| Revision as of 00:56, 12 April 2009 view sourceTDL42 (talk | contribs)3 editsmNo edit summary← Previous edit | Revision as of 00:58, 12 April 2009 view source TDL42 (talk | contribs)3 editsmNo edit summaryNext edit → | ||
| Line 169: | Line 169: | ||
| Many of these species have adaptations to help in locomotion under water. The water beetles and water bugs have legs adapted into paddle like structures. Dragonfly naiads, use jet propulsion, forcibly expelling water out of the rectal chamber.<ref>{{cite journal|last=Mill|first=P. J.|coauthors=R. S. Pickard|year=1975|title=Jet-propulsion in anisopteran dragonfly larvae|journal=Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology|volume=97|issue=4|pages=329–338|doi=10.1007/BF00631969}}</ref> | Many of these species have adaptations to help in locomotion under water. The water beetles and water bugs have legs adapted into paddle like structures. Dragonfly naiads, use jet propulsion, forcibly expelling water out of the rectal chamber.<ref>{{cite journal|last=Mill|first=P. J.|coauthors=R. S. Pickard|year=1975|title=Jet-propulsion in anisopteran dragonfly larvae|journal=Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology|volume=97|issue=4|pages=329–338|doi=10.1007/BF00631969}}</ref> | ||
| Some species like the ]s are capable of walking on the surface of water. They can do this because their claws are not at the tips of the legs as in most insects, but recessed in a special groove further up the leg; this prevents the claws from piercing the water's surface film.<ref name=aquins /> Other insects such as the ] '']'' are known to emit salivary secretions that reduce surface tension making it possible for them to move on the surface of water by ] (also known by the ] term ''Entspannungsschwimmen'').<ref>{{cite journal|last=Linsenmair|first=K.|coauthors=Jander R.|year=1976|title=Das "entspannungsschwimmen" von ''Velia'' and ''Stenus''|journal=Naturwissenschaften|volume=50|pages=231|doi=10.1007/BF00639292}}</ref><ref>{{cite journal|last=Bush|first=J. W. M.|coauthors=David L. Hu|year=2006|title=Walking on Water: Biolocomotion at the Interface|journal=Annu. Rev. Fluid Mech.|volume=38|pages=339–369|url=http://www.cims.nyu.edu/~dhu/Pubweb/Bush_Hu_06.pdf|doi=10.1146/annurev.fluid.38.050304.092157}}</ref> | Some species like the ]s are capable of walking on the surface of water. They can do this because their claws are not at the tips of the legs as in most insects, but recessed in a special groove further up the leg; this prevents the claws from piercing the water's surface film.<ref name=aquins /> Other insects such as the ] '']'' are known to emit salivary secretions that reduce surface tension making it possible for them to move on the surface of water by ] (also known by the ] term ''Entspannungsschwimmen'').<ref>{{cite journal|last=Linsenmair|first=K.|coauthors=Jander R.|year=1976|title=Das "entspannungsschwimmen" von ''Velia'' and ''Stenus''|journal=Naturwissenschaften|volume=50|pages=231|doi=10.1007/BF00639292}}</ref><ref>{{cite journal|last=Bush|first=J. W. M.|coauthors=David L. Hu|year=2006|title=Walking on Water: Biolocomotion at the Interface|journal=Annu. Rev. Fluid Mech.|volume=38|pages=339–369|url=http://www.cims.nyu.edu/~dhu/Pubweb/Bush_Hu_06.pdf|doi=10.1146/annurev.fluid.38.050304.092157}}</ref> | ||
| Species that are submerged also have adaptations to aid in respiration. Many larval forms have gills that can extract oxygen dissolved in water, while others need to rise to the water surface to replenish air supplies which may be held or trapped in special structures.<ref name=aquins /> | Species that are submerged also have adaptations to aid in respiration. Many larval forms have gills that can extract oxygen dissolved in water, while others need to rise to the water surface to replenish air supplies which may be held or trapped in special structures.<ref name=aquins /> | ||
Revision as of 00:58, 12 April 2009
| Insects Temporal range: 396–0 Ma PreꞒ Ꞓ O S D C P T J K Pg N Early Devonian (but see text) - Recent | |
|---|---|

| |
| Western honey bee (Order Hymenoptera) | |
| Scientific classification | |
| Kingdom: | Animalia |
| Phylum: | Arthropoda |
| Subphylum: | Mandibulata |
| Class: | Insecta Linnaeus, 1758 |

A- Head B- Thorax C- Abdomen
1. antenna
2. ocelli (lower)
3. ocelli (upper)
4. compound eye
5. brain (cerebral ganglia)
6. prothorax
7. dorsal blood vessel
8. tracheal tubes (trunk with spiracle)
9. mesothorax
10. metathorax
11. forewing
12. hindwing
13. mid-gut (stomach)
14. dorsal blood vessel ("aorta")
15. ovary
16. hind-gut (intestine, rectum & anus)
17. anus
18. oviduct
19. nerve chord (abdominal ganglia)
20. Malpighian tubes
21. tarsal pads
22. claws
23. tarsus
24. tibia
25. femur
26. trochanter
27. fore-gut (crop, gizzard)
28. thoracic ganglion
29. coxa
30. salivary gland
31. subesophageal ganglion
32. mouthparts
Insects (Class Insecta) are the biggest class of arthropods and the only ones with wings. They are the most diverse group of animals on the planet. They are most diverse at the equator and their diversity declines toward the poles. With over a million described species—more than half of all known living organisms—with estimates of undescribed species as high as 30 million, thus potentially representing over 90% of the differing life forms on the planet. Insects may be found in nearly all environments on the planet, although only a small number of species occur in the oceans, a habitat dominated by another arthropod group, the crustaceans.
There are approximately 2,200 species of praying mantis, 5,000 dragonfly species, 20,000 grasshopper, 82,000 true bug, 120,000 fly, 110,000 bee, wasp ant and sawfly, 170,000 butterfly and moth, and 360,000 beetle species described to date. Estimates of the total number of current species, including those not yet known to science, range from two million to fifty million, with newer studies favouring a lower figure of about six to ten million. Adult modern insects range in size from a 0.139 mm (0.00547 in) fairyfly (Dicopomorpha echmepterygis) to a 56.7 centimetres (22.3 in) long stick insect (Phobaeticus chani). The heaviest documented insect was a Giant Weta of 70 g (2½ oz), but other possible candidates include the Goliath beetles Goliathus goliatus, Goliathus regius and Cerambycid beetles such as Titanus giganteus, though no one is certain which is truly the heaviest.
The study of insects (from Latin insectus, meaning "cut into sections") is called entomology, from the Greek έντομον, also meaning "cut into sections".
Body structure
Insects possess segmented bodies supported by an exoskeleton, a hard outer covering made mostly of chitin. The segments of the body are organized into three distinctive but interconnected units, or tagmata; a head, a thorax, and an abdomen. The head supports a pair of sensory antennae, a pair of compound eyes, if present, one to three simple eyes, if present, ("ocelli") and three sets of variously modified appendages that form the mouthparts. The thorax has six segmented legs (one pair each for the prothorax, mesothorax and the metathorax segments making up the thorax) and two or four wings (if present in the species). The abdomen (made up of eleven segments some of which may be reduced or fused) has most of the digestive, respiratory, excretory and reproductive internal structures.
Nervous system
Their nervous system can be divided into a brain and a ventral nerve cord. The head capsule (made up of six fused segments) has six pairs of ganglia. The first three pairs are fused into the brain, while the three following pairs are fused into a structure called the subesophageal ganglion.
The thoracic segments have one ganglion on each side, which are connected into a pair, one pair per segment. This arrangement is also seen in the abdomen but only in the first eight segments. Many species of insects have reduced numbers of ganglia due to fusion or reduction. Some cockroaches have just six ganglia in the abdomen, whereas the wasp Vespa crabro has only two in the thorax and three in the abdomen. And some, like the house fly Musca domestica, have all the body ganglia fused into a single large thoracic ganglion.
Until very recently, no one had ever documented the presence of nociceptors (the cells that detect and transmit sensations of pain) in insects (e.g., ), though recent findings of nociception in larval fruit flies challenges this and raises the possibility that some insects may be capable of feeling pain.
Digestive system

A- Head B- Thorax C- Abdomen
13. mid-gut (stomach)
15. ovary
16. hind-gut (intestine, rectum & anus)
17. anus
27. fore-gut (crop, gizzard)
30. salivary gland
An insect uses its digestive system to extract nutrients and other substances from the food it consumes. Most of this food is ingested in the form of macromolecules and other complex substances (such as proteins, polysaccharides, fats, nucleic acids, etc.) which must be broken down by catabolic reactions into smaller molecules (i.e. amino acids, simple sugars, etc.) before being used by cells of the body for energy, growth, or reproduction. This break-down process is known as digestion.
The insect's digestive system is a closed system, with one long enclosed tube called the alimentary canal which runs lengthwise through the body. The alimentary canal only allows food to enter the mouth, and then gets processed as it travels toward the anus. Each of the three sections of the alimentary canal performs a different process of digestion. In addition to the alimentary canal, insects also have paired salivary glands and salivary reservoirs. These structures usually reside in the thorax (adjacent to the fore-gut).
The salivary glands (30) produce saliva, the salivary ducts lead from the glands to the reservoirs and then forward, through the head, to an opening called the salivarium behind the hypopharynx; which movements of the mouthparts help mix saliva with food in the buccal cavity. Saliva mixes with food which travels through salivary tubes into the mouth; Beginning the process of breaking it down.
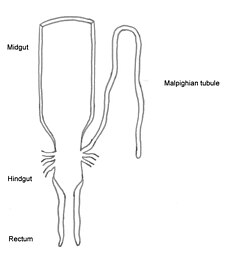
The first section of the alimentary canal is the fore-gut (27) or stomodaeum. In the fore-gut, initial breakdown of large food particles occurs, mostly by saliva. The fore-gut includes the Buccal cavity, the esophagus, and the crop, which stores food before it passes to the mid-gut.
Once food leaves the crop, it passes to the mid-gut (13) or mesenteron. The mid-gut is where digestion really happens, through enzymatic action. Microscopic projections from the mid-gut wall, called microvilli, increase surface area and allow for maximum absorption of nutrients.
In the hind-gut (16) or proctodaeum, is where undigested food particles join uric acid from Malphigian tubules to form fecal pellets. The rectum absorbs most of the water in this waste matter, and the dry pellet is then eliminated through the anus (17), there by completing the process of digestion.
Respiration and circulation
Insect respiration is accomplished without lungs, using a system of internal tubes and sacs through which gases either diffuse or are actively pumped, delivering oxygen directly to the adjoining body tissues (see Invertebrate trachea). Since oxygen is delivered directly, the circulatory system is not used to carry oxygen, and is therefore greatly reduced; it has no closed vessels (i.e., no veins or arteries), consisting of little more than a single, perforated dorsal tube which pulses peristaltically, and in doing so helps circulate the hemolymph inside the body cavity. Air is taken in through spiracles, openings on the sides of the abdomen. There are many different patterns of gas exchange demonstrated by different groups of insects. Gas exchange patterns in insects can range from continuous, diffusive ventilation, to discontinuous gas exchange.
Exoskeleton

Most higher insects have two pairs of wings located on the second and third thoracic segments. Insects are the only invertebrates to have developed flight capability, and this has played an important part in their success. The winged insects, and their wingless relatives, make up the subclass Pterygota. Insect flight is not very well understood, relying heavily on turbulent aerodynamic effects. The primitive insect groups use muscles that act directly on the wing structure. The more advanced groups making up the Neoptera have foldable wings and their muscles act on the thorax wall and power the wings indirectly. These muscles are able to contract multiple times for each single nerve impulse, allowing the wings to beat faster than would ordinarily be possible (see insect flight).
Their outer skeleton, the cuticle, is made up of two layers; the epicuticle which is a thin and waxy water resistant outer layer and contains no chitin, and another layer under it called the procuticle. This is chitinous and much thicker than the epicuticle and has two layers, the outer being the exocuticle while the inner is the endocuticle. The tough and flexible endocuticle is built from numerous layers of fibrous chitin and proteins, criss-crossing each others in a sandwich pattern, while the exocuticle is rigid and sclerotized. The exocuticle is greatly reduced in many soft-bodied insects, especially the larval stages (e.g., caterpillars).
Reproduction

Most insects hatch from eggs, but some are ovoviviparous or viviparous, and all undergo a series of moults as they develop and grow in size. This manner of growth is necessitated by the inelastic exoskeleton. Moulting is a process by which the individual escapes the confines of the exoskeleton in order to increase in size, then grows a new and larger outer covering. In some insects, the young are called nymphs and are similar in form to the adult except that the wings are not developed until the adult stage. This is called incomplete metamorphosis and insects showing this are termed hemimetabolous. Holometabolous insects show complete metamorphosis, which distinguishes the Endopterygota and includes many of the most successful insect groups. In these species, an egg hatches to produce a larva, which is generally worm-like in form, and can be divided into five different forms; eruciform (caterpillar-like), scarabaeiform (grublike), campodeiform (elongated, flattened, and active), elateriform (wireworm-like) and vermiform (maggot-like). The larva grows and eventually becomes a pupa, a stage marked by reduced movement and often sealed within a cocoon.

There are three types of pupae; obtect (the pupa is compact with the legs and other appendages enclosed), exarate (where the pupa has the legs and other appendages free and extended) and coarctate (where the pupa develops inside the larval skin). In the pupal stage, the insect undergoes considerable change in form to emerge as an adult, or imago. Butterflies are an example of an insect that undergoes complete metamorphosis. Some insects have even evolved hypermetamorphosis.
Some insects (parastic wasps) show polyembryony where a single fertilized egg can divide into many and in some cases thousands of separate embryos. Other developmental and reproductive variations include haplodiploidy, polymorphism, paedomorphosis (metathetely and prothetely), sexual dimorphism, parthenogenesis and more rarely hermaphroditism.
Senses and communication

Many insects possess very sensitive and/or specialized organs of perception. Some insects such as bees can perceive ultraviolet wavelengths, or detect polarized light, while the antennae of male moths can detect the pheromones of female moths over distances of many kilometres. There is a pronounced tendency for there to be a trade-off between visual acuity and chemical or tactile acuity, such that most insects with well-developed eyes have reduced or simple antennae, and vice-versa. There are a variety of different mechanisms by which insects perceive sound, and it is by no means universal; the general pattern, however, is that if an insect can produce sound, then it can also hear sound, though the range of frequencies they can hear is often quite narrow (and may in fact be limited to only the frequency that they themselves produce). Some nocturnal moths can perceive the ultrasonic emissions of bats, a mechanism which helps them avoid predation. Certain predatory and parasitic insects can detect the characteristic sounds made by their prey/hosts. Bloodsucking insects have special sensory structures that can detect infrared emissions, and use them to home in on their hosts.

A few such insects also have a well-developed number sense, among the solitary wasps that provision with a single species of prey. The mother wasp lays her eggs in individual cells and provides each egg with a number of live caterpillars on which the young feed when hatched. Some species of wasp always provide five, others twelve, and others as high as twenty-four caterpillars per cell. The number of caterpillars is different among species, but it is always the same for each sex of larvae. The male solitary wasp in the genus Eumenes is smaller than the female, so the mother of one species supplies him with only five caterpillars; the larger female receives ten caterpillars in her cell. She can in other words distinguish between both the numbers five and ten in the caterpillars she is providing and which cell contains a male or a female.
Light production and vision
A few insects, such as members of the families Poduridae and Onychiuridae (Collembola), Mycetophilidae (Diptera), and the beetle families Lampyridae, Phengodidae, Elateridae and Staphylinidae are bioluminescent. The most familiar group are the fireflies, beetles of the family Lampyridae. Some species are able to control this light generation to produce flashes. The function varies with some species using them to attract mates, while others use them to lure prey. Cave dwelling larvae of Arachnocampa (Mycetophilidae, Fungus gnats) glow to lure small flying insects into sticky strands of silk. Some fireflies of the genus Photuris mimic the flashing of female Photinus species to attract males of that species, which are then captured and devoured. The colours of emitted light vary from dull blue (Orfelia fultoni, Mycetophilidae) to the familiar greens and the rare reds (Phrixothrix tiemanni, Phengodidae).
Most insects except some species of cave dwelling crickets are able to perceive light and dark. Many species have acute vision capable of detecting minute movements. The eyes include simple eyes or ocelli as well as compound eyes of varying sizes. Many species are able to detect light in the infrared, ultraviolet as well as the visible light wavelengths. Colour vision has been demonstrated in many species and phylogenetic analysis suggests that the basic bauplan of UV-green-blue trichromacy existed from at least the Devonian period.
Sound production and hearing
Insects were the earliest organisms to produce sounds and to sense them. Soundmaking in insects is achieved mostly by mechanical action of appendages. In the grasshoppers and crickets this is achieved by stridulation. The cicadas have the loudest sounds among the insects and have special modifications to their body and musculature to produce and amplify sounds. Some species such as the African cicada, Brevisana brevis have been measured at 106.7 decibels at a distance of 50 cm (20 in). Some insects, such as the hawk moths and Hedylid butterflies, can hear ultrasound and take evasive action when they sense detection by bats. Some moths produce ultrasound clicks and these were earlier thought to have a role in jamming the bat echolocation, but it was subsequently found that these are produced mostly by unpalatable moths to warn bats, just as warning colourations are used against predators that hunt by sight. These calls are also made by other moths involved in mimicry.
Very low sounds are also produced in various species of Neuroptera, Lepidoptera (butterflies and moths), Coleoptera and Hymenoptera produced by the mechanical actions of movement often aided by special microscopic stridulatory structures.
Most sound-making insects also have tympanal organs that can perceive airborne sounds. Most insects are also able to sense vibrations transmitted by the substrate. Communication using substrate-borne vibrational signals is more widespread among insects because of the size constraints in producing air-borne sounds. Insects cannot effectively produce low-frequency sounds, and high-frequency sounds tend to disperse more in a dense environment (such as foliage), so insects living in such environments communicate primarily using substrate-borne vibrations. The mechanisms of production of vibrational signals are just as diverse as those for producing sound in insects.
Some species use vibrations for communicating within members of the same species, such as to attract mates as in the songs of the shield bug Nezara viridula while it can also be used to communicate between entirely different species, such as between ants and myrmecophilous lycaenid caterpillars.
The Madagascar hissing cockroach has the ability to press air through the spiracles to make a hissing noise, and the Death's-head Hawkmoth makes a squeaking noise by forcing air out of their pharynx.
Chemical communication
In addition to the use of sound for communication, a wide range of insects have evolved chemical means for communication. These chemicals, termed semiochemicals, are often derived from plant metabolites include those meant to attract, repel and provide other kinds of information. While some chemicals are targeted at individuals of the same species, others are used for communication across species. The use of scents is especially well known to have developed in social insects.
Social behaviour

Social insects, such as the termites, ants and many bees and wasps, are the most familiar species of eusocial animal. They live together in large well-organized colonies that may be so tightly integrated and genetically similar that the colonies of some species are sometimes considered superorganisms. It is sometimes argued that the various species of honey bee are the only invertebrates (and indeed one of the few non-human groups) to have evolved a system of abstract symbolic communication (i.e., where a behaviour is used to represent and convey specific information about something in the environment), called the "dance language" - the angle at which a bee dances represents a direction relative to the sun, and the length of the dance represents the distance to be flown.
Only those insects which live in nests or colonies demonstrate any true capacity for fine-scale spatial orientation or "homing" - this can be quite sophisticated, however, and allow an insect to return unerringly to a single hole a few millimetres in diameter among a mass of thousands of apparently identical holes all clustered together, after a trip of up to several kilometres' distance, and (in cases where an insect hibernates) as long as a year after last viewing the area (a phenomenon known as philopatry). A few insects migrate, but this is a larger-scale form of navigation, and often involves only large, general regions (e.g., the overwintering areas of the Monarch butterfly).
Care of young
Most insects lead short lives as adults, and rarely interact with one another except to mate, or compete for mates. A small number exhibit some form of parental care, where they will at least guard their eggs, and sometimes continue guarding their offspring until adulthood, and possibly even actively feeding them. Another simple form of parental care is to construct a nest (a burrow or an actual construction, either of which may be simple or complex), store provisions in it, and lay an egg upon those provisions. The adult does not contact the growing offspring, but it nonetheless does provide food. This sort of care is typical of bees and various types of wasps.
Locomotion
Flight
Main article: Insect flight
Insects are the only group of invertebrates to have developed flight. The evolution of insect wings has been a subject of debate. Some proponents suggest that the wings are para-notal in origin while others have suggested they are modified gills. In the Carboniferous age, some of the Meganeura dragonflies had as much as a 50 cm (20 in) wide wingspan. The appearance of gigantic insects has been found to be consistent with high atmospheric oxygen. The percentage of oxygen in the atmosphere found from ice core-samples was as high as 35% compared to the current 21%. The respiratory system of insects constrains their size, however the high oxygen in the atmosphere allowed larger sizes. The largest flying insects today are much smaller and include several moth species such as the Atlas moth and the White Witch (Thysania agrippina).
Insect flight has been a topic of great interest in aerodynamics due partly to the inability of steady-state theories to explain the lift generated by the tiny wings of insects.
In addition to powered flight, many of the smaller insects are also dispersed by winds. These include the aphids, which are often transported long distances by low-level jet streams.
Walking
Many adult insects use six legs for walking and have adopted a tripedal gait. The tripedal gait allows for rapid walking whilst always having a stable stance and has been studied extensively in cockroaches. The legs are used in alternate triangles touching the ground. For the first step the middle right leg and the front and rear left legs are in contact with the ground and move the insect forward, whilst the front and rear right leg and the middle left leg are lifted and moved forward to a new position. When they touch the ground to form a new stable triangle the other legs can be lifted and brought forward in turn and so on.
The purest form of the tripedal gait is seen in insects moving at speed. However, this type of locomotion is not rigid and insects can adapt a variety of gaits; for example, when moving slowly, turning, or avoiding obstacles, four or more feet may be touching the ground. Insects can also adapt their gait to cope with the loss of one or more limbs.
Cockroaches are amongst the fastest insect runners and at full speed actually adopt a bipedal run to reach a high velocity in proportion to their body size. As Cockroaches move extremely rapidly, they need recording at several hundred frames per second to reveal their gait. More sedate locomotion is also studied by scientists in stick insects Phasmatodea.
A few insects have evolved to walk on the surface of the water, especially the bugs of the family, Gerridae, also known as water striders. A few species of ocean-skaters in the genus Halobates even live on the surface of open oceans, a habitat that has few insect species.
Insect walking is of particular interest as an alternative form of locomotion to the use of wheels for robots (Robot locomotion).
Swimming

A large number of insects live either parts or the whole of their lives underwater. In many of the more primitive orders the immature stages are aquatic while some other groups have aquatic adults as well.
Many of these species have adaptations to help in locomotion under water. The water beetles and water bugs have legs adapted into paddle like structures. Dragonfly naiads, use jet propulsion, forcibly expelling water out of the rectal chamber.
Some species like the water striders are capable of walking on the surface of water. They can do this because their claws are not at the tips of the legs as in most insects, but recessed in a special groove further up the leg; this prevents the claws from piercing the water's surface film. Other insects such as the Rove beetle Stenus are known to emit salivary secretions that reduce surface tension making it possible for them to move on the surface of water by Marangoni propulsion (also known by the Germany term Entspannungsschwimmen).
Species that are submerged also have adaptations to aid in respiration. Many larval forms have gills that can extract oxygen dissolved in water, while others need to rise to the water surface to replenish air supplies which may be held or trapped in special structures.
Evolution
Main article: Insect evolutionThe relationships of insects to other animal groups remain unclear. Although more traditionally grouped with millipedes and centipedes, evidence has emerged favoring closer evolutionary ties with the crustaceans. In the Pancrustacea theory, insects, together with Remipedia and Malacostraca, make up a natural clade.
Other terrestrial arthropods, such as centipedes, millipedes, scorpions and spiders, are sometimes confused with insects since their body plans can appear similar, sharing (as do all arthropods) a jointed exoskeleton. However upon closer examination their features differ significantly; most noticeably they do not have the six legs characteristic of adult insects.
The higher level phylogeny of the arthropods continues to be a matter of debate and research.
A phylogenetic tree of the arthropods and related groups. |
In 2008, a Tufts University student and a faculty lecturer uncovered what they believe is the world's oldest known full-body impression of a primitive flying insect, a 300 million-year-old specimen from the Carboniferous Period. The scientists presented the fossil at the Second International Congress on Ichnology, in Krakow, Poland in September 2008.
The oldest definitive insect fossil is the Devonian Rhyniognatha hirsti, from the 396 million year old Rhynie chert. This species already possessed dicondylic mandibles, a feature associated with winged insects, suggesting that wings may already have evolved at this time. Thus, the first insects probably appeared earlier, in the Silurian period.
The origins of insect flight remain obscure, since the earliest winged insects currently known appear to have been capable fliers. Some extinct insects had an additional pair of winglets attaching to the first segment of the thorax, for a total of three pairs. So far, there is nothing that suggests that the insects were a particularly successful group of animals before they got their wings.
Late Carboniferous and Early Permian insect orders include both several current very long-lived groups and a number of Paleozoic forms. During this era, some giant dragonfly-like forms reached wingspans of 55 to 70 cm, (22-28 in) making them far larger than any living insect. This gigantism may have been due to higher atmospheric oxygen levels that allowed increased respiratory efficiency relative to today. The lack of flying vertebrates could have been another factor.
Most extant orders of insects developed during the Permian era that began around 270 million years ago. Many of the early groups became extinct during the Permian-Triassic extinction event, the largest mass extinction in the history of the Earth, around 252 million years ago.
The remarkably successful Hymenopterans appeared in the Cretaceous but achieved their diversity more recently, in the Cenozoic. A number of highly-successful insect groups evolved in conjunction with flowering plants, a powerful illustration of co-evolution.
Many modern insect genera developed during the Cenozoic; insects from this period on are often found preserved in amber, often in perfect condition. Such specimens are easily compared with modern species. The study of fossilized insects is called paleoentomology.
Coevolution
- See also: Coevolution
Insects were among the earliest terrestrial herbivores and acted as major selection agents on plants. Plants evolved chemical defenses against this herbivory and the insects in turn evolved mechanisms to deal with plant toxins. Many insects make use of these toxins to protect themselves from their predators. Such insects often advertise their toxicity using warning colours. This successful evolutionary pattern has also been utilized by mimics. Over time, this has led to complex groups of co-evolved species. Conversely, some interactions between plants and insects are beneficial (see pollination), and coevolution has led to the development of very specific mutualisms in such systems.
Classification
Simplified Cladogram of insect groups and very simplified. Note that Apterygota, Palaeoptera and Exopterygota are possibly paraphyletic groups. |
Traditional morphology-based systematics has included in the subphylum Hexapoda four groups - Insects (Ectognatha), springtails (Collembola), Protura and Diplura, the latter three being grouped together as Entognatha on the basis of internalized mouthparts. Supraordinal relationships have undergone numerous changes with the advent of cladistic methods and genetic data. A recent hypothesis is that Hexapoda is polyphyletic, with the entognath classes having separate evolutionary histories from Insecta.
As many of the traditional morphology-based taxa have been shown to be paraphyletic, it is best to avoid using terms such as subclass, superorder and infraorder and instead focus on monophyletic groupings. The following list represents the best supported monophyletic groupings for the Insecta.
† signifies an extinct taxon.
- Monura †
- Archaeognatha=Microcoryphia (bristletails)
- Thysanura=Zygentoma (silverfish)
- Paleoptera
- Ephemeroptera (mayflies)
- Palaeodictyoptera †
- Megasecoptera †
- Archodonata †
- Diaphanopterodea †
- Protodonata=Meganisoptera †
- Protanisoptera †
- Triadophlebioptera †
- Protozygoptera=Archizygoptera †
- Odonata (dragonflies, damselflies)
- Paleoptera
- Polyneoptera
- Caloneurodea †
- Titanoptera †
- Protorthoptera †
- Plecoptera (stoneflies)
- Embiidina=Embioptera (webspinners)
- Zoraptera (angel insects)
- Dermaptera (earwigs)
- Orthoptera (grasshoppers, crickets, katydids)
- Phasmatodea (stick insects)
- Notoptera (ice-crawlers & gladiators)
- Blattaria (cockroaches)
- Isoptera (termites - included in Blattaria)
- Mantodea (mantids)
- Paraneoptera
- Psocodea (booklice, barklice)
- Mallophaga (chewing lice - in Psocodea)
- Anoplura (sucking lice - in Psocodea)
- Thysanoptera (thrips)
- Hemiptera (true bugs, aphids, cicadas)
- Endopterygota=Holometabola
- Glosselytrodea †
- Miomoptera †
- Hymenoptera (wasps, bees, ants)
- Coleoptera (beetles)
- Strepsiptera (twisted-winged parasites)
- Neuropterida
- Raphidioptera (snakeflies)
- Megaloptera (alderflies, dobsonflies)
- Neuroptera (lacewings, antlions)
- Antliophora/Mecopteroidea
- Mecoptera (scorpionflies, hangingflies)
- Siphonaptera (fleas - in Mecoptera)
- Diptera (true flies)
- Protodiptera †
- Amphiesmenoptera
- Trichoptera (caddisflies)
- Lepidoptera (butterflies, moths, skippers)
- Polyneoptera
Insects can be divided into two groups, historically treated as subclasses: Apterygota (wingless) and Pterygota (winged). The Apterygota consists of two primitively wingless orders - Archaeognatha (bristletails) and Thysanura (silverfish). Archaeognatha makes up the Monocondylia (based on mandibular morphology) while Thysanura and Pterygota are grouped together as Dicondylia. It is possible that the Thysanura itself is not monophyletic, with the family Lepidotrichidae a sister group to the Dicondylia (Pterygota + the remaining Thysanura).
Paleoptera and Neoptera are the winged orders of insects, separated by the presence of sclerites and musculature that allow for folding of the wings flat over the abdomen in the latter group. Neoptera can further be divided into hemimetabolous (Polyneoptera & Paraneoptera) and Holometabolous groups. It has proven particularly difficult to elucidate interordinal relationships within Polyneoptera. Phasmatodea and Embiidina have been suggested to form Eukinolabia. Mantodea, Blattodea & Isoptera are thought to form a monophyletic group termed Dictyoptera. Paraneoptera has turned out to be more closely related to Endopterygota than to the rest of the Exopterygota. The recent molecular finding that the traditional louse orders Mallophaga and Anoplura are derived from within Psocoptera has led to the new taxon Psocodea.
It is quite likely that Exopterygota is paraphyletic in regards to Endopterygota. Contentious matters include Strepsiptera and Diptera grouped together as Halteria based on a reduction of one of the wing pairs - a position not well-supported in the entomological community. The Neuropterida are often "lumped" or "split" on the whims of the taxonomist. Fleas are now thought to be closely related to boreid mecopterans. Many questions remain to be answered when it comes to basal relationships amongst endopterygote orders, particularly Hymenoptera.
Insects and Cosmetics
Insects are used in the cosmetic industry to fabricate makeup, the technique used in this process is maleleuocolakastasis, the chemical transition of pigments - This process is used to retain consistency of colour when making insect feaces into makeup, another material used would be the urine of the insect and substances produced during sexual intercourse.
Relationship to humans

Many insects are considered pests by humans. Insects commonly regarded as pests include those that are parasitic (mosquitoes, lice, bed bugs), transmit diseases (mosquitoes, flies), damage structures (termites), or destroy agricultural goods (locusts, weevils). Many entomologists are involved in various forms of pest control, often using insecticides, but more and more relying on methods of biocontrol.
Although pest insects attract the most attention, many insects are beneficial to the environment and to humans. Some pollinate flowering plants (for example wasps, bees, butterflies, ants). Pollination is a trade between plants that need to reproduce, and pollinators that receive rewards of nectar and pollen. A serious environmental problem today is the decline of populations of pollinator insects, and a number of species of insects are now cultured primarily for pollination management in order to have sufficient pollinators in the field, orchard or greenhouse at bloom time.
Insects also produce useful substances such as honey, wax, lacquer and silk. Honey bees have been cultured by humans for thousands of years for honey, although contracting for crop pollination is becoming more significant for beekeepers. The silkworm has greatly affected human history, as silk-driven trade established relationships between China and the rest of the world. Fly larvae (maggots) were formerly used to treat wounds to prevent or stop gangrene, as they would only consume dead flesh. This treatment is finding modern usage in some hospitals. Adult insects such as crickets, and insect larvae of various kinds are also commonly used as fishing bait.

In some parts of the world, insects are used for human food, while being a taboo in other places. There are proponents of developing this use to provide a major source of protein in human nutrition. Since it is impossible to entirely eliminate pest insects from the human food chain, insects already are present in many foods, especially grains. Most people do not realize that food safety laws in many countries do not prohibit insect parts in food, but rather limit the quantity. According to cultural materialist anthropologist Marvin Harris, the eating of insects is taboo in cultures that have protein sources that require less work, like farm birds or cattle.
Many insects, especially beetles, are scavengers, feeding on dead animals and fallen trees, recycling the biological materials into forms found useful by other organisms, and insects are responsible for much of the process by which topsoil is created. The ancient Egyptian religion adored dung beetles and represented them as beetle-shaped amulets, or scarabs.
The most useful of all insects are insectivores, those that feed on other insects. Many insects can potentially reproduce so quickly that if all of their offspring were to survive, they could literally bury the earth in a single season. However, for any given insect one can name, whether it is considered a pest or not, there will be one to hundreds of species of insects that are either parasitoids or predators upon it, and play a significant role in controlling it. This role in ecology is usually assumed to be primarily one of birds, but insects, though less glamorous, are much more significant.
Human attempts to control pests by insecticides can backfire, because important but unrecognised insects already helping to control pest populations are also killed by the poison, leading eventually to population explosions of the pest species.
Quotations
- "Something in the insect seems to be alien to the habits, morals, and psychology of this world, as if it had come from some other planet: more monstrous, more energetic, more insensate, more atrocious, more infernal than our own."
- —Maurice Maeterlinck (1862–1949)
- When asked what can be learned about the Creator by examining His work, J.B.S. Haldane said "an inordinate fondness for beetles."
- "To understand the success of insects is to appreciate our own shortcomings" —Thomas Eisner
See also
- Aquatic insect
- Flying and gliding animals
- Entomology
- Invertebrate
- Prehistoric insect
- Insect flight
- Insect ecology
- Category:Insect-borne diseases
- Chrysomya megacephala
References
- ^ Engel, Michael S. (2004). "New light shed on the oldest insect". Nature. 427: 627–630. doi:10.1038/nature02291.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Chapman, A. D. (2006). Numbers of living species in Australia and the World. Canberra: Australian Biological Resources Study. pp. 60pp. ISBN 978-0-642-56850-2.
- Threats to Global Biodiversity (Accessed December 2007
- Erwin, Terry L. (1982). "Tropical forests: their richness in Coleoptera and other arthropod species". Coleopt. Bull. 36: 74–75.
- Vojtech Novotny, Yves Basset, Scott E. Miller, George D. Weiblen, Birgitta Bremer, Lukas Cizek & Pavel Drozd (2002). "Low host specificity of herbivorous insects in a tropical forest". Nature. 416: 841–844. doi:10.1038/416841a.
{{cite journal}}: Unknown parameter|quotes=ignored (help)CS1 maint: multiple names: authors list (link) - Erwin, Terry L. (1997). Biodiversity at its utmost: Tropical Forest Beetles. pp. 27–40. In: Reaka-Kudla, M. L., D. E. Wilson & E. O. Wilson (eds.). Biodiversity II. Joseph Henry Press, Washington, D.C.
{{cite book}}:|author=has generic name (help)CS1 maint: multiple names: authors list (link) - "World's longest insect revealed". Natural History Museum. 2008-10-16. Retrieved 2008-10-16.
- ^ Walker, T.J., ed. 2001. University of Florida Book of Insect Records, 2001.
- Oxford English Dictionary. Oxford University Press.
- Gullan, P.J. (2005). The Insects: An Outline of Entomology (3 ed.). Oxford: Blackwell Publishing. pp. 22–48. ISBN 1-4051-1113-5.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Gullan and Cranston, 57.
- C. H. Eisemann, W. K. Jorgensen, D. J. Merritt, M. J. Rice, B. W. Cribb, P. D. Webb and M. P. Zalucki (1984) Do insects feel pain? — A biological view. Cellular and Molecular Life Sciences 40: 1420-1423
- Tracey, J., W. Daniel, R. I. Wilson, G. Laurent, and S. Benzer (2003) painless, a Drosophila gene essential for nociception. Cell 113: 261-273. http://dx.doi.org/10.1016/S0092-8674(03)00272-1
- Gullan and Cranston, 61, 62–65.
- Gullan and Cranston, 65–68.
- Gullan and Cranston, 38.
- Gullan and Cranston, 186.
- Gullan and Cranston, 22–24.
- Gullan and Cranston, 129, 131, 134–135.
- Gullan and Cranston, 142.
- Gullan and Cranston, 143.
- Pugsley, Chris W. (1983). "Literature review of the New Zealand glowworm Arachnocampa luminosa (Diptera: Keroplatidae) and related cave-dwelling Diptera" (PDF). New Zealand Entomologist. 7 (4): 419–424.
- Lloyd, James E. (1984). "Occurrence of Aggressive Mimicry in Fireflies". The Florida Entomologist. 67 (3): 368–376. doi:10.2307/3494715.
- Lloyd, James E. in Resh, V.H. and R.C. Cardé (editors) 2003. The Encyclopedia of Insects. Academic Press. pp. 115–120.
{{cite book}}: Unknown parameter|coauthors=ignored (|author=suggested) (help); line feed character in|title=at position 3 (help) - Briscoe, AD & L Chittka (2001). "The evolution of color vision in insects". Annu. Rev. Entomol. 46: 471–510. doi:10.1146/annurev.ento.46.1.471.
- Hristov, N.I. (2005). "Sound strategy: acoustic aposematism in the bat–tiger moth arms race". Naturwissenschaften. 92: 164–169. doi:10.1007/s00114-005-0611-7.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Barber, J. R. (2007). "Acoustic mimicry in a predator–prey interaction". Proc. Nat. Acad. Sci. 104 (22): 9331–9334. doi:10.1073/pnas.0703627104. PMID 17517637.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Virant-Doberlet, M. (2004). "Vibrational communication in insects" (PDF). Neotropical Entomology. 33 (2): 121–134. doi:10.1590/S1519-566X2004000200001.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Bennet-Clark, H.C. (1998). "Size and scale effects as constraints in insect sound communication". Phil. Trans. R. Soc. Lond. B. 353: 407–419. doi:10.1098/rstb.1998.0219.
- Miklas, Nadège (2001). "The Influence of Substrate on Male Responsiveness to the Female Calling Song in Nezara viridula". Journal of Insect Behavior. 14 (3): 313–332. doi:10.1023/A:1011115111592.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - DeVries, P. J. (1990). "Enhancement of symbiosis between butterfly caterpillars and ants by vibrational communication". Science. 248: 1104–1106. doi:10.1126/science.248.4959.1104. PMID 17733373.
- Gullan and Cranston, 96–105.
- Dudley, R. (1998). "Atmospheric oxygen, giant Paleozoic insects and the evolution of aerial locomotor performance" (PDF). Journal of Experimental Biology. 201 (8): 1043–1050.
- Drake, V. A. (1988). "The Influence of Atmospheric Structure and Motions on Insect Migration". Annual Review of Entomology. 33: 183–210. doi:10.1146/annurev.en.33.010188.001151.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - ^ Richard W. Merritt, Kenneth W. Cummins, and Martin B. Berg (editors) (2007). An Introduction to the Aquatic Insects of North America (4th ed.). Kendall Hunt Publishers. ISBN 9780757550492.
{{cite book}}:|author=has generic name (help)CS1 maint: multiple names: authors list (link) - Mill, P. J. (1975). "Jet-propulsion in anisopteran dragonfly larvae". Journal of Comparative Physiology A: Neuroethology, Sensory, Neural, and Behavioral Physiology. 97 (4): 329–338. doi:10.1007/BF00631969.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Linsenmair, K. (1976). "Das "entspannungsschwimmen" von Velia and Stenus". Naturwissenschaften. 50: 231. doi:10.1007/BF00639292.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Bush, J. W. M. (2006). "Walking on Water: Biolocomotion at the Interface" (PDF). Annu. Rev. Fluid Mech. 38: 339–369. doi:10.1146/annurev.fluid.38.050304.092157.
{{cite journal}}: Unknown parameter|coauthors=ignored (|author=suggested) (help) - Tree of Life Web Project. 1995. Arthropoda. Version 01 January 1995 (temporary). in The Tree of Life Web Project,
- Researchers Discover Oldest Fossil Impression of a Flying Insect Newswise, Retrieved on October 19, 2008.
- Rice, C. M., Ashcroft, W. A., Batten, D. J., Boyce, A. J., Caulfield, J. B. D., Fallick, A. E., Hole, M. J., Jones, E., Pearson, M. J., Rogers, G., Saxton, J. M., Stuart, F. M., Trewin, N. H. & Turner, G. (1995). "A Devonian auriferous hot spring system, Rhynie, Scotland". Journal of the Geological Society, London. 152: 229–250. doi:10.1144/gsjgs.152.2.0229.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Tree of Life, Insecta
- Terry, M. D. and M. F. Whiting. 2005. Mantophasmatodea and phylogeny of the lower neopterous insects. Cladistics 21(3): 240-257
- Lo, N., G. Tokuda, H. Watanabe, H. Rose, M. Slaytor, K. Maekawa, C. Bandi, and H. Noda. 2000. Evidence from multiple gene sequences indicates that termites evolved from wood-feeding cockroaches. Current Biology 10(13):801-804.
- Johnson, K. P., Yoshizawa, K. and V. S. Smith. 2004. Multiple origins of parasitism in lice. Proceedings of the Royal Society of London 271: 1771-1776.
- Bonneton, F., F. G. Brunet, J. Kathirithamby, V. Laudet. 2006. The rapid divergence of the ecdysone receptor is a synapomorphy for Mecopterida that clarifies the Strepsiptera problem. Insect Molecular Biology 15(3):351-362.
- Whiting, M.F. 2002. Mecoptera is paraphyletic: multiple genes and phylogeny of Mecoptera and Siphonaptera. Zoologica Scripta 31(1): 93-104.
- Gullan and Cranston, 10–13.
- Gullan and Cranston, 3, 218–228.
- Gullan and Cranston, 328–348.
- Gullan and Cranston, 400.
Further reading
- Davidson, E. (ed.) 1981. Pathogenesis of Invertebrate Micorobial Diseases. Allanheld, Osmun & Co. Publishers, Inc., Totowa, New Jersey, USA. 562 pages.
- Davidson, E. 2006. Big Fleas Have Little Fleas: How Discoveries of Invertebrate Diseases Are Advancing Modern Science University of Arizona Press, Tucson, 208 pages, ISBN 0-8165-2544-7
- Davidson, RH and William F. Lyon. 1979 Insect Pests of Farm, Garden, and Orchard. John Wiley & Sons., New York. 596 pages, ISBN 0-471-86314-9.
- Grimaldi, D. and Engel, M.S. (2005). Evolution of the Insects. Cambridge University Press. ISBN 0-521-82149-5.
{{cite book}}: CS1 maint: multiple names: authors list (link) — an up to date review of the evolutionary history of the insects - Reimer, N.J., J.W. Beardsley, and G. C. Jahn 1990. Pest ants in the Hawaiian Islands. In R. Vander Meer, K. Jaffe, and A. Cedena , "Applied Myrmecology: a world perspective." Westview Press, Oxford, 40-50.
- Triplehorn, Charles A. and Norman F. Johnson (2005-05-19). Borror and DeLong's Introduction to the Study of Insects, 7th edition, Thomas Brooks/Cole. ISBN 0-03-096835-6. — a classic textbook in North America
- Rasnitsyn, A.P. and Quicke, D.L.J. (2002). History of Insects. Kluwer Academic Publishers. ISBN 1-4020-0026-X.
{{cite book}}: CS1 maint: multiple names: authors list (link) — detail coverage of various aspects of the evolutionary history of the insects - Biewener, Andrew A. (2003). Animal Locomotion. Oxford University Press. ISBN 0-19-850022-X.
- Merritt, RW, KW Cummins, and MB Berg (2007). An Introduction To The Aquatic Insects Of North America. Kendall Hunt Publishing Company. ISBN 0-7575-4128-3.
{{cite book}}: CS1 maint: multiple names: authors list (link)
External links
- [http://tolweb.org/Insecta/8205 Tree of Life Proje
ct] – Insecta, Insecta Movies
- Insect Morphology Overview of insect external and internal anatomy
- Let's Talk About Insects For students ages 9-11 years, learn how insects grow and
develop (metamorphosis), and learn the importance of insects in our environment. (Also in
- Meganeura Insect evolution and fossil record.
- IPS International Palaeoentological Society.
- UF Book of Insect Records Insect records
- Insect Walking Research into Insect Locomotion at the University
of Illinois
- [http://www.berkeley.edu/news/media/releases/2002/09/rfull/locomotion.html/ Press Release 2002 on research into polypodal
locomotion]
- Insectclopedia Insect research portal
- Insects as Food Insects as a food resource.
- Information on keeping, breeding and rearing Insects and invertebrates.
- Bug Bytes Reference library of digitized insect sounds.
- INSECTS .org Insect appreciation.
- [http://www.nzzfolio.ch/www/d80bd71b-b264-4db4-afd0-277884b93470/showarticle/d217322c-e20e-4870-84c4-ec8839e4ee02.aspx The
Unexpected Apocalypse]. Entomologist May Berenbaum about a world without insects.
- Priscilla Wakefield, [http://www.antiquebooks.net/readpage.html#insects Introduction to the Natural History and
Classification of Insects in a Series of Familiar Letters with Engravings], 1816. A free to read book
- Earthlife Web - Insect Morphology and Anatomy
- Featured Creatures Hundreds of species and groups
- WoodyBug S.E. U.S. woody ornamental pests and beneficials
- Public Health Pest Control - EPA national manual on public health arthropods
Image resources
- BugGuide Photographs, life history and identification of North American arthropods, especially insects
- LiveScience: Insects Insect information and user-submitted insect pictures
- ESALQ Entomological Museum 6000 insect pictures. Brazilian collection (ESALQ/USP).
- North American Insects 4,000 large format insect pictures. Creative Commons licensed
- InsectImages.org 24,000 high resolution insect photographs. Free for non-profit educational
uses.
- Pest Control The pest control library offers the latest information on
insects. Learn how to identify the insect, test your home and even create your own bug!]
| Extant Arthropoda classes by subphylum | |||||
|---|---|---|---|---|---|
| |||||
| Chelicerata |
| ||||
| Mandibulata | |||||
| italic are paraphyletic groups | |||||
Template:Link FA Template:Link FA Template:Link FA
[
Categories: