| Revision as of 02:47, 16 April 2011 editRifleman 82 (talk | contribs)Extended confirmed users32,435 edits →Reactions: whitespace← Previous edit | Revision as of 12:45, 20 April 2012 edit undoMSBOT (talk | contribs)13,809 editsm r2.7.3) (Robot: Adding fa:نیتروزونیوم تترافلوروبوراتNext edit → | ||
| Line 40: | Line 40: | ||
| ] | ] | ||
| ] | ] | ||
| ] | |||
Revision as of 12:45, 20 April 2012
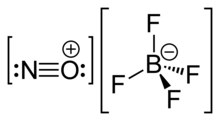
| |
| Names | |
|---|---|
| IUPAC name nitrosonium tetrafluoroborate | |
| Other names nitrosyl tetrafluoroborate | |
| Identifiers | |
| CAS Number | |
| ECHA InfoCard | 100.035.148 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
| Properties | |
| Chemical formula | BF4NO |
| Molar mass | 116.81 g·mol |
| Appearance | colourless crystalline solid |
| Density | 2.185 g cm |
| Melting point | 250 °C (sublimes) |
| Solubility in water | decomposes |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Nitrosonium tetrafluoroborate, also called nitrosyl tetrafluoroborate, is a chemical compound with the chemical formula NOBF4. This colourless solid finds use in organic synthesis as a nitrosating agent.
NOBF4 is the nitrosonium salt of fluoroboric acid, and is composed of a nitrosonium cation, , and a tetrafluoroborate anion, .
Reactions
Nitrosonium tetrafluoroborate may be used to prepare metal salts of the type 2 (M = Cr, Mn, Fe, Co, Ni, Cu). The nitrosonium cation acts as the oxidizer, itself being reduced to nitric oxide gas: With ferrocene the ferrocenium tetrafluoroborate is formed.
- M + NOBF4 + xCH3CN → (BF4)2 + NO
References
- "A15806 Nitrosonium tetrafluoroborate, 98%". Alfa Aesar website. Retrieved 2010-09-04.
- Robert A. Heintz, Jennifer A. Smith, Paul S. Szalay, Amy Weisgerber, And Kim R. Dunbar. "11. Homoleptic Transition Metal Acetonitrile Cations with Tetrafluoroborate or Trifluoromethanesulfonate Anions". Inorg. Synth. 33: 75–83. doi:10.1002/0471224502.ch2.
{{cite journal}}: CS1 maint: multiple names: authors list (link) - Roger M. Nielson, George E. McManis, Lance K. Safford, Michael J. Weaver (1989). "Solvent and electrolyte effects on the kinetics of ferrocenium-ferrocene self-exchange. A reevaluation". J. Phys. Chem. 93 (5): 2152. doi:10.1021/j100342a086.
{{cite journal}}: CS1 maint: multiple names: authors list (link)
This inorganic compound–related article is a stub. You can help Misplaced Pages by expanding it. |