| Revision as of 09:23, 23 February 2011 editAnypodetos (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers39,350 editsm Sufficient refs for the contents of this stub← Previous edit | Revision as of 07:58, 30 August 2011 edit undoBogBot (talk | contribs)Bots53,132 edits populated new fields in {{drugbox}} and reordered per bot approval. Report errors and suggestions to User_talk:BogBotNext edit → | ||
| Line 2: | Line 2: | ||
| | Watchedfields = changed | | Watchedfields = changed | ||
| | verifiedrevid = 398777189 | | verifiedrevid = 398777189 | ||
| ⚫ | | image = Amlodipine.svg | ||
| ⚫ | | type |
||
| | |
| image2 = Benazepril.svg | ||
| ⚫ | | |
||
| <!--Combo data--> | |||
| ⚫ | | |
||
| ⚫ | | type = combo | ||
| ⚫ | | class1 |
||
| | component1 = Amlodipine | |||
| | component2 = Benazepril | |||
| ⚫ | | class1 = ] | ||
| ⚫ | | class2 |
||
| ⚫ | | component2 = Benazepril | ||
| ⚫ | | class2 = ] | ||
| <!--Clinical data--> | |||
| | tradename = | |||
| ⚫ | | pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | ||
| ⚫ | | pregnancy_US = D | ||
| ⚫ | | pregnancy_category = | ||
| ⚫ | | legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S5 / S6 / S7 / S8 / S9 --> | ||
| ⚫ | | legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> | ||
| ⚫ | | legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> | ||
| ⚫ | | legal_US = Rx-only | ||
| ⚫ | | legal_status = | ||
| ⚫ | | routes_of_administration = Oral | ||
| <!--Identifiers--> | |||
| ⚫ | | CAS_number = | ||
| ⚫ | | ATC_prefix = None | ||
| ⚫ | | ATC_suffix = | ||
| ⚫ | | PubChem = 5746247 | ||
| ⚫ | | DrugBank = | ||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | ChemSpiderID = 4676979 | | ChemSpiderID = 4676979 | ||
| ⚫ | | InChI = 1/C24H28N2O5.C20H25ClN2O5/c1-2-31-24(30)20(14-12-17-8-4-3-5-9-17)25-19-15-13-18-10-6-7-11-21(18)26(23(19)29)16-22(27)28;1-4-28-20(25)18-15(11-27-10-9-22)23-12(2)16(19(24)26-3)17(18)13-7-5-6-8-14(13)21/h3-11,19-20,25H,2,12-16H2,1H3,(H,27,28);5-8,17,23H,4,9-11,22H2,1-3H3/t19-,20-;/m0./s1 | ||
| <!--Chemical data--> | |||
| | smiles = Clc1ccccc1C2C(\C(=O)OC)=C(/N\C(=C2\C(=O)OCC)COCCN)C.O=C(OCC)(N2C(=O)N(c1ccccc1CC2)CC(=O)O)CCc3ccccc3 | | smiles = Clc1ccccc1C2C(\C(=O)OC)=C(/N\C(=C2\C(=O)OCC)COCCN)C.O=C(OCC)(N2C(=O)N(c1ccccc1CC2)CC(=O)O)CCc3ccccc3 | ||
| ⚫ | | InChI = 1/C24H28N2O5.C20H25ClN2O5/c1-2-31-24(30)20(14-12-17-8-4-3-5-9-17)25-19-15-13-18-10-6-7-11-21(18)26(23(19)29)16-22(27)28;1-4-28-20(25)18-15(11-27-10-9-22)23-12(2)16(19(24)26-3)17(18)13-7-5-6-8-14(13)21/h3-11,19-20,25H,2,12-16H2,1H3,(H,27,28);5-8,17,23H,4,9-11,22H2,1-3H3/t19-,20-;/m0./s1 | ||
| | InChIKey = SRTZYSFUFGOMFR-FKLPMGAJBV | | InChIKey = SRTZYSFUFGOMFR-FKLPMGAJBV | ||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | ||
| Line 18: | Line 41: | ||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| | StdInChIKey = SRTZYSFUFGOMFR-FKLPMGAJSA-N | | StdInChIKey = SRTZYSFUFGOMFR-FKLPMGAJSA-N | ||
| ⚫ | | CAS_number |
||
| ⚫ | | ATC_prefix |
||
| ⚫ | | ATC_suffix |
||
| ⚫ | | PubChem |
||
| ⚫ | | DrugBank |
||
| ⚫ | | pregnancy_AU |
||
| ⚫ | | pregnancy_US |
||
| ⚫ | | pregnancy_category= | ||
| ⚫ | | legal_AU |
||
| ⚫ | | legal_CA |
||
| ⚫ | | legal_UK |
||
| ⚫ | | legal_US |
||
| ⚫ | | legal_status |
||
| ⚫ | | routes_of_administration = Oral | ||
| }} | }} | ||
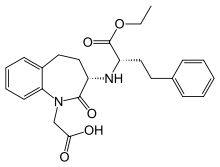
| '''Amlodipine/benazepril''', marketed in the U.S. as '''Lotrel''' by ] and manufactured as a ] by ] and ], is an ] medication which combines a ] (] besilate) with an ] (]).<ref></ref> This drug, like similar combinations, is prescribed when either agent alone is not sufficient to bring a person's blood pressure down to target range. As a combination agent, Lotrel shares the ] profile of both of its individual parts.<ref>Drugs.com: </ref><ref>RxList.com: </ref> | '''Amlodipine/benazepril''', marketed in the U.S. as '''Lotrel''' by ] and manufactured as a ] by ] and ], is an ] medication which combines a ] (] besilate) with an ] (]).<ref></ref> This drug, like similar combinations, is prescribed when either agent alone is not sufficient to bring a person's blood pressure down to target range. As a combination agent, Lotrel shares the ] profile of both of its individual parts.<ref>Drugs.com: </ref><ref>RxList.com: </ref> | ||
Revision as of 07:58, 30 August 2011
Pharmaceutical compound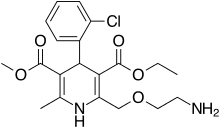 | |
 | |
| Combination of | |
|---|---|
| Amlodipine | Calcium channel blocker |
| Benazepril | ACE inhibitor |
| Clinical data | |
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| PubChem CID | |
| ChemSpider | |
| CompTox Dashboard (EPA) | |
| Chemical and physical data | |
| 3D model (JSmol) | |
SMILES
| |
InChI
| |
| (verify) | |
Amlodipine/benazepril, marketed in the U.S. as Lotrel by Novartis and manufactured as a generic drug by Teva and Sandoz, is an antihypertensive medication which combines a calcium channel blocker (amlodipine besilate) with an angiotensin converting enzyme inhibitor (benazepril). This drug, like similar combinations, is prescribed when either agent alone is not sufficient to bring a person's blood pressure down to target range. As a combination agent, Lotrel shares the adverse reaction profile of both of its individual parts.
See also
References
- Lotrel Prescribing Information
- Drugs.com: Lotrel
- RxList.com: Lotrel
External links
| Antihypertensive drugs acting on the renin–angiotensin system (C09) | |
|---|---|
| ACE inhibitors ("-pril") |
|
| AIIRAs ("-sartan") |
|
| Renin inhibitors ("-kiren") | |
| Dual ACE/NEP inhibitors | |
| Neprilysin inhibitors | |
| Other | |
| |
This drug article relating to the cardiovascular system is a stub. You can help Misplaced Pages by expanding it. |