| Revision as of 00:29, 22 October 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (changes to verified fields - updated 'DrugBank_Ref', 'UNII_Ref', 'ChEBI_Ref', 'CASNo_Ref') per Chem/Drugbox validation (report errors or bugs)← Previous edit | Revision as of 09:34, 7 January 2012 edit undoKlemen Kocjancic (talk | contribs)Extended confirmed users136,513 editsm clean up, replaced: US → United States using AWBNext edit → | ||
| Line 46: | Line 46: | ||
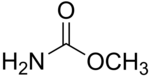
| '''Methyl carbamate''' (also called '''methylurethane''', or '''urethylane''') is an ] and the simplest ester of the hypothetical ] (NH<sub>2</sub>COOH). Its sum formula is C<sub>2</sub>H<sub>5</sub>NO<sub>2</sub>. | '''Methyl carbamate''' (also called '''methylurethane''', or '''urethylane''') is an ] and the simplest ester of the hypothetical ] (NH<sub>2</sub>COOH). Its sum formula is C<sub>2</sub>H<sub>5</sub>NO<sub>2</sub>. | ||
| Methyl carbamate is formed by the reaction of ] with ] or ]. According to ] ] number 2834799 ] can be reacted with ] to form it using ] as a reagent. Unlike its close relative ] it is not mutagenic in ] (it tested negative in the ]), but it is mutagenic in ].<ref>P. Foureman, J.M. Mason, R. Valencia and S. Zimmering, ''Environ. Mol. Mutagen.'', 1994, '''23''' (1), 51 - 63.</ref> Experimental evidence does show that it is a carcinogen in ], and not carcinogenic in mice. The compound is "known to the state of California to cause cancer" per ].<ref></ref> | Methyl carbamate is formed by the reaction of ] with ] or ]. According to ] ] number 2834799 ] can be reacted with ] to form it using ] as a reagent. Unlike its close relative ] it is not mutagenic in ] (it tested negative in the ]), but it is mutagenic in ].<ref>P. Foureman, J.M. Mason, R. Valencia and S. Zimmering, ''Environ. Mol. Mutagen.'', 1994, '''23''' (1), 51 - 63.</ref> Experimental evidence does show that it is a carcinogen in ], and not carcinogenic in mice. The compound is "known to the state of California to cause cancer" per ].<ref></ref> | ||
| The compound was detected in ]s preserved with ].<ref></ref> | The compound was detected in ]s preserved with ].<ref></ref> | ||
| Line 52: | Line 52: | ||
| Methyl carbamate is used by the textile industry to manufacture resins to be applied on polyester/cotton blend fabrics as durable-press ].<ref></ref> | Methyl carbamate is used by the textile industry to manufacture resins to be applied on polyester/cotton blend fabrics as durable-press ].<ref></ref> | ||
| ] are widely used as insecticides.<ref></ref> |
] are widely used as insecticides.<ref></ref> | ||
| ==See also== | ==See also== | ||
Revision as of 09:34, 7 January 2012
| |

| |
| Names | |
|---|---|
| IUPAC name Methyl carbamate | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.009.037 |
| KEGG | |
| PubChem CID | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
| Chemical formula | C2H5NO2 |
| Molar mass | 75 g/mol |
| Melting point | 52 °C (126 °F; 325 K) |
| Boiling point | 177 °C (351 °F; 450 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Methyl carbamate (also called methylurethane, or urethylane) is an organic compound and the simplest ester of the hypothetical carbamic acid (NH2COOH). Its sum formula is C2H5NO2.
Methyl carbamate is formed by the reaction of ammonia with methyl chloroformate or methyl carbonate. According to United States patent number 2834799 urea can be reacted with methanol to form it using boron trifluoride as a reagent. Unlike its close relative ethyl carbamate it is not mutagenic in salmonella (it tested negative in the Ames test), but it is mutagenic in Drosophila. Experimental evidence does show that it is a carcinogen in rat, and not carcinogenic in mice. The compound is "known to the state of California to cause cancer" per Proposition 65.
The compound was detected in wines preserved with dimethyl dicarbonate.
Methyl carbamate is used by the textile industry to manufacture resins to be applied on polyester/cotton blend fabrics as durable-press finishes.
N-Methyl carbamates are widely used as insecticides.
See also
References
- P. Foureman, J.M. Mason, R. Valencia and S. Zimmering, Environ. Mol. Mutagen., 1994, 23 (1), 51 - 63.
- OEHHA
- Inchem.org
- National Toxocology Program
- National Pesticide Information Center at Oregon State University