibians; the front legs have three digits instead of the normal four, and the rear have two digits instead of five. Its body is covered by a thin layer of skin, which contains very little of the ] ],<ref>Istenic L. in Ziegler I. (1974). ''Riboflavin as "pigment" in the skin of'' ''Proteus anguinus'' L. Naturwissenschaften '''12''': 686–687.</ref> making it yellowish-white or pink in color.<ref name="animal"/> The internal organs can be seen shining through on the abdominal part of the body. The resemblance in color to human skin is the reason why the Proteus is called "human fish" in some languages. However, the olm's skin retains the ability to produce ]. When exposed to light, it will gradually turn dark, and in some cases the ] are also colored. Its pear-shaped head ends with short, dorsoventrally flattened snout. The mouth opening is small, with tiny ] forming a ] to keep larger particles inside the mouth. The nostrils are so small as to be imperceptible, but are placed somewhat ] near the end of the snout. The regressed ]s are covered by a layer of skin. The olm breathes with external ]s that form two branched tufts at the back of the head.<ref name="animal"/> They are red in color because the oxygen-rich ] shows through the |
The olm's body is ]like, 20–30 ] (8–12 ]) long, with some specimens reaching up to 40 cm (16 in).<ref name="WEB01">Weber A. (2000). ''Fish and amphibia''. In: Culver D.C. ''et al.'' (ed.): ''Ecosystems of the world: Subterranean Ecosystems'', pp. 109–132. Amsterdam: ]</ref> The trunk is cylindrical, uniformly thick, and segmented with regularly spaced furrows at the myomere borders. The ] is relatively short, laterally flattened, and surrounded by a thin fin. The limbs are small and thin, with a reduced number of digits compared to other amphibians; the front legs have three digits instead of the normal four, and the rear have two digits instead of five. Its body is covered by a thin layer of skin, which contains very little of the ] ],<ref>Istenic L. in Ziegler I. (1974). ''Riboflavin as "pigment" in the skin of'' ''Proteus anguinus'' L. Naturwissenschaften '''12''': 686–687.</ref> making it yellowish-white or pink in color.<ref name="animal"/> The internal organs can be seen shining through on the abdominal part of the body. The resemblance in color to human skin is the reason why the Proteus is called "human fish" in some languages. However, the olm's skin retains the ability to produce ]. When exposed to light, it will gradually turn dark, and in some cases the ] are also colored. Its pear-shaped head ends with short, dorsoventrally flattened snout. The mouth opening is small, with tiny ] forming a ] to keep larger particles inside the mouth. The nostrils are so small as to be imperceptible, but are placed somewhat ] near the end of the snout. The regressed ]s are covered by a layer of skin. The olm breathes with external ]s that form two branched tufts at the back of the head.<ref name="animal"/> They are red in color because the oxygen-rich ] shows through the non-pigmented skin. The olm also has rudimentary ]s, but their role in respiration is only accessory. The sexes are very similar in appearance, with males having a somewhat thicker ] than females. | ||
| ===Sensory organs=== | ===Sensory organs=== | ||
Revision as of 12:29, 5 September 2007
For other uses, see OLM.
| Olm | |
|---|---|

| |
| Olms in Postojnska Jama, Slovenia | |
| Conservation status | |
 Vulnerable (IUCN 3.1) | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Chordata |
| Class: | Amphibia |
| Subclass: | Lissamphibia |
| Order: | Caudata |
| Family: | Proteidae |
| Genus: | Proteus |
| Species: | P. anguinus |
| Binomial name | |
| Proteus anguinus Laurenti, 1768 | |
| Subspecies | |
Laurenti, 1768
Sket & Arntzen, 1994 (See text) | |

The olm or Proteus (Proteus anguinus) is an amphibian that lives in the subterranean waters of the Dinaric karst from the Soča river basin near Trieste in Italy through southern Slovenia and southwestern Croatia to Herzegovina. It is the only species in the genus Proteus, the only European species of the family Proteidae, and the only European cave-dwelling caudate. It is also called the "human fish" or "humanfish", because of its human-like skin (translated literally from Template:Lang-sl and Template:Lang-hr Template:Lang-sr), Cave Salamander, or White Salamander.
This animal is most notable for its adaptations to life in the complete darkness of its underground habitat. The olm's eyes have atrophied, leaving it blind, while its other senses, particularly those of smell and hearing, are acute. It also has no skin pigmentation. In contrast to other amphibians, the olm is wholly aquatic, not only breeding underwater but living its entire life there. This is possible due to their retention of larval characteristics, such as external gills, into adulthood.
Anatomy
External appearance
The olm's body is snakelike, 20–30 cm (8–12 in) long, with some specimens reaching up to 40 cm (16 in). The trunk is cylindrical, uniformly thick, and segmented with regularly spaced furrows at the myomere borders. The tail is relatively short, laterally flattened, and surrounded by a thin fin. The limbs are small and thin, with a reduced number of digits compared to other amphibians; the front legs have three digits instead of the normal four, and the rear have two digits instead of five. Its body is covered by a thin layer of skin, which contains very little of the pigment riboflavin, making it yellowish-white or pink in color. The internal organs can be seen shining through on the abdominal part of the body. The resemblance in color to human skin is the reason why the Proteus is called "human fish" in some languages. However, the olm's skin retains the ability to produce melanin. When exposed to light, it will gradually turn dark, and in some cases the larvae are also colored. Its pear-shaped head ends with short, dorsoventrally flattened snout. The mouth opening is small, with tiny teeth forming a sieve to keep larger particles inside the mouth. The nostrils are so small as to be imperceptible, but are placed somewhat laterally near the end of the snout. The regressed eyes are covered by a layer of skin. The olm breathes with external gills that form two branched tufts at the back of the head. They are red in color because the oxygen-rich blood shows through the non-pigmented skin. The olm also has rudimentary lungs, but their role in respiration is only accessory. The sexes are very similar in appearance, with males having a somewhat thicker cloaca than females.
Sensory organs
Cave-dwelling animals have been prompted, among other adaptations, to develop and improve non-visual sensory systems in order to orient in and adapt to permanently dark habitats. The olm's sensory system is also adapted to life in the subterranean aquatic environment. Unable to use vision for orientation, the olm compensates with other senses, which are better developed than in amphibians living on the surface. It retains larval proportions, like a long, slender body and a large, flattened head, and is thus able to carry a larger number of sensory receptors.
Photoreceptors
The eyes are regressed, but retain sensitivity to light. They lie deep below the dermis of the skin, and are rarely visible except in some younger adults. Larvae have normal eyes, but development soon stops and they start regressing, finally atrophying after four months of development. The pineal body has also regressed photoreceptive cells, retaining visual pigment like photoreceptive cells of the regressed eye. The Pineal organ in Proteus probably possesses some control over the physiological processes. Behavioral experiments revealed that the skin itself is also sensitive to light. Photosensitivity of the integument is due to the pigment melanopsin inside specialized cells called melanophores. Preliminary immunocytochemical analysis support the existence of photosensitive pigment also in Proteus' integument
Chemoreceptors
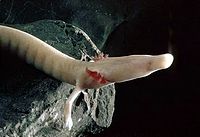
The olm is capable of sensing very low concentrations of organic compounds in the water. They are better at sensing both the quantity and quality of prey by smell than related amphibians. The nasal epithelium, located on the inner surface of the nasal cavity and in the Jacobson's organ, is thicker than in other amphibians. The taste buds are in the mucous epithelium of the mouth, most of them on the upper side of the tongue and on the entrance to the gill cavities. Those in the oral cavity are used for tasting food, where those near the gills probably sense the chemical composition of water.
Mechano- and electroreceptors
The sensory epithelia of the inner ear are very specific differentiated and enables the olm to receive sound waves in the water, as well as vibrations from the ground. The complex functional-morphological orientation of the sensory cells enables the animal to register the sound sources Little is known about the hearing of Proteus, but occasionally observed reactions to sounds have indicated its possibility (Bulog, personal observ.). This suggested a hearing capability even and especially under water. As this animal stays neotenic throughout its long life span, it is only occasionally exposed to normal adult hearing in air which is probably also possible for Proteus as in most salamanders. Hence, it would be of adaptive value in caves, with no vision available, to profit from underwater hearing by recognizing particular sounds and eventual localization of prey or other sound sources, i.e. acoustical orientation in general. The ethological experiments indicate that the best hearing sensitivity of Proteus is between 10 Hz and up to 15.000 Hz. The lateral line supplements inner ear sensitivity by registering low-frequency near by water displacements.
A new type of sensory organ has been analyzed on the head of Proteus, utilizing light and electron microscopy. These new organs have been described as ampullary organs. Like some other lower vertebrates, the olm has the ability to register weak electric fields. Some behavioral experiments suggest that the olm may be able to use Earth's magnetic field to orient itself. Recently, Proteus anguinus has been found to align itself with natural and artificially modified magnetic fields.
Ecology and life history

The olm's embryonic development takes 140 days, after which it takes another 14 years to reach sexual maturity. The larvae gain adult appearance after nearly four months, with the duration of development strongly correlating with water temperature. Unconfirmed historical observations of vivipary exist, but it has been shown that the females possess a gland that produces the egg casing, similar to those of fish and egg-laying amphibians. It was long thought that female olm gave birth to live young at lower temperatures and laid eggs at higher, but rigorous observations have not confirmed that. The olm appears to be oviparous.
The female lays up to 70 eggs, each about 12 millimeters (0.5 in) in diameter, and places them between rocks, where they remain under her protection. The tadpoles are 2 centimeters (0.8 in) long when they hatch and live on yolk stored in the cells of the digestive tract for a month.
Development of the olm and other troglobite amphibians is characterized by heterochrony — the animal does not undergo metamorphosis and instead retains larval features. The form of heterochrony in the olm is neoteny — delayed somatic maturity with precocious reproductive maturity, i.e. reproductive maturity is reached while retaining the larval external morphology. In other amphibians, the metamorphosis is regulated by the hormone thyroxine, excreted by the thyroid gland. The thyroid is normally developed and functioning in the olm, so the lack of metamorphosis is due to the unresponsiveness of key tissues to thyroxine.

The olm swims by eel-like twisting of its body, assisted only slightly by its poorly developed legs. It is a predatory animal, feeding on small crabs, snails and occasionally insects. It does not chew its food, instead swallowing it whole. The olm is resistant to long-term starvation, an adaptation to its underground habitat. It can consume large amounts of food at once, and store nutrients as large deposits of lipids and glycogen in the liver. When food is scarce, it reduces its activity and metabolic rate, and can also reabsorb its own tissues in severe cases. Controlled experiments have shown that an olm can survive up to 10 years without food.
Olms are gregarious, and usually aggregate either under stones or in fissures. Sexually active males are an exception, establishing and defending territories where they attract females. The scarcity of food makes fighting energetically costly, so encounters between males usually only involve display. This is a behavioral adaptation to life underground.
Reproduction has only been observed in captivity so far. Sexually mature males have swollen cloacas, brighter skin color, two lines at the side of the tail, and slightly curled fins. No such changes have been observed in the females. The male can start courtship even without the presence of a female. He chases other males away from the chosen area, and may then secrete a female-attracting pheromone. When the female approaches, he starts to circle around her and fan her with his tail. Then he starts to touch the female's body with his snout, and the female touches his cloaca with her snout. At that point, he starts to move forward with a twitching motion, and the female follows. He then deposits the spermatophore, and the animals keep moving forward until the female hits it with her cloaca, after which she stops and stands still. The spermatophore sticks to her and the sperm cells swim inside her cloaca where they attempt to fertilize her eggs. The courtship ritual can be repeated several times over a couple of hours.
Taxonomic history
Olms from different cave systems differ substantially in body measurements, color and some microscopic characters. Earlier researchers used these differences to support the division into five different species, while modern herpetologists understand that external morphology is not reliable for amphibian systematics and can be extremely variable, depending on nourishment, illness, and other factors even varying among individuals in a single population. Proteus anguinus is now considered a single species. The length of the head is the most obvious difference among various populations — individuals from Stična, Slovenia, have shorter heads on average than those from Tržič, Slovenia, and the Istrian peninsula, for example.
Black Proteus



The Black Proteus (Proteus anguinus parkelj Sket & Arntzen, 1994) is the only recognized subspecies of the olm, endemic to the underground waters near Črnomelj, Slovenia, an area smaller than 100 square kilometers (39 mi²). It was first found in 1986 by members of the Slovenian Karst Research Institute who were exploring the water from Dobličice karst spring in the Bela krajina region.
It has several features separating it from the type subspecies:
| Feature | Proteus anguinus anguinus | Proteus anguinus parkelj | Notes |
|---|---|---|---|
| Skin | Not pigmented. | Normally pigmented, dark brown or black in color. | The most obvious difference. |
| Head shape | Long, slender. | Shorter, equally thick. Stronger jaw muscles visible as two bulbs on the top of the head. | |
| Body length | Shorter, 29–32 vertebrae. | Longer, 34–35 vertebrae. | Amphibians do not have a fixed number of vertebrae. |
| Appendages | Longer. | Shorter. | |
| Tail | Longer in proportion to the rest of the body. | Shorter in proportion. | |
| Eyes | Regressed. | Almost normally developed, although still small compared to other amphibians. Covered by a thin layer of transparent skin, no eyelids. | Regressed eye of White Proteus shows first of all immunolabelling for the red-sensitive cone opsin. The eye of Black Proteus has principal rods, red-sensitive cones and blue- or UV- sensitive cones. |
| Other senses | Specific and highly sensitive. | Some sensory organs, particularly electroreceptors, less sensitive. |
These features suggest that the Black Proteus has probably colonized underground habitats more recently and still retains some nontroglomorphic characteristics.
Research history
The olm is supposedly first pictured as two pairs of snakes with wings, which could represent gills, in a relief on a Venetian stone fountain, probably originating from Karst.
The first written mention of the olm is in Janez Vajkard Valvasor's The Glory of the Duchy of Carniola (1689) as a baby dragon. This was a reference to a folk story he did not really believe in. The first researcher to retrieve a live olm was a physician and researcher from Idrija, G.A. Scopoli; he sent dead specimens and drawings to colleagues and collectors.
Joseph Nicolai Laurenti, though, was the first to briefly describe the olm in 1768 and give it the scientific name Proteus anguinus. It wasn't until the end of the century that Carl Franz Anton Ritter von Schreibers from the Naturhistorisches Museum of Vienna started to look into this animal's anatomy. The animals were sent to him by Žiga Zois. Schreibers presented his findings in 1801 to The Royal Society in London, and later also in Paris. Soon the olm started to gain wide recognition and attract significant attention, resulting in thousands of animals being sent to researchers and collectors worldwide. The basis of functional morphological investigations in Slovenia was set up by Prof. dr. Lili Istenič in the 1980s. More than twenty years the Research Group for functional morphological Studies of the Vertebrates in the Department of Biology (Biotechnical Faculty, University of Ljubljana), is one of the leading groups studying the olm under the guidance of Prof.dr. Boris Bulog. There are also several cave laboratories in Europe, where olms have been introduced and are being studied. These are Moulis (France), Kent's Cavern (England), Han-sur-Lesse (Belgium) and Aggtelek (Hungary). They were also introduced into the Hermannshöhle (Germany) and Grote Oliero (Italy) caves, where they still live today.
The olm was used by Charles Darwin in his famous The Origin of Species as an example for the reduction of structures through disuse:
Far from feeling surprise that some of the cave-animals should be very anomalous...as is the case with blind Proteus with reference to the reptiles of Europe, I am only surprised that more wrecks of ancient life have not been preserved, owing to the less severe competition to which the scanty inhabitants of these dark abodes will have been exposed.
Conservation

The olm is extremely vulnerable to changes in its environment due to its adaptation to the specific conditions in caves. Water resources in the karst are extremely sensitive to all kinds of pollution. The contamination of the karst underground waters is due to the large number of waste disposal sites leached by rainwater, as well as to the accidental overflow of various liquids. The reflection of such pollution in the karst underground waters depends on the type and quantity of pollutants, and on the rock structure through which the waters penetrate. Self-purification processes in the underground waters are not completely understood, but they are quite different from those in surface waters. Among the most serious chemical pollutants are chlorinated hydrocarbon pesticides, fertilizers, polychlorinated biphenyls (PCBs), which are or were used in a variety of industrial processes and in the manufacture of many kinds of materials; and metals such as mercury, lead, cadmium, and arsenic. All of these substances persist in the environment, being slowly, if at all, degraded by natural processes. In addition, all are toxic to life if they accumulate in any appreciable quantity. Slovenian caves became famous for the animals they contained and which could not be found elsewhere. Due to its rarity the olm is also popular among collectors, which can threaten the species by taking too many animals out of the habitat.
The olm is included in Appendices II and IV of the EU Habitats Directive (92/43/EEC). Appendix II seeks to preserve favorable conservation status in animal and plant species along with their habitats by protecting the species or defining special areas of conservation. These areas of conservation form the Natura 2000 network. Appendix IV further defines "animal and plant species of community interest in need of strict protection." Hunting or keeping a limited number of olm is allowed only under strictly controlled circumstances, determined by local authorities.
The olm was first protected in Slovenia in 1922 along with all cave fauna, but the protection was not effective and a substantial black market came into existence. In 1982 it was placed on a list of rare and endangered species. This list also had the effect of prohibiting trade of the species. After joining the European Union, Slovenia had to establish mechanisms for protection of the species included in the EU Habitats Directive. The olm is included in a Slovenian red list of endangered species. The Postojna cave and other caves inhabited by olm were also included in the Slovenian part of the Natura 2000 network.
In Croatia, the olm is protected by the legislation designed to protect amphibians — collecting is possible only for research purposes by permission of the National Administration for Nature and Environment Protection. Conservation status in Bosnia and Herzegovina and Montenegro has not yet been defined.
On the IUCN Red List, the olm is listed as vulnerable because of its fragmented and limited distribution and ever-decreasing population.
Cultural significance
The olm is a symbol of Slovenian natural heritage. The enthusiasm of scientists and the broader public about this inhabitant of Slovenian caves is still strong 300 years after its discovery. The Postojna cave is one of the birthplaces of speleobiology due to the olm and other rare cave inhabitants. The image of the olm contributes significantly to the fame of the Postojna cave, which Slovenia successfully utilizes for the promotion of eco-tourism in Postojna and other parts of Slovenian karst. Tours of the Postojna cave also include a tour around the speleobiological station — the Proteus vivarium, showing different aspects of the cave environment.
The olm was also depicted on one of the Slovenian Tolar coins, and was the namesake of Proteus, the oldest Slovenian popular science magazine, first published in 1933.
References
- ^ Template:IUCN2006 Database entry includes a range map and justification for why this species is vulnerable
- Istria on the Internet — Proteus anguinus (Olm). Retrieved on 7 June 2007.
- ^ Burnie D. & Wilson D.E. (eds.) (2001). Animal. London: DK. pp. 61, 435. ISBN 0-7894-7764-5.
{{cite book}}:|author=has generic name (help) - Weber A. (2000). Fish and amphibia. In: Culver D.C. et al. (ed.): Ecosystems of the world: Subterranean Ecosystems, pp. 109–132. Amsterdam: Elsevier
- Istenic L. in Ziegler I. (1974). Riboflavin as "pigment" in the skin of Proteus anguinus L. Naturwissenschaften 12: 686–687.
- Schlegel P.A., Briegleb W., Bulog B., Steinfartz S. (2006). Error: {{Lang}}: text has italic markup (help). Bulletin de la Société herpétologique de France, 118, pp. 1–31. Template:Fr icon
- Durand J.P. (1973). Error: {{Lang}}: text has italic markup (help). Ann. Spéléol. 28, 193–208 Template:Fr icon
- ^ Langecker T.G. (2000). The effects of continuous darkness on cave ecology and caverniculous evolution. In: Culver D.C. et al. (eds.): Ecosystems of the world: Subterranean Ecosystems, pp. 135–157. Amsterdam: Elsevier
- Hawes R.S. (1945). On the eyes and reactions to light of Proteus anguinus. Quart. Journ. Micr. Sc. N.S. 86:1–53
- Kos M. (2000). Error: {{Lang}}: text has italic markup (help) (Immunocitochemical analysis of the visual pigments in the sensory cells of the eye and the pineal organ of the olm (Proteus anguinus, Amphibia, Urodela).) PhD thesis. Ljubljana: University of Ljubljana. Template:Sl icon
- Kos, M., Bulog, B. et al. (2001) Immunocytochemical demonstration of visual pigments in the degenerate retinal and pineal photoreceptors of the blind cave salamander (Proteus anguinus). Cell Tissue Res, 303, pp. 15–25.
- Hüpop K. (2000). How do cave animals cope with the food scarcity in caves?. In: Culver D.C. et al. (ed.): Ecosystems of the world: Subterranean Ecosystems, pp. 159–188. Amsterdam: Elsevier
- Dumas P. in Chris B. (1998). The olfaction in Proteus anguinus. Behavioural Processes 43: 107–113
- Bulog B. (1989). Differentiation of the inner ear sensory epithelia of Proteus anguinus (Urodela, Amphibia). Journal of Morphology, 202, pp. 325–338.
- Bulog B. (1990). Error: {{Lang}}: text has italic markup (help) (Sense organs of the octavolateral system in proteus Proteus anguinus (Urodela, Amphibia). I. Otic labyrinth). Biološki vestnik 38: 1–16 Template:Sl icon
- Bulog B. & Schlegel P. (2000). Functional morphology of the inner ear and underwater audiograms of Proteus anguinus (Amphibia, Urodela). Pflügers Arch 439(3), suppl., pp. R165–R167.
- Istenič L. & Bulog B. (1984). Some evidence for the ampullary organs in the European cave salamander Proteus anguinus (Urodela, Amphibia). Cell Tissue Res. 235, pp. 393–402.
- Schegel P. & Bulog B. (1997). Population-specific behavioral electrosensitivity of the European blind cave salamander, Proteus anguinus. Journal of Physiology (Paris) 91: 75–79
- Bulog B., Schlegel P. et al. (2002). Non-visual orientation and light-sensitivity in the blind cave salamander, Proteus anguinus (Amphibia, Caudata). In: Latella L., Mezzanotte E., Tarocco M. (eds.). 16th international symposium of biospeleology; 2002 Sep 8–15; Verona: Societé Internationale de Biospéologie, pp. 31–32.
- Durand J.P. & Delay B. (1981). Influence of temperature on the development of Proteus anguinus (Caudata: Proteidae) and relation with its habitat in the subterranean world. Journal of Thermal Biology 6(1): 53–57
- ^ Aljančič M., Bulog B. et al. (1993). Proteus — mysterious ruler of Karst darkness. Ljubljana: Vitrium d.o.o. Template:Sl icon
- Aljančič G. and Aljančič M. (1998). Error: {{Lang}}: text has italic markup (help) (The animal of the month of October: olm). Proteus 61(2): 83–87 Template:Sl icon
- Bulog B. (1994). Error: {{Lang}}: text has italic markup (help) (Two decades of functional-morphological research on the olm (Proteus anguinus, Amphibia, Caudata). Acta Carsologica XXIII/19. Template:Sl icon
- Guillaume O. (2000). Role of chemical communication and behavioural interactions among conspecifics in the choice of shelters by the cave-dwelling salamander Proteus anguinus (Caudata, Proteidae). Can. J. Zool. 78(2): 167–173
- Sket B. et al. (ed.) (2003). Error: {{Lang}}: text has italic markup (help) (The animals of Slovenia). Ljubljana: Tehniška založba Slovenije. ISBN 86-365-0410-4 Template:Sl icon
- Sket B. & Arntzen J.W. (1994). A black, non-troglomorphic amphibian from the karst of Slovenia: Proteus anguinus parkelj n. ssp (Urodela: Proteidae). Bijdragen tot de Dierkunde 64:33–53.
- Bulog B. et al. (2003). Black Proteus: mysterious dweller of the Karst in Bela krajina . Ljubljana: TV Slovenia, Video tape.
- Darwin C. (1859). On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. London: John Murray.
- Bulog B., Mihajl K. et al. (2002). Trace element concentrations in the tissues of Proteus anguinus (Amphibia, Caudata) and the surrounding environment. Water air soil pollut., 136(1–4), pp. 147–163
- EU Habitats directive (1992). .
- Slovenian official gazette (2002). no. 82, tuesday 24th september 2002. Template:Sl icon
- Error: {{Lang}}: text has italic markup (help). Template:Hr icon
- Destinacija Postojna. Retrieved 7 June 2007.
External links
- AmphibiaWeb page for the olm
- Flora and Fauna of Caves: Proteus anguinus
- Template:Sl icon Proteus magazine
- Slovenian practice example: Human Fish (Proteus anguinus)
- The olm on ARKive — pictures, films
- Template:Sl icon The olm on the pages of the Slovenian Natural history museum.