| Revision as of 03:03, 26 October 2011 editCheMoBot (talk | contribs)Bots141,565 edits Updating {{chembox}} (changes to watched fields - updated 'DrugBank_Ref') per Chem/Drugbox validation (report errors or bugs)← Previous edit | Revision as of 22:15, 20 March 2012 edit undoYobot (talk | contribs)Bots4,733,870 editsm WP:CHECKWIKI error 61 fixes + general fixes using AWB (8032)Next edit → | ||
| Line 36: | Line 36: | ||
| | MolarMass = 135.17 g/mol | | MolarMass = 135.17 g/mol | ||
| | Density = 1.219 g/cm<sup>3</sup> | | Density = 1.219 g/cm<sup>3</sup> | ||
| | MeltingPt = 114.3 |
| MeltingPt = 114.3 °C (236.7 °F) | ||
| | BoilingPt = 304 °C (579 |
| BoilingPt = 304 °C (579 °F) | ||
| | Solubility = <0.1 g/100 mL at 22 °C | | Solubility = <0.1 g/100 mL at 22 °C | ||
| | SolubleOther = Soluble in ], ], ], ] | | SolubleOther = Soluble in ], ], ], ] | ||
| | LogP = 1.16 (23 |
| LogP = 1.16 (23 °C) | ||
| | VaporPressure = 2 Pa (20 |
| VaporPressure = 2 Pa (20 °C) | ||
| }} | }} | ||
| | Section7 = {{Chembox Hazards | | Section7 = {{Chembox Hazards | ||
| Line 51: | Line 51: | ||
| | HPhrases = {{H-phrases|302}} | | HPhrases = {{H-phrases|302}} | ||
| | PPhrases = {{P-phrases|264|270|301+312|330|501}} | | PPhrases = {{P-phrases|264|270|301+312|330|501}} | ||
| | Autoignition = 545 |
| Autoignition = 545 °C (1013 °F) | ||
| }} | }} | ||
| }} | }} | ||
| Line 65: | Line 65: | ||
| ==Applications== | ==Applications== | ||
| Acetanilide is used as an ] in ] and is used to stabilize ] ] ]es. It has also found uses in the intermediation in ] accelerator synthesis, dyes and ] intermediate synthesis, and ] synthesis. Acetanilide is used for the production of 4-acetamidobenzenesulfonyl chloride, a key intermediate for the manufacture of the ]<ref>, Ashford's Dictionary of Industrial Chemicals, Third edition, 2011, page 33</ref> |
Acetanilide is used as an ] in ] and is used to stabilize ] ] ]es. It has also found uses in the intermediation in ] accelerator synthesis, dyes and ] intermediate synthesis, and ] synthesis. Acetanilide is used for the production of 4-acetamidobenzenesulfonyl chloride, a key intermediate for the manufacture of the ].<ref>, Ashford's Dictionary of Industrial Chemicals, Third edition, 2011, page 33</ref> It is also a precursor in the synthesis of ] and other ]s.<ref name="SIDS"/> | ||
| In the 19th century acetanilide was one of a large number of compounds used as experimental ]s. | In the 19th century acetanilide was one of a large number of compounds used as experimental ]s. | ||
Revision as of 22:15, 20 March 2012
| |

| |
| Names | |
|---|---|
| IUPAC names
N-phenylacetamide N-phenylethanamide | |
| Identifiers | |
| CAS Number | |
| 3D model (JSmol) | |
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | 100.002.864 |
| KEGG | |
| UNII | |
| CompTox Dashboard (EPA) | |
InChI
| |
SMILES
| |
| Properties | |
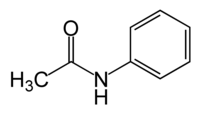
| Chemical formula | C6H5NH(COCH3) |
| Molar mass | 135.17 g/mol |
| Density | 1.219 g/cm |
| Melting point | 114.3 °C (236.7 °F) |
| Boiling point | 304 °C (579 °F) |
| Solubility in water | <0.1 g/100 mL at 22 °C |
| Solubility | Soluble in ethanol, diethyl ether, acetone, benzene |
| log P | 1.16 (23 °C) |
| Vapor pressure | 2 Pa (20 °C) |
| Hazards | |
| GHS labelling: | |
| Pictograms | 
|
| Signal word | Warning |
| Hazard statements | H302 |
| Precautionary statements | P264, P270, P301+P312, P330, P501 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |
Acetanilide is an odourless solid chemical of leaf or flake-like appearance. It is also known as N-phenylacetamide, acetanil, or acetanilid, and was formerly known by the trade name Antifebrin.
Preparation and properties
Acetanilide can be produced by reacting acetic anhydride with aniline:
- C6H5NH2 + (CH3CO)2O → C6H5NHCOCH3 + CH3COOH
The preparation used to be a traditional experiment in introductory organic chemistry lab classes, but it has now been widely replaced by the preparation of either paracetamol or aspirin, both of which teach the same practical techniques (especially recrystallization of the product) but which avoid the use of aniline, a suspected carcinogen.
Acetanilide is slightly soluble in water, and stable under most conditions. Pure crystals are plate shaped and colorless to white.
Applications
Acetanilide is used as an inhibitor in hydrogen peroxide and is used to stabilize cellulose ester varnishes. It has also found uses in the intermediation in rubber accelerator synthesis, dyes and dye intermediate synthesis, and camphor synthesis. Acetanilide is used for the production of 4-acetamidobenzenesulfonyl chloride, a key intermediate for the manufacture of the sulfa drugs. It is also a precursor in the synthesis of penicillin and other pharmaceuticals.
In the 19th century acetanilide was one of a large number of compounds used as experimental photographic developers.
Pharmaceutical use
Acetanilide was the first aniline derivative serendipitously found to possess analgesic as well as antipyretic properties, and was quickly introduced into medical practice under the name of Antifebrin by A. Cahn and P. Hepp in 1886. But its (apparent) unacceptable toxic effects, the most alarming being cyanosis due to methemoglobinemia, prompted the search for supposedly less toxic aniline derivatives such as phenacetin. After several conflicting results over the ensuing fifty years, it was established in 1948 that acetanilide was mostly metabolized to paracetamol (USAN: acetaminophen) in the human body, and that it was the paracetamol that was responsible for the analgesic and antipyretic properties. The observed methemoglobinemia after acetanilide administration was ascribed to the small proportion of acetanilide that is hydrolyzed to aniline in the body. Acetanilide is no longer used as a drug in its own right, although the success of its metabolite – paracetamol (acetaminophen) – is well known.
Notes
- The presence of aniline as an impurity in 19th century batches of acetanilide drugs cannot be ruled out. In this sense as well, paracetamol (acetaminophen) is safer than acetanilide, as (1) the corresponding impurity would be 4-aminophenol, which is less toxic than aniline; and (2) in vivo hydrolysis of the amide group in paracetamol appears to be negligible.
References
- Weast, Robert C., ed. (1981). CRC Handbook of Chemistry and Physics (62nd ed.). Boca Raton, Florida: CRC Press. p. C-67. ISBN 0-8493-0462-8..
- ^ Acetanilide (PDF), SIDS Initial Assessment Report, Geneva: United Nations Environment Programme, September 2003.
- HSNO Chemical Classification Information Database, New Zealand Environmental Risk Management Authority, retrieved 2009-08-26.
- ^ Safety data for acetanilide, Physical Chemistry Laboratory, University of Oxford.
- Acetanilide.
- See, e.g., The preparation of acetanilide from aniline, Department of Chemistry, University of the West Indies at Mona, Jamaica, retrieved 2009-08-26; Reeve, Wilkins; Lowe, Valerie C. (1979), "Preparation of Acetanilide from Nitrobenzene", J. Chem. Educ., 56 (7): 488, doi:10.1021/ed056p488
{{citation}}: Unknown parameter|unused_data=ignored (help): the latter preparation includes the reduction of nitrobenzene to aniline. - , Ashford's Dictionary of Industrial Chemicals, Third edition, 2011, page 33
- Cahn, A.; Hepp, P. (1886), "Das Antifebrin, ein neues Fiebermittel", Centralbl. Klin. Med., 7: 561–64.
- Bertolini, A.; Ferrari, A.; Ottani, A.; Guerzoni, S.; Tacchi, R.; Leone, S. (2006), "Paracetamol: new vistas of an old drug", CNS drug reviews, 12 (3–4): 250–75, doi:10.1111/j.1527-3458.2006.00250.x, PMID 17227290.
- Lester, D.; Greenberg, L. A. (1947), "Metabolic fate of acetanilide and other aniline derivatives. II. Major metabolites of acetanilide in the blood", J. Pharmacol. Exp. Ther., 90 (1): 68, PMID 20241897.
- Brodie, B. B.; Axelrod, J. (1948), "The estimation of acetanilide and its metabolic products, aniline, N-acetyl p-aminophenol and p-aminophenol (free and total conjugated) in biological fluids and tissues", J. Pharmacol. Exp. Ther., 94 (1): 22–28, PMID 18885610.
- Brodie, B. B.; Axelrod, J. (1948), "The fate of acetanilide in man" (PDF), J. Pharmacol. Exp. Ther., 94 (1): 29–38, PMID 18885611
- Flinn, Frederick B.; Brodie, Bernard B. (1948), "The effect on the pain threshold of N-acetyl p-aminophenol, a product derived in the body from acetanilide", J. Pharmacol. Exp. Ther., 94 (1): 76–77, PMID 18885618.