| Revision as of 16:07, 12 January 2011 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Script assisted update of identifiers from ChemSpider, CommonChemistry and FDA for the Chem/Drugbox validation project - Updated: ChEMBL ChEMBL.← Previous edit | Revision as of 16:18, 12 January 2011 edit undoCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'ChEMBL_Ref') per Chem/Drugbox validation (report errors or [[user talk:CheMoBot|Next edit → | ||
| Line 1: | Line 1: | ||
| {{Refimprove|date=July 2009}} | {{Refimprove|date=July 2009}} | ||
| {{chembox | {{chembox | ||
| | verifiedrevid = |
| verifiedrevid = 407488762 | ||
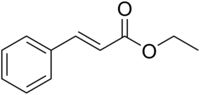
| | Name = Ethyl cinnamate | | Name = Ethyl cinnamate | ||
| | ImageFile = Ethyl_cinnamate.png | | ImageFile = Ethyl_cinnamate.png | ||
| Line 18: | Line 18: | ||
| | InChI = 1/C11H12O2/c1-2-13-11(12)9-8-10-6-4-3-5-7-10/h3-9H,2H2,1H3/b9-8+ | | InChI = 1/C11H12O2/c1-2-13-11(12)9-8-10-6-4-3-5-7-10/h3-9H,2H2,1H3/b9-8+ | ||
| | InChIKey = KBEBGUQPQBELIU-CMDGGOBGBD | | InChIKey = KBEBGUQPQBELIU-CMDGGOBGBD | ||
| | ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| | ChEMBL = 318196 | | ChEMBL = 318196 | ||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
Revision as of 16:18, 12 January 2011
| This article needs additional citations for verification. Please help improve this article by adding citations to reliable sources. Unsourced material may be challenged and removed. Find sources: "Ethyl cinnamate" – news · newspapers · books · scholar · JSTOR (July 2009) (Learn how and when to remove this message) |
| |

| |
| Names | |
|---|---|
| IUPAC name Ethyl 3-phenylprop-2-enoate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.822 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C11H12O2 |
| Molar mass | 176.21 g/mol |
| Density | 1.046 g/cm |
| Melting point | 6.5-8 °C |
| Boiling point | 271 °C |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Ethyl cinnamate is the ester of cinnamic acid and ethanol. It is present in the essential oil of cinnamon ; pure ethyl cinnamate has a "fruity and balsamic odor, reminiscent of cinnamon with an amber note" .
The p-methoxy substitute is reported to be a monoamine oxidase inhibitor.
List of plants that contain the chemical
Toxicology
| This section is empty. You can help by adding to it. (September 2009) |
Notes and references
| This article has an unclear citation style. The references used may be made clearer with a different or consistent style of citation and footnoting. (September 2007) (Learn how and when to remove this message) |
- Budavari, Susan (2001). "Merck Index 13th Ed". Merck & co., Inc.
- Noro T, Miyase T, Kuroyanagi M, Ueno A, Fukushima S. (1983). "Monoamine oxidase inhibitor from the rhizomes of Kaempferia galanga L.". Chem Pharm Bull (Tokyo). 31 (8): 2708–11. PMID 6652816.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Wong, K. C.; Ong, K. S.; Lim, C. L.; et al. (2006). "Compositon of the essential oil of rhizomes of kaempferia galanga L.". Flavour and Fragrance Journal. 7 (5): 263–266. doi:10.1002/ffj.2730070506.
{{cite journal}}:|access-date=requires|url=(help); Cite has empty unknown parameter:|coauthors=(help); Explicit use of et al. in:|first=(help) - Othman, R.; Ibrahim, H; Mohd, MA; Mustafa, MR; Awang, K; et al. (2006). "Bioassay-guided isolation of a vasorelaxant active compound from Kaempferia galanga L.". Phytomedicine. 13 (1–2): 61–66. doi:10.1016/j.phymed.2004.07.004. PMID 16360934.
{{cite journal}}:|access-date=requires|url=(help); Cite has empty unknown parameter:|coauthors=(help); Explicit use of et al. in:|first=(help)
This article about an ester is a stub. You can help Misplaced Pages by expanding it. |