| Revision as of 10:45, 9 August 2011 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Script assisted update of identifiers for the Chem/Drugbox validation project (updated: 'DrugBank', 'ChEBI').← Previous edit | Revision as of 10:56, 9 August 2011 edit undoCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (no changed fields - added verified revid - updated 'DrugBank_Ref', 'ChEBI_Ref', 'KEGG_Ref') per Chem/Drugbox validation (report [[Wikipedia_talk:WikiProject_Chemicals|errorsNext edit → | ||
| Line 1: | Line 1: | ||
| {{chembox | {{chembox | ||
| | verifiedrevid = |
| verifiedrevid = 443849964 | ||
| | Name = Guanidine | | Name = Guanidine | ||
| | ImageFile = Guanidine-2D-skeletal.png | | ImageFile = Guanidine-2D-skeletal.png | ||
| Line 25: | Line 25: | ||
| | CASNo = 113-00-8 | | CASNo = 113-00-8 | ||
| | CASNo_Ref = {{cascite|correct|CAS}} | | CASNo_Ref = {{cascite|correct|CAS}} | ||
| | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| | |
| DrugBank = DB00536 | ||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | ChEBI = 42820 | | ChEBI = 42820 | ||
| | SMILES = C(=N)(N)N | | SMILES = C(=N)(N)N | ||
Revision as of 10:56, 9 August 2011

| |

| |
| Names | |
|---|---|
| IUPAC name Guanidine | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| DrugBank | |
| ECHA InfoCard | 100.003.656 |
| PubChem CID | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | CH5N3 |
| Molar mass | 59.07 g/mol |
| Melting point | 50 °C |
| Acidity (pKa) | 13.6 (guanidinium cation) |
| Related compounds | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Guanidine is a crystalline compound of strong alkalinity formed by the oxidation of guanine. It is used in the manufacture of plastics and explosives. It is found in urine as a normal product of protein metabolism. The molecule was first synthesized in 1861 by the oxidative degradation of an aromatic natural product, guanine, isolated from Peruvian guano. Despite the simplicity of the molecule, the crystal structure was first described 148 years later.

Guanidinium cation
Guanidine is protonated in physiological conditions. This conjugate acid is called the guanidinium cation, . The guanidinium cation has a charge of +1. It is a highly stable cation in aqueous solution due to the efficient resonance stabilization of the charge and efficient solvation by water molecules. As a result, its pKa is 13.6 meaning that guanidine is a very strong base in water.
-
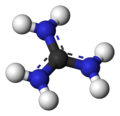
ball-and-stick model -

resonance hybrid -

canonical forms
Notable guanidinium salts include guanidinium chloride (GndCl), which has chaotropic properties and is used to denature proteins. Empirically, guanidine hydrochloride is known to denature proteins with a linear relationship between concentration and free energy of unfolding. Another such salt is guanidinium thiocyanate.
Guanidine derivatives
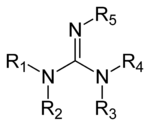
Guanidines are a group of organic compounds sharing a common functional group with the general structure (RRN)(RRN)C=N-R. The central bond within this group is that of an imine, and the group is related structurally to amidines and ureas. Examples of guanidines are arginine, triazabicyclodecene and saxitoxin. Another derivative is guanidinium hydroxide, the active ingredient in some non-lye hair relaxers. Guanidinium salts are well known for their denaturing action on proteins; guanidinium chloride is one of the most effective denaturants. In 6 M aqueous GndHCl almost all proteins lose their ordered "secondary structure" (that results from intramolecular noncovalent interactions) and become "randomly coiled"; that is, their secondary structure interactions are disrupted by the dissolved guanidinium, leaving only the primary covalent structure of their polyamide backbones.
See also
References
- ^ Perrin, D.D., Dissociation Constants of Organic Bases in Aqueous Solution, Butterworths, London, 1965; Supplement, 1972.
- A. Strecker, Liebigs Ann. Chem. 1861, 118, 151.
- T. Yamada, X. Liu, U. Englert, H. Yamane, R. Dronskowski, Chem. Eur. J. 2009, 15, 5651.
| Sympatholytic (and closely related) antihypertensives (C02) | |||||
|---|---|---|---|---|---|
| Sympatholytics (antagonize α-adrenergic vasoconstriction) | |||||
| Other antagonists |
| ||||
| |||||