| Revision as of 08:25, 22 August 2011 editNao1958 (talk | contribs)Extended confirmed users546 editsNo edit summary← Previous edit | Revision as of 19:43, 8 February 2012 edit undo129.137.214.56 (talk) I could not find any evidence that AIBN explodes when dissolved in acetone.Next edit → | ||
| Line 55: | Line 55: | ||
| ==Safety== | ==Safety== | ||
| AIBN is safer to use than ] (another ]) because the risk of explosion is far smaller. However, it is considered a flammable solid. | AIBN is safer to use than ] (another ]) because the risk of explosion is far smaller. However, it is considered a flammable solid. | ||
| It is soluble in methanol and ethanol, but is insoluble in water. |
It is soluble in methanol and ethanol, but is insoluble in water. | ||
| AIBN is highly toxic. A respirator/dust mask, protective gloves, & safety glasses should be worn when handling AIBN. | AIBN is highly toxic. A respirator/dust mask, protective gloves, & safety glasses should be worn when handling AIBN. | ||
Revision as of 19:43, 8 February 2012

| |
| |
| Names | |
|---|---|
| IUPAC name 2,2′-Azobis(2-methylpropionitrile) | |
| Other names
Azobisisobutyronitrile Azobisisobutylonitrile AIBN | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| Abbreviations | AIBN |
| ChemSpider | |
| ECHA InfoCard | 100.001.030 |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C8H12N4 |
| Molar mass | 164.21 g/mol |
| Appearance | white crystalline |
| Density | 1.1 g cm |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
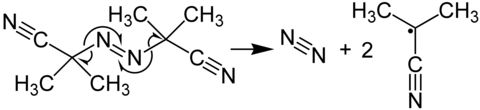
Azobisisobutyronitrile is a compound often used as a foamer in plastics and rubber and as a radical initiator. It is commonly known as AIBN. Its most common chemical reaction is one of decomposition, eliminating a molecule of nitrogen gas to form two 2-cyanoprop-2-yl radicals:
These radicals can be used to initiate free radical polymerizations and other radical reactions. For instance a mixture of styrene and maleic anhydride in toluene will react if heated, forming the polystyrene polymer, only very slowly unless an initiator such an AIBN is present. Another example of a radical reaction that can be initiated by AIBN is the anti-Markovnikov hydrohalogenation of alkenes.
Safety
AIBN is safer to use than benzoyl peroxide (another radical initiator) because the risk of explosion is far smaller. However, it is considered a flammable solid. It is soluble in methanol and ethanol, but is insoluble in water. AIBN is highly toxic. A respirator/dust mask, protective gloves, & safety glasses should be worn when handling AIBN.
Several water-soluble azo initiators similar to AIBN are manufactured by DuPont and Wako.
See also
- 1,1'-Azobis(cyclohexanecarbonitrile) or ABCN is another free radical initiator
References
External links
- SIDS Initial Assessment Report for 2,2’-Azobis(2-methylpropionitrile) from the Organisation for Economic Co-operation and Development (OECD)