| Revision as of 00:58, 1 March 2011 editKupirijo (talk | contribs)Extended confirmed users9,131 edits Category:Carboxylic acids← Previous edit | Revision as of 17:17, 21 April 2011 edit undoEdgar181 (talk | contribs)Extended confirmed users196,325 edits chembox data correctionsNext edit → | ||
| Line 1: | Line 1: | ||
| {{ |
{{Chembox | ||
| | ImageFile = Beta-oxalylamino-L-alanine.svg | |||
| |ImageFile=Oxalyldiaminopropionic acid.png | |||
| |ImageSize= | | ImageSize = 200px | ||
| |IUPACName=3-amino- |
| IUPACName = 3--<small>L</small>-alanine | ||
| | OtherNames = β-Oxalylamino-<small>L</small>-alanine; BOAA; L-BOAA; β-''N''-Oxalyl-<small>L</small>-α,β-diaminopropionic acid; ODAP; beta-ODAP | |||
| |OtherNames= | |||
| |Section1= {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| | CASNo= |
| CASNo = 5302-45-4 | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | PubChem= |
| PubChem = 440259 | ||
| ⚫ | | SMILES=C(C(C(=O) |
||
| | ChemSpiderID = 389238 | |||
| ⚫ | | SMILES = O=C(NC(N)C(=O)O)C(=O)O | ||
| | InChI = InChI=1S/C5H8N2O5/c6-2(4(9)10)1-7-3(8)5(11)12/h2H,1,6H2,(H,7,8)(H,9,10)(H,11,12)/t2-/m0/s1 | |||
| }} | }} | ||
| |Section2= {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| | C=5|H=8|N=2|O=5 | |||
| | Formula=C<sub>5</sub>H<sub>8</sub>N<sub>2</sub>O<sub>5</sub> | |||
| | Appearance = | |||
| | MolarMass=176.127 | |||
| | |
| Density = | ||
| | |
| MeltingPt = | ||
| | |
| BoilingPt = | ||
| | |
| Solubility = | ||
| | Solubility= | |||
| }} | }} | ||
| |Section3= {{Chembox Hazards | | Section3 = {{Chembox Hazards | ||
| | MainHazards= | | MainHazards = | ||
| | FlashPt= | | FlashPt = | ||
| | Autoignition= | | Autoignition = | ||
| }} | }} | ||
| }} | }} | ||
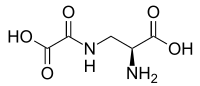
| ⚫ | '''Oxalyldiaminopropionic acid''' is the ] responsible for ]. | ||
| ⚫ | '''Oxalyldiaminopropionic acid''' ('''ODAP''') is the ] responsible for ].<ref>{{Cite journal | pmid = 11850107}}</ref> | ||
| ⚫ | ] | ||
| ==References== | |||
| {{reflist}} | |||
| {{biochem-stub}} | |||
| {{organic-compound-stub}} | |||
| ⚫ | ] | ||
| ] | |||
| ] | ] | ||
Revision as of 17:17, 21 April 2011
| |
| Names | |
|---|---|
| IUPAC name 3--L-alanine | |
| Other names β-Oxalylamino-L-alanine; BOAA; L-BOAA; β-N-Oxalyl-L-α,β-diaminopropionic acid; ODAP; beta-ODAP | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChemSpider | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C5H8N2O5 |
| Molar mass | 176.128 g·mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa). Infobox references | |
Oxalyldiaminopropionic acid (ODAP) is the neurotoxin responsible for lathyrism.
References
- . PMID 11850107.
{{cite journal}}: Cite journal requires|journal=(help); Missing or empty|title=(help)
This article about an organic compound is a stub. You can help Misplaced Pages by expanding it. |