| Revision as of 14:41, 21 November 2011 editBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 443861708 of page Hydramethylnon for the Chem/Drugbox validation project (updated: 'ChEMBL').← Previous edit | Revision as of 14:57, 21 November 2011 edit undoBeetstra (talk | contribs)Edit filter managers, Administrators172,031 edits Saving copy of the {{chembox}} taken from revid 455934086 of page Hydroxylamine for the Chem/Drugbox validation project (updated: 'ChEMBL').Next edit → | ||
| Line 1: | Line 1: | ||
| {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | {{ambox | text = This page contains a copy of the infobox ({{tl|chembox}}) taken from revid of page ] with values updated to verified values.}} | ||
| {{ |
{{Chembox | ||
| | Verifiedfields = changed | |||
| | Watchedfields = changed | | Watchedfields = changed | ||
| | verifiedrevid = |
| verifiedrevid = 398741254 | ||
| | ImageFileL1 = Hydroxylamine-2D.png | |||
| | Name = Hydramethylnon | |||
| | ImageFileL1_Ref = {{chemboximage|correct|??}} | |||
| | ImageFile = Hydramethylnon.png | |||
| | ImageSizeL1 = 121 | |||
| | IUPACName = 2(1''H'')-pyrimidinone, tetrahydro-5,5-dimethyl-, | |||
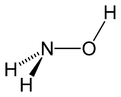
| | ImageNameL1 = Stereo, skeletal formula of hydroxylamine with all explicit hydrogens added | |||
| (3-(4-(trifluoromethyl)phenyl) | |||
| | ImageFileR1 = Hydroxylamine-3D-balls.png | |||
| -1-(2-(4-(trifluoromethyl)phenyl)ethenyl) | |||
| | ImageFileR1_Ref = {{chemboximage|correct|??}} | |||
| -2-propenylidene)hydrazone | |||
| | ImageSizeR1 = 121 | |||
| | ImageNameR1 = Spacefill model of hydroxylamine | |||
| | ImageFile2 = Hydroxylamine-dimensions-2D.png | |||
| | ImageFile2_Ref = {{chemboximage|correct|??}} | |||
| | ImageSize2 = 242 | |||
| | ImageName2 = Stereo, skeletal formula of hydroxylamine with all explicit hydrogens added and assorted dimensions | |||
| | IUPACName = Hydroxylamine | |||
| | SystematicName = Hydroxylamine<ref>{{Cite web|title = Hydroxylamine - PubChem Public Chemical Database|url = http://pubchem.ncbi.nlm.nih.gov/summary/summary.cgi?cid=787|work = The PubChem Project|location = USA|publisher = National Center for Biotechnology Information}}</ref> | |||
| | OtherNames = Aminol<br /> | |||
| Azanol<br /> | |||
| Hydroxyamine<br /> | |||
| Hydroxyazane<br /> | |||
| Hydroxylazane<br /> | |||
| Nitrinous acid | |||
| | Section1 = {{Chembox Identifiers | | Section1 = {{Chembox Identifiers | ||
| | CASNo = 7803-49-8 | |||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | PubChem = 787 | |||
| | PubChem_Ref = {{Pubchemcite|correct|pubchem}} | |||
| | ChemSpiderID = 766 | |||
| | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | | ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | ||
| | EINECS = 232-259-2 | |||
| | ChemSpiderID = 4445168 | |||
| | |
| KEGG = C00192 | ||
| | KEGG_Ref = {{keggcite|correct|kegg}} | |||
| | PubChem = 5281875 | |||
| | MeSHName = Hydroxylamine | |||
| | InChIKey = IQVNEKKDSLOHHK-FNCQTZNRBM | |||
| | |
| ChEBI_Ref = {{ebicite|correct|EBI}} | ||
| | ChEBI = 15429 | |||
| | StdInChI = 1S/C25H24F6N4/c1-23(2)15-32-22(33-16-23)35-34-21(13-7-17-3-9-19(10-4-17)24(26,27)28)14-8-18-5-11-20(12-6-18)25(29,30)31/h3-14H,15-16H2,1-2H3,(H2,32,33,35)/b13-7+,14-8+ | |||
| | ChEMBL = <!-- blanked - oldvalue: 1191361 --> | |||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| | StdInChIKey = IQVNEKKDSLOHHK-FNCQTZNRSA-N | |||
| | RTECS = NC2975000 | |||
| | CASNo_Ref = {{cascite|correct|CAS}} | |||
| | |
| Gmelin = 478 | ||
| | 3DMet = B01184 | |||
| | KEGG_Ref = {{keggcite|correct|kegg}} | |||
| | UNII_Ref = {{fdacite|changed|FDA}} | |||
| | KEGG = C10994 | |||
| | UNII = 2FP81O2L9Z | |||
| | ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| | |
| SMILES = NO | ||
| | SMILES1 = ON | |||
| | SMILES = FC(F)(F)c1ccc(cc1)\C=C\C(=N/N/C2=N/CC(C)(C)CN2)/C=C/c3ccc(cc3)C(F)(F)F | |||
| | StdInChI = 1S/H3NO/c1-2/h2H,1H2 | |||
| | InChI = 1/C25H24F6N4/c1-23(2)15-32-22(33-16-23)35-34-21(13-7-17-3-9-19(10-4-17)24(26,27)28)14-8-18-5-11-20(12-6-18)25(29,30)31/h3-14H,15-16H2,1-2H3,(H2,32,33,35)/b13-7+,14-8+ | |||
| | StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| | InChI = 1/H3NO/c1-2/h2H,1H2 | |||
| | StdInChIKey = AVXURJPOCDRRFD-UHFFFAOYSA-N | |||
| | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| | InChIKey = AVXURJPOCDRRFD-UHFFFAOYAD | |||
| }} | }} | ||
| | Section2 = {{Chembox Properties | | Section2 = {{Chembox Properties | ||
| | H = 3 | |||
| | Formula = C<sub>25</sub>H<sub>24</sub>F<sub>6</sub>N<sub>4</sub> | |||
| | N = 1 | |||
| | Appearance = yellow to orange crystalline solid | |||
| | |
| O = 1 | ||
| | ExactMass = 33.021463723 g mol<sup>-1</sup> | |||
| | Density = | |||
| | Appearance = Vivid white, opaque crystals | |||
| | MeltingPt = 185-190 °C | |||
| | Density = 1.21 g cm<sup>-3</sup> (at 20 °C)<ref name="RubberBible87th">{{RubberBible87th}}</ref> | |||
| | BoilingPt = | |||
| | MeltingPtC = 33 | |||
| }} | |||
| | BoilingPtC = 58 | |||
| | Section7 = {{Chembox Hazards | |||
| | |
| Boiling_notes = decomposes | ||
| | |
| LogP = -0.758 | ||
| | |
| pKa = 13.7 | ||
| | pKb = 0.3 | |||
| }} | |||
| | Section3 = {{Chembox Structure | |||
| | Coordination = Trigonal at N | |||
| | MolShape = Tetrahedral at N | |||
| | Dipole = 0.67553 D | |||
| }} | |||
| | Section4 = {{Chembox Thermochemistry | |||
| | DeltaHf = -39.9 kJ mol<sup>-1</sup> | |||
| | Entropy = 236.18 J K<sup>-1</sup> mol<sup>-1</sup> | |||
| | HeatCapacity = 46.47 J K<sup>-1</sup> mol<sup>-1</sup> | |||
| }} | |||
| | Section5 = {{Chembox Hazards | |||
| | ExternalMSDS = | |||
| | EUIndex = 612-122-00-7 | |||
| | EUClass = E {{Hazchem Xn}} {{Hazchem Xi}} {{Hazchem N}} | |||
| | RPhrases = {{R2}}, {{R21/22}}, {{R37/38}}, {{R40}}, {{R41}}, {{R43}}, {{R48/22}}, {{R50}} | |||
| | SPhrases = {{S2}}, {{S26}}, {{S36/37/39}}, {{S61}} | |||
| | NFPA-H = 2 | |||
| | NFPA-F = 1 | |||
| | NFPA-R = 3 | |||
| | FlashPt = 129 °C | |||
| | Autoignition = 265 °C | |||
| | LD50 = 408 mg/kg (oral, mouse); 59–70 mg/kg (intraperitoneal mouse, rat); 29 mg/kg (subcutaneous, rat)<ref>{{cite book |author=Martel, B.; Cassidy, K. |title=Chemical Risk Analysis: A Practical Handbook |publisher=Butterworth–Heinemann |year=2004 |pages=362 |isbn=1903996651}}</ref> | |||
| }} | }} | ||
| | Section6 = {{Chembox Related | |||
| | Function = hydroxylammonium salts | |||
| | OtherFunctn = ]<br/>]<br/>] | |||
| | OtherCpds = ]<br /> | |||
| ] | |||
| }} | |||
| }} | }} | ||
Revision as of 14:57, 21 November 2011
| This page contains a copy of the infobox ({{chembox}}) taken from revid 455934086 of page Hydroxylamine with values updated to verified values. |
| |||

| |||
| Names | |||
|---|---|---|---|
| IUPAC name Hydroxylamine | |||
| Systematic IUPAC name Hydroxylamine | |||
| Other names
Aminol Azanol | |||
| Identifiers | |||
| CAS Number | |||
| 3D model (JSmol) | |||
| ChEBI | |||
| ChemSpider | |||
| EC Number |
| ||
| Gmelin Reference | 478 | ||
| KEGG | |||
| MeSH | Hydroxylamine | ||
| PubChem CID | |||
| RTECS number |
| ||
| UNII | |||
InChI
| |||
SMILES
| |||
| Properties | |||
| Chemical formula | H3NO | ||
| Molar mass | 33.030 g·mol | ||
| Appearance | Vivid white, opaque crystals | ||
| Density | 1.21 g cm (at 20 °C) | ||
| Melting point | 33 °C (91 °F; 306 K) | ||
| Boiling point | 58 °C (136 °F; 331 K) | ||
| log P | -0.758 | ||
| Acidity (pKa) | 13.7 | ||
| Basicity (pKb) | 0.3 | ||
| Structure | |||
| Coordination geometry | Trigonal at N | ||
| Molecular shape | Tetrahedral at N | ||
| Dipole moment | 0.67553 D | ||
| Thermochemistry | |||
| Heat capacity (C) | 46.47 J K mol | ||
| Std molar entropy (S298) |
236.18 J K mol | ||
| Std enthalpy of formation (ΔfH298) |
-39.9 kJ mol | ||
| Hazards | |||
| NFPA 704 (fire diamond) |
 | ||
| Flash point | 129 °C | ||
| Lethal dose or concentration (LD, LC): | |||
| LD50 (median dose) | 408 mg/kg (oral, mouse); 59–70 mg/kg (intraperitoneal mouse, rat); 29 mg/kg (subcutaneous, rat) | ||
| Related compounds | |||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C , 100 kPa).
| |||
Chemical compound
- "Hydroxylamine - PubChem Public Chemical Database". The PubChem Project. USA: National Center for Biotechnology Information.
- Lide, David R., ed. (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, Florida: CRC Press. ISBN 0-8493-0487-3.
- Martel, B.; Cassidy, K. (2004). Chemical Risk Analysis: A Practical Handbook. Butterworth–Heinemann. p. 362. ISBN 1903996651.
{{cite book}}: CS1 maint: multiple names: authors list (link)