| Revision as of 17:26, 14 July 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (changes to watched fields - updated 'ChEMBL_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report errors or [[user talk:CheMoBot|bug← Previous edit | Revision as of 15:26, 28 August 2011 edit undoSmokefoot (talk | contribs)Autopatrolled, Extended confirmed users, Pending changes reviewers, Rollbackers74,550 edits rm warning about exergonic rxn with base, some sectioning and add refNext edit → | ||
| Line 55: | Line 55: | ||
| }} | }} | ||
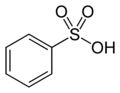
| '''Benzenesulfonic acid''' is an ] with the formula C<sub>6</sub>H<sub>5</sub>SO<sub>3</sub>H. It is the simplest ] ]. | '''Benzenesulfonic acid''' is an ] with the formula C<sub>6</sub>H<sub>5</sub>SO<sub>3</sub>H. It is the simplest ] ]. It forms colorless ] sheet crystals or a white waxy solid that is soluble in water and ethanol, slightly soluble in ] and insoluble in ] and ]. It is often stored in the form of alkali metal salts. Its aqueous solution is ]. | ||
| Benzenesulfonic acid forms colorless ] sheet crystals or a white waxy solid that is soluble in water and ethanol, slightly soluble in ] and insoluble in ] and ]. It is often stored in the form of alkali metal salts. Its aqueous solution is ]. It has the ability to react violently with bases and attack metals. | |||
| ==Preparation== | ==Preparation== | ||
| Benzenesulfonic acid is prepared from the ] of ] |
Benzenesulfonic acid is prepared from the ] of ] using concentrated ]: | ||
| :] | :] | ||
| This conversion illustrates aromatic sulfonation, which has been called "one of the most important reactions in industrial organic chemistry."<ref>Otto Lindner, Lars Rodefeld "Benzenesulfonic Acids and Their Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry 2005, Wiley-VCH, Weinheim. {{DOI|10.1002/14356007.a03 507}}</ref> | |||
| == |
==Reactions== | ||
| ⚫ | Benzenesulfonic acid exhibits the reactions typical of other aromatic ]s, forming sulfonamides, sulfonyl chloride, and esters. The sulfonation is reversed above 220 °C. Dehydration with ] gives benzenesulfonic ] ((C<sub>6</sub>H<sub>5</sub>SO<sub>2</sub>)<sub>2</sub>O). Conversion to the corresponding benzenesulfonyl chloride (C<sub>6</sub>H<sub>5</sub>SO<sub>2</sub>Cl) is effected with ]. | ||
| The alkali metal salt of benzenesulfonic acid is used in the production of ] and ]. Benzenesulfonic acid is also used as an acidic catalyst in ] and ]s. | |||
| It is a strong acid, being dissociated in water. | |||
| ⚫ | Benzenesulfonic acid |
||
| ==Applications== | |||
| ⚫ | A variety of pharmaceutical drugs are prepared as ] of benzenesulfonic acid and are known as besylates or besilates. | ||
| The alkali metal salt of benzenesulfonic acid was once widely used in the production of ]: | |||
| :C<sub>6</sub>H<sub>5</sub>SO<sub>3</sub>Na + 2 NaOH → C<sub>6</sub>H<sub>5</sub>ONa + Na<sub>2</sub>SO<sub>3</sub> | |||
| :C<sub>6</sub>H<sub>5</sub>ONa + 2 HCl → C<sub>6</sub>H<sub>5</sub>OH + NaCl | |||
| The process has been largely displaced by the ], which generates less waste. | |||
| ⚫ | Benzenesulfonic acid is mainly consumed by conversion to other specialty chemicals. A variety of pharmaceutical drugs are prepared as ] of benzenesulfonic acid and are known as besylates or besilates. | ||
| ==References== | ==References== | ||
Revision as of 15:26, 28 August 2011
| |||
| Names | |||
|---|---|---|---|
| Other names Benzene sulphonic acid; Benzenesulphonic acid; Phenylsulfonic acid | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChemSpider | |||
| ECHA InfoCard | 100.002.399 | ||
| UNII | |||
| CompTox Dashboard (EPA) | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | C6H6O3S | ||
| Molar mass | 158.17 g·mol | ||
| Appearance | Colorless crystalline solid | ||
| Density | 1.32 g/cm (47 °C) | ||
| Melting point | * 44 °C (hydrate)
| ||
| Boiling point | 190 °C (374 °F; 463 K) | ||
| Solubility in water | Soluble | ||
| Solubility in other solvents | Soluble in alcohol, insoluble in non-polar solvents | ||
| Acidity (pKa) | -2.8 | ||
| Hazards | |||
| Occupational safety and health (OHS/OSH): | |||
| Main hazards | Corrosive | ||
| Flash point | >113 °C | ||
| Related compounds | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Benzenesulfonic acid is an organosulfur compound with the formula C6H5SO3H. It is the simplest aromatic sulfonic acid. It forms colorless deliquescent sheet crystals or a white waxy solid that is soluble in water and ethanol, slightly soluble in benzene and insoluble in carbon disulfide and diethyl ether. It is often stored in the form of alkali metal salts. Its aqueous solution is strongly acidic.
Preparation
Benzenesulfonic acid is prepared from the sulfonation of benzene using concentrated sulfuric acid:
This conversion illustrates aromatic sulfonation, which has been called "one of the most important reactions in industrial organic chemistry."
Reactions
Benzenesulfonic acid exhibits the reactions typical of other aromatic sulfonic acids, forming sulfonamides, sulfonyl chloride, and esters. The sulfonation is reversed above 220 °C. Dehydration with phosphorus pentoxide gives benzenesulfonic acid anhydride ((C6H5SO2)2O). Conversion to the corresponding benzenesulfonyl chloride (C6H5SO2Cl) is effected with phosphorus pentachloride.
It is a strong acid, being dissociated in water.
Applications
The alkali metal salt of benzenesulfonic acid was once widely used in the production of phenol:
- C6H5SO3Na + 2 NaOH → C6H5ONa + Na2SO3
- C6H5ONa + 2 HCl → C6H5OH + NaCl
The process has been largely displaced by the Hock process, which generates less waste. Benzenesulfonic acid is mainly consumed by conversion to other specialty chemicals. A variety of pharmaceutical drugs are prepared as salts of benzenesulfonic acid and are known as besylates or besilates.
References
- Benzenesulfonic acid, Sigma-Aldrich
- Guthrie, J. P. Hydrolysis of esters of oxy acids: pKa values for strong acids. Can. J. Chem. 1978, 56, 2342-2354.
- Otto Lindner, Lars Rodefeld "Benzenesulfonic Acids and Their Derivatives" in Ullmann's Encyclopedia of Industrial Chemistry 2005, Wiley-VCH, Weinheim. doi:10.1002/14356007.a03 507